false 0001531978 0001531978 2023-11-07 2023-11-07
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): November 07, 2023
Paragon 28, Inc.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
| Delaware |
|
001-40902 |
|
27-3170186 |
(State or Other Jurisdiction
of Incorporation) |
|
(Commission File Number) |
|
(IRS Employer
Identification No.) |
|
|
|
| 14445 Grasslands Drive |
|
|
| Englewood, Colorado |
|
80112 |
| (Address of Principal Executive Offices) |
|
(Zip Code) |
Registrant’s Telephone Number, Including Area Code: (720) 912-1332
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
| Title of each class |
|
Trading
Symbol(s) |
|
Name of each exchange on which registered |
| Common Stock, $0.01 par value |
|
FNA |
|
The New York Stock Exchange |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 2.02 |
Results of Operations and Financial Condition. |
On November 7, 2023, Paragon 28, Inc. (the “Company”) issued a press release announcing certain financial results for the third quarter ended September 30, 2023. A copy of the Company’s press release is furnished as Exhibit 99.1 hereto.
The information in Item 2.02 of this Current Report on Form 8-K, including Exhibit 99.1, shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liabilities of that section or Section 11 and 12(a)(2) of the Securities Act of 1933, as amended. The information contained in Item 2.02 of this Current Report on Form 8-K, including Exhibit 99.1, shall not be incorporated by reference into any filing with the U.S. Securities and Exchange Commission made by the Company, whether made before or after the date hereof, regardless of any general incorporation language in such filing, except as expressly set forth by specific reference in such a filing.
| Item 7.01 |
Regulation FD Disclosure. |
On November 7, 2023, the Company posted a presentation to its website relating to the Company’s quarterly discussion of its financial profile, market strategy, products and recent developments. A copy of the presentation is attached as Exhibit 99.2 to this Current Report on Form 8-K.
The information in Item 7.01 of this Current Report on Form 8-K, including Exhibit 99.2, shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liabilities of that section or Section 11 and 12(a)(2) of the Securities Act of 1933, as amended. The information contained in Item 7.01 of this Current Report on Form 8-K, including Exhibit 99.2, shall not be incorporated by reference into any filing with the U.S. Securities and Exchange Commission made by the Company, whether made before or after the date hereof, regardless of any general incorporation language in such filing, except as expressly set forth by specific reference in such a filing.
| Item 9.01 |
Financial Statements and Exhibits. |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
|
|
PARAGON 28, INC. |
|
|
|
|
| Date: November 7, 2023 |
|
|
|
By: |
|
/s/ Robert S. McCormack |
|
|
|
|
|
|
General Counsel & Corporate Secretary |
Exhibit 99.1
Paragon 28 Reports Third Quarter 2023 Financial Results and Reaffirms 2023 Net Revenue Guidance
ENGLEWOOD, CO., November 7, 2023 — Paragon 28, Inc. (NYSE: FNA) (“Paragon 28” or “Company”), a leading medical device
company exclusively focused on the foot and ankle orthopedic market, today reported financial results for the quarter ended September 30, 2023 and reaffirmed its 2023 net revenue guidance.
Third Quarter 2023 and Nine Months Ended September 30, 2023 Financial Results
| |
• |
|
Consolidated net revenue for the third quarter of 2023 was $52.8 million, representing 14.7% and 14.5%
reported and constant currency growth, respectively, compared to the third quarter of 2022. Consolidated net revenue for the nine months ended September 30, 2023 was $155.8 million, representing 20.0% and 20.6% reported and constant
currency growth, respectively, compared to the nine months ended September 30, 2022. |
| |
• |
|
U.S. net revenue for the third quarter of 2023 and nine months ended September 30, 2023 was
$44.6 million and $131.8 million, respectively, representing 11.5% and 16.9% reported growth, respectively, compared to the prior year periods. |
| |
• |
|
International net revenue for the third quarter of 2023 and nine months ended September 30, 2023 was
$8.2 million and $24.0 million, respectively, representing 36.2% and 40.6% reported growth respectively, compared to the prior year periods. |
| |
• |
|
Gross margin was 80.3% for the third quarter of 2023 compared to 81.5% in the third quarter of 2022. Gross margin
was 81.9% for the nine months ended September 30, 2023, compared to 82.4% for the nine months ended September 30, 2022. |
| |
• |
|
Operating expenses were $51.4 million for the third quarter of 2023, an increase of 11.7%, compared to
$46.0 million for the third quarter of 2022. Operating expenses were $153.7 million for the nine months ended September 30, 2023, an increase of 15.6%, compared to $133.0 million for the nine months ended September 30, 2022.
|
| |
• |
|
Net loss was $8.3 million for the third quarter of 2023, a decrease of 14.3%, compared to a net loss of
$9.7 million for the third quarter of 2022. Net loss was $28.3 million for the nine months ended September 30, 2023, a decrease of 1%, compared to net a loss of $28.6 million for the nine months ended September 30, 2022.
|
| |
• |
|
Adjusted EBITDA was a $1.2 million loss for the third quarter of 2023, an improvement of 54.1%, compared to
a $2.7 million loss in the third quarter of 2022. Adjusted EBITDA was a $5.3 million loss for the nine months ended September 30, 2023, an improvement of 42.4%, compared to a $9.1 million loss for the nine months ended
September 30, 2022. |
“Paragon 28’s business fundamentals are as strong as ever and continue to position us for
sustainable long-term growth. Through the third quarter, we grew revenue 20% and ended the quarter with record numbers of U.S. sales representatives and surgeon customers while also improving EBITDA by over 50% compared the third quarter of
2022.” said Albert DaCosta, Chairman and Chief Executive Officer. “Finally, our new product pipeline is filled with meaningful and innovative technologies, and we are excited to be bringing several of these important new products to market
in the next few quarters.”
2023 Net Revenue Guidance
The Company reaffirms its prior 2023 net revenue guidance, and expects net revenue to be $214 million to $218 million, representing 19% and 20%
reported and constant currency growth at the midpoint, respectively, compared to 2022.
The Company’s 2023 net revenue guidance assumes foreign
currency translation rates remain consistent with current foreign currency translation rates.
1
Webcast and Conference Call Information
Paragon 28 will host a conference call to discuss third quarter 2023 financial results on Tuesday, November 7, 2023, at 2:30 p.m. Mountain Time / 4:30
p.m. Eastern Time. Investors interested in listening to the conference call may do so by dialing (833-470-1428) for domestic callers or (646-904-5544) for international callers, using conference ID: 308457. Live audio of the webcast will be available on the “Investors” section of the company’s website at: ir.paragon28.com. The
webcast will be archived and available for replay for at least 90 days after the event.
About Paragon 28, Inc.
Based in Englewood, Colo., Paragon 28, is a leading medical device company exclusively focused on the foot and ankle orthopedic market and is dedicated to
improving patient lives. From the onset, Paragon 28® has provided innovative orthopedic solutions, procedural approaches and instrumentation that cover a wide range of foot and ankle ailments
including fracture fixation, forefoot, ankle, progressive collapsing foot deformity (PCFD) or flatfoot, charcot foot and orthobiologics. The company designs products with both the patient and surgeon in mind, with the goal of improving outcomes,
reducing ailment recurrence and complication rates, and making the procedures simpler, consistent, and reproducible.
Forward Looking Statements
Except for the historical information contained herein, the matters set forth in this press release are forward-looking statements within the meaning
of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995, including, but not limited to: Paragon 28’s potential to shape a better future for foot and ankle patients and its estimated net revenue for full
year 2023. You are cautioned not to place undue reliance on these forward-looking statements. Forward-looking statements are only predictions based on our current expectations, estimates, and assumptions, valid only as of the date they are made, and
subject to risks and uncertainties, some of which we are not currently aware. Forward-looking statements should not be read as a guarantee of future performance or results and may not necessarily be accurate
indications of the times at, or by, which such performance or results will be achieved. These forward-looking statements are based on Paragon 28’s current expectations and inherently involve significant
risks and uncertainties. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of these risks and uncertainties. These risks and
uncertainties are described more fully in the section titled “Risk Factors” in Paragon 28’s filings with the Securities and Exchange Commission (the “SEC”), including Paragon 28’s annual report on Form 10-K filed with the SEC on March 2, 2023. Paragon 28 does not undertake any obligation to update forward-looking statements and expressly disclaims any obligation or
undertaking to release publicly any updates or revisions to any forward-looking statements contained herein. These forward-looking statements should not be relied upon as representing Paragon 28’s views
as of any date subsequent to the date of this press release. Paragon 28’s results for the quarter ended September 30, 2023 are not necessarily indicative of our operating results for any future periods.
2
Use of Non-GAAP Financial Measures and Their Limitations
In addition to our results and measures of performance determined in accordance with U.S. GAAP presented in this press release, we believe that certain non-GAAP financial measures are useful in evaluating and comparing our financial and operational performance over multiple periods, identifying trends affecting our business, formulating business plans and making
strategic decisions.
Adjusted EBITDA is a key performance measure that our management uses to assess our financial performance and is also used for
internal planning and forecasting purposes. We define Adjusted EBITDA as earnings (loss) before interest expense, income tax expense (benefit), depreciation and amortization, stock-based compensation expense, employee stock purchase plan expense, non-recurring expenses and certain other non-cash expenses.
We believe that
Adjusted EBITDA, together with a reconciliation to net income, helps identify underlying trends in our business and helps investors make comparisons between our company and other companies that may have different capital structures, tax rates, or
different forms of employee compensation. Accordingly, we believe that Adjusted EBITDA provides useful information to investors and others in understanding and evaluating our operating results, enhancing the overall understanding of our past
performance and future prospects, and allowing for greater transparency with respect to a key financial metric used by our management in its financial and operational decision-making. Our use of Adjusted EBITDA has limitations as an analytical tool,
and you should not consider these measures in isolation or as a substitute for analysis of our financial results as reported under U.S. GAAP. Some of these potential limitations include:
| |
• |
|
other companies, including companies in our industry which have similar business arrangements, may report
Adjusted EBITDA, or similarly titled measures but calculate them differently, which reduces their usefulness as comparative measures; |
| |
• |
|
although depreciation and amortization expenses are non-cash charges, the
assets being depreciated and amortized may have to be replaced in the future, and Adjusted EBITDA does not reflect cash capital expenditures for such replacements or for new capital expenditure requirements; |
| |
• |
|
Adjusted EBITDA also does not reflect changes in, or cash requirements for, our working capital needs or the
potentially dilutive impact of stock-based compensation; and |
| |
• |
|
Adjusted EBITDA does not reflect the interest expense, or the cash requirements necessary to service interest or
principal payments, on our debt that we may incur. |
Additionally, we report revenue growth on a constant-currency basis in order to
facilitate period-to-period comparisons of results without regard to the impact of fluctuating foreign currency exchange rates. The term foreign currency exchange rates
refers to the exchange rates used to translate the company’s operating results for all countries where the functional currency is not the U.S. dollar into U.S. dollars. Because we are a global company, foreign currency exchange rates used for
translation may have a significant effect on our reported results. References to revenue growth on a constant-currency basis means without the impact of foreign currency exchange rate fluctuations.
The company believes disclosure of constant-currency revenue growth rates is helpful to investors because it facilitates period-to-period comparisons. However, constant-currency revenue growth rates are non-GAAP financial measures and are not meant to be considered as an alternative or
substitute for comparable measures prepared in accordance with GAAP. Constant-currency growth has no standardized meaning prescribed by GAAP and should be read in conjunction with our consolidated financial statements prepared in accordance with
GAAP. We calculate constant-currency growth rates by translating local currency amounts in the current period at actual foreign exchange rates for the prior period.
Because of these and other limitations, you should consider our non-GAAP measures only as supplemental to other
GAAP-based financial measures.
Investor Contact:
Matt Brinckman
Senior Vice President, Strategy and Investor
Relations
mbrinckman@paragon28.com
3
PARAGON 28, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
(in thousands, unaudited)
|
|
|
|
|
|
|
|
|
| |
|
September 30, 2023 |
|
|
December 31, 2022 |
|
| ASSETS |
|
|
|
|
|
|
| Current assets: |
|
|
|
|
|
|
|
|
| Cash |
|
$ |
34,949 |
|
|
$ |
38,468 |
|
| Trade receivables |
|
|
33,615 |
|
|
|
37,687 |
|
| Inventories, net |
|
|
94,380 |
|
|
|
60,948 |
|
| Income taxes receivable |
|
|
1,022 |
|
|
|
615 |
|
| Other current assets |
|
|
4,826 |
|
|
|
4,658 |
|
|
|
|
|
|
|
|
|
|
| Total current assets |
|
|
168,792 |
|
|
|
142,376 |
|
| Property and equipment, net |
|
|
73,530 |
|
|
|
61,938 |
|
| Intangible assets, net |
|
|
21,802 |
|
|
|
22,387 |
|
| Goodwill |
|
|
25,465 |
|
|
|
25,465 |
|
| Deferred income taxes |
|
|
132 |
|
|
|
148 |
|
| Other assets |
|
|
3,634 |
|
|
|
1,795 |
|
|
|
|
|
|
|
|
|
|
| Total assets |
|
$ |
293,355 |
|
|
$ |
254,109 |
|
|
|
|
|
|
|
|
|
|
| LIABILITIES & STOCKHOLDERS’ EQUITY |
|
|
|
|
|
|
| Current liabilities: |
|
|
|
|
|
|
|
|
| Accounts payable |
|
$ |
27,395 |
|
|
$ |
14,939 |
|
| Accrued expenses |
|
|
24,966 |
|
|
|
26,807 |
|
| Accrued legal settlement |
|
|
— |
|
|
|
22,000 |
|
| Other current liabilities |
|
|
1,893 |
|
|
|
3,844 |
|
| Current maturities of long-term debt |
|
|
640 |
|
|
|
728 |
|
| Income taxes payable |
|
|
— |
|
|
|
184 |
|
|
|
|
|
|
|
|
|
|
| Total current liabilities |
|
|
54,894 |
|
|
|
68,502 |
|
| Long-term liabilities: |
|
|
|
|
|
|
|
|
| Long-term debt net, less current maturities |
|
|
42,288 |
|
|
|
42,182 |
|
| Other long-term liabilities |
|
|
1,467 |
|
|
|
1,628 |
|
| Deferred income taxes |
|
|
327 |
|
|
|
342 |
|
| Income taxes payable |
|
|
635 |
|
|
|
527 |
|
|
|
|
|
|
|
|
|
|
| Total liabilities |
|
|
99,611 |
|
|
|
113,181 |
|
|
|
|
|
|
|
|
|
|
| Stockholders’ equity: |
|
|
|
|
|
|
|
|
| Common stock, $0.01 par value, 300,000,000 shares authorized; 83,469,426 and 78,684,107 shares
issued, and 82,555,907 and 77,770,588 shares outstanding as of September 30, 2023 and December 31, 2022, respectively |
|
|
824 |
|
|
|
776 |
|
| Additional paid in capital |
|
|
296,018 |
|
|
|
213,956 |
|
| Accumulated deficit |
|
|
(96,071 |
) |
|
|
(67,789 |
) |
| Accumulated other comprehensive loss |
|
|
(1,045 |
) |
|
|
(33 |
) |
| Treasury stock, at cost; 913,519 shares as of September 30, 2023 and December 31,
2022 |
|
|
(5,982 |
) |
|
|
(5,982 |
) |
|
|
|
|
|
|
|
|
|
| Total stockholders’ equity |
|
|
193,744 |
|
|
|
140,928 |
|
|
|
|
|
|
|
|
|
|
| Total liabilities & stockholders’ equity |
|
$ |
293,355 |
|
|
$ |
254,109 |
|
|
|
|
|
|
|
|
|
|
4
PARAGON 28, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS
(in thousands, unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Three Months Ended September 30, |
|
|
Nine Months Ended September 30, |
|
| |
|
2023 |
|
|
2022 |
|
|
2023 |
|
|
2022 |
|
| Net revenue |
|
$ |
52,783 |
|
|
$ |
46,006 |
|
|
$ |
155,828 |
|
|
$ |
129,875 |
|
| Cost of goods sold |
|
|
10,394 |
|
|
|
8,491 |
|
|
|
28,158 |
|
|
|
22,920 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Gross profit |
|
|
42,389 |
|
|
|
37,515 |
|
|
|
127,670 |
|
|
|
106,955 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Research and development costs |
|
|
7,244 |
|
|
|
6,337 |
|
|
|
21,976 |
|
|
|
18,100 |
|
| Selling, general, and administrative |
|
|
44,126 |
|
|
|
39,667 |
|
|
|
131,773 |
|
|
|
114,857 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total operating expenses |
|
|
51,370 |
|
|
|
46,004 |
|
|
|
153,749 |
|
|
|
132,957 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Operating loss |
|
|
(8,981 |
) |
|
|
(8,489 |
) |
|
|
(26,079 |
) |
|
|
(26,002 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Other income (expense): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Other income, net |
|
|
1,660 |
|
|
|
59 |
|
|
|
1,014 |
|
|
|
610 |
|
| Interest expense, net |
|
|
(1,119 |
) |
|
|
(1,093 |
) |
|
|
(3,127 |
) |
|
|
(2,865 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total other income (expense) |
|
|
541 |
|
|
|
(1,034 |
) |
|
|
(2,113 |
) |
|
|
(2,255 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Loss before income taxes |
|
|
(8,440 |
) |
|
|
(9,523 |
) |
|
|
(28,192 |
) |
|
|
(28,257 |
) |
| Income tax (benefit) expense |
|
|
(108 |
) |
|
|
201 |
|
|
|
90 |
|
|
|
306 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net loss |
|
$ |
(8,332 |
) |
|
$ |
(9,724 |
) |
|
$ |
(28,282 |
) |
|
$ |
(28,563 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
5
PARAGON 28, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands, unaudited)
|
|
|
|
|
|
|
|
|
| |
|
Nine Months Ended September 30, |
|
| |
|
2023 |
|
|
2022 |
|
| Cash flows from operating activities |
|
|
|
|
|
|
|
|
| Net loss |
|
$ |
(28,282 |
) |
|
$ |
(28,563 |
) |
| Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
|
|
| Depreciation and amortization |
|
|
10,602 |
|
|
|
9,624 |
|
| Allowance for doubtful accounts |
|
|
147 |
|
|
|
— |
|
| Provision for excess and obsolete inventories |
|
|
2,053 |
|
|
|
(91 |
) |
| Stock-based compensation |
|
|
10,294 |
|
|
|
7,052 |
|
| Other |
|
|
(1,428 |
) |
|
|
(1,295 |
) |
| Changes in other assets and liabilities, net of acquisitions: |
|
|
|
|
|
|
|
|
| Accounts receivable |
|
|
3,706 |
|
|
|
(10,227 |
) |
| Inventories |
|
|
(35,558 |
) |
|
|
(15,316 |
) |
| Accounts payable |
|
|
12,468 |
|
|
|
951 |
|
| Accrued expenses |
|
|
3,718 |
|
|
|
176 |
|
| Accrued legal settlement |
|
|
(22,000 |
) |
|
|
— |
|
| Income tax receivable/payable |
|
|
(533 |
) |
|
|
297 |
|
| Other assets and liabilities |
|
|
(2,704 |
) |
|
|
1,442 |
|
|
|
|
|
|
|
|
|
|
| Net cash used in operating activities |
|
|
(47,517 |
) |
|
|
(35,950 |
) |
|
|
|
|
|
|
|
|
|
| Cash flows from investing activities |
|
|
|
|
|
|
|
|
| Purchase of office building |
|
|
— |
|
|
|
(18,300 |
) |
| Purchases of property and equipment |
|
|
(21,893 |
) |
|
|
(15,637 |
) |
| Proceeds from sale of property and equipment |
|
|
795 |
|
|
|
642 |
|
| Purchases of intangible assets |
|
|
(933 |
) |
|
|
(1,720 |
) |
| Acquisition of Disior, net of cash received |
|
|
— |
|
|
|
(18,504 |
) |
|
|
|
|
|
|
|
|
|
| Net cash used in investing activities |
|
|
(22,031 |
) |
|
|
(53,519 |
) |
|
|
|
|
|
|
|
|
|
| Cash flows from financing activities |
|
|
|
|
|
|
|
|
| Proceeds from draw on term loan |
|
|
— |
|
|
|
20,000 |
|
| Proceeds from issuance of long-term debt |
|
|
— |
|
|
|
16,000 |
|
| Payments on long-term debt |
|
|
(568 |
) |
|
|
(367 |
) |
| Payments of debt issuance costs |
|
|
— |
|
|
|
(420 |
) |
| Proceeds from issuance of common stock, net of issuance costs |
|
|
68,453 |
|
|
|
— |
|
| Proceeds from exercise of stock options |
|
|
2,535 |
|
|
|
2,224 |
|
| Proceeds from employee stock purchase plan |
|
|
560 |
|
|
|
— |
|
| Payments on earnout liability |
|
|
(5,500 |
) |
|
|
(500 |
) |
|
|
|
|
|
|
|
|
|
| Net cash provided by financing activities |
|
|
65,480 |
|
|
|
36,937 |
|
|
|
|
|
|
|
|
|
|
| Effect of exchange rate changes on cash |
|
|
549 |
|
|
|
(495 |
) |
|
|
|
|
|
|
|
|
|
| Net decrease in cash |
|
|
(3,519 |
) |
|
|
(53,027 |
) |
| Cash at beginning of period |
|
|
38,468 |
|
|
|
109,352 |
|
|
|
|
|
|
|
|
|
|
| Cash at end of period |
|
$ |
34,949 |
|
|
$ |
56,325 |
|
|
|
|
|
|
|
|
|
|
6
PARAGON 28, INC. AND SUBSIDIARIES
RECONCILIATION OF NET LOSS TO NON-GAAP ADJUSTED EBITDA
(in thousands, unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Three Months Ended
September 30, |
|
|
Nine Months Ended
September 30, |
|
| |
|
2023 |
|
|
2022 |
|
|
2023 |
|
|
2022 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
(in thousands) |
|
| Net loss |
|
$ |
(8,332 |
) |
|
$ |
(9,724 |
) |
|
$ |
(28,282 |
) |
|
$ |
(28,563 |
) |
| Interest expense, net |
|
|
1,119 |
|
|
|
1,093 |
|
|
|
3,127 |
|
|
|
2,865 |
|
| Income tax (benefit) expense |
|
|
(108 |
) |
|
|
201 |
|
|
|
90 |
|
|
|
306 |
|
| Depreciation and amortization expense |
|
|
4,188 |
|
|
|
3,058 |
|
|
|
10,602 |
|
|
|
9,624 |
|
| Stock based compensation expense |
|
|
3,512 |
|
|
|
2,587 |
|
|
|
10,294 |
|
|
|
7,052 |
|
| Employee stock purchase plan expense |
|
|
86 |
|
|
|
100 |
|
|
|
268 |
|
|
|
100 |
|
| Change in fair value (1) |
|
|
(1,714 |
) |
|
|
(35 |
) |
|
|
(1,394 |
) |
|
|
(575 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Adjusted EBITDA |
|
$ |
(1,249 |
) |
|
$ |
(2,720 |
) |
|
$ |
(5,295 |
) |
|
$ |
(9,191 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (1) |
Represents non-cash change in the fair value of earnout liabilities and
interest rate swap contract. |
7
PARAGON 28, INC. AND SUBSIDIARIES
Constant-Currency Revenue Growth
(in thousands, unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Three Months Ended
September 30, |
|
|
Change |
|
|
Nine Months Ended
September 30, |
|
|
Change |
|
| |
|
2023 |
|
|
2022 |
|
|
% |
|
|
2023 |
|
|
2022 |
|
|
% |
|
| Total Consolidated Revenues |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| As Reported |
|
$ |
52,783 |
|
|
$ |
46,006 |
|
|
|
14.7 |
% |
|
$ |
155,828 |
|
|
$ |
129,875 |
|
|
|
20.0 |
% |
| Impact of foreign currency exchange rates |
|
|
(92 |
) |
|
|
— |
|
|
|
* |
|
|
|
804 |
|
|
|
— |
|
|
|
* |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Constant-currency net revenues |
|
$ |
52,691 |
|
|
$ |
46,006 |
|
|
|
14.5 |
% |
|
$ |
156,632 |
|
|
$ |
129,875 |
|
|
|
20.6 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total International Revenues |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| As Reported |
|
$ |
8,235 |
|
|
$ |
6,046 |
|
|
|
36.2 |
% |
|
$ |
24,035 |
|
|
$ |
17,094 |
|
|
|
40.6 |
% |
| Impact of foreign currency exchange rates |
|
|
(92 |
) |
|
|
— |
|
|
|
* |
|
|
|
804 |
|
|
|
— |
|
|
|
* |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Constant-currency net revenues |
|
$ |
8,143 |
|
|
$ |
6,046 |
|
|
|
34.7 |
% |
|
$ |
24,839 |
|
|
$ |
17,094 |
|
|
|
45.3 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
8

3Q 2023 Update November 7, 2023
Exhibit 99.2

Forward Looking Statements Except for
the historical information contained herein, the matters set forth in this presentation are forward-looking statements within the meaning of the "safe harbor" provisions of the Private Securities Litigation Reform Act of 1995, including, but not
limited to: Paragon 28’s potential to shape a better future for foot and ankle patients and its estimated net revenue for full year 2023. You are cautioned not to place undue reliance on these forward-looking statements. Forward-looking
statements are only predictions based on our current expectations, estimates, and assumptions, valid only as of the date they are made, and subject to risks and uncertainties, some of which we are not currently aware. Forward‐looking
statements should not be read as a guarantee of future performance or results and may not necessarily be accurate indications of the times at, or by, which such performance or results will be achieved. These forward‐looking statements are
based on Paragon 28’s current expectations and inherently involve significant risks and uncertainties. Actual results and the timing of events could differ materially from those anticipated in such forward‐looking statements as a result
of these risks and uncertainties. These risks and uncertainties are described more fully in the section titled “Risk Factors” in Paragon 28’s filings with the Securities and Exchange Commission (the “SEC”), including
Paragon 28’s annual report on Form 10-K filed with the SEC on March 2, 2023. Paragon 28 does not undertake any obligation to update forward‐looking statements and expressly disclaims any obligation or undertaking to release publicly any
updates or revisions to any forward‐looking statements contained herein. These forward-looking statements should not be relied upon as representing Paragon 28’s views as of any date subsequent to the date of this presentation. Paragon
28’s results for the quarter ended September 30, 2023 are not necessarily indicative of our operating results for any future periods.

Non-GAAP Financial Measures In
addition to our results and measures of performance determined in accordance with U.S. GAAP presented in this press release, we believe that certain non-GAAP financial measures are useful in evaluating and comparing our financial and operational
performance over multiple periods, identifying trends affecting our business, formulating business plans and making strategic decisions. Adjusted EBITDA is a key performance measure that our management uses to assess our financial performance
and is also used for internal planning and forecasting purposes. We define Adjusted EBITDA as earnings (loss) before interest expense, income tax expense (benefit), depreciation and amortization, stock-based compensation expense, employee stock
purchase plan expense, non-recurring expenses and certain other non-cash expenses. We believe that Adjusted EBITDA, together with a reconciliation to net income, helps identify underlying trends in our business and helps investors make
comparisons between our company and other companies that may have different capital structures, tax rates, or different forms of employee compensation. Accordingly, we believe that Adjusted EBITDA provides useful information to investors and others
in understanding and evaluating our operating results, enhancing the overall understanding of our past performance and future prospects, and allowing for greater transparency with respect to a key financial metric used by our management in its
financial and operational decision-making. Our use of Adjusted EBITDA has limitations as an analytical tool, and you should not consider these measures in isolation or as a substitute for analysis of our financial results as reported under U.S.
GAAP. Some of these potential limitations include: other companies, including companies in our industry which have similar business arrangements, may report Adjusted EBITDA, or similarly titled measures but calculate them differently, which reduces
their usefulness as comparative measures; although depreciation and amortization expenses are non-cash charges, the assets being depreciated and amortized may have to be replaced in the future, and Adjusted EBITDA does not reflect cash capital
expenditures for such replacements or for new capital expenditure requirements; Adjusted EBITDA also does not reflect changes in, or cash requirements for, our working capital needs or the potentially dilutive impact of stock-based compensation; and
Adjusted EBITDA does not reflect the interest expense, or the cash requirements necessary to service interest or principal payments, on our debt that we may incur. Additionally, we report revenue growth on a constant-currency basis in order
to facilitate period-to-period comparisons of results without regard to the impact of fluctuating foreign currency exchange rates. The term foreign currency exchange rates refers to the exchange rates used to translate the company's operating
results for all countries where the functional currency is not the U.S. dollar into U.S. dollars. Because we are a global company, foreign currency exchange rates used for translation may have a significant effect on our reported results. References
to revenue growth on a constant-currency basis means without the impact of foreign currency exchange rate fluctuations. The company believes disclosure of constant-currency revenue growth rates is helpful to investors because it facilitates
period-to-period comparisons. However, constant-currency revenue growth rates are non-GAAP financial measures and are not meant to be considered as an alternative or substitute for comparable measures prepared in accordance with GAAP.
Constant-currency growth has no standardized meaning prescribed by GAAP and should be read in conjunction with the our consolidated financial statements prepared in accordance with GAAP. We calculate constant-currency growth rates by translating
local currency amounts in the current period at actual foreign exchange rates for the prior period. Because of these and other limitations, you should consider our non-GAAP measures only as supplemental to other GAAP-based financial measures.

3Q 2023 Update – Table of
Contents 3Q 2023 and YTD Highlights 3Q 2023 and YTD Revenue Performance 2020 – 2023 YTD Operating Cash Flow Trends Ares Credit Facility: Reinforced Pathway to Cash Flow Break-even The Global Foot & Ankle Market Weighing Potential GLP-1
Impact on F&A Market Continued Strong Product Launch Cadence & Pipeline
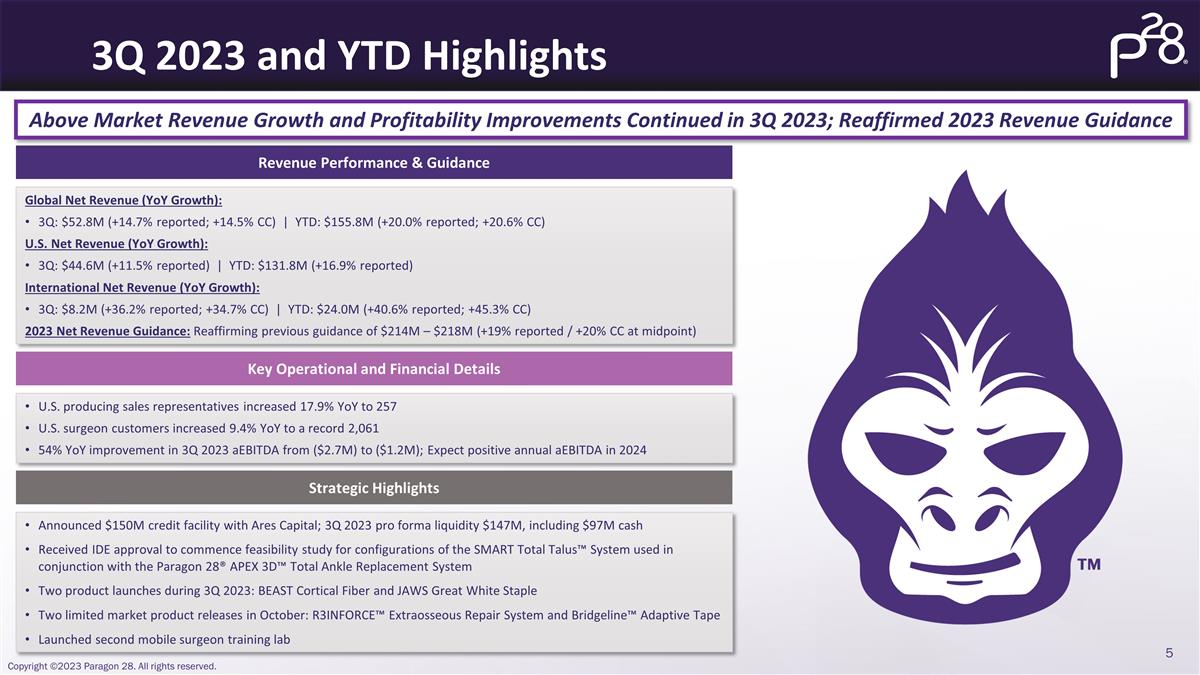
3Q 2023 and YTD Highlights Above
Market Revenue Growth and Profitability Improvements Continued in 3Q 2023; Reaffirmed 2023 Revenue Guidance Revenue Performance & Guidance Key Operational and Financial Details Strategic Highlights Global Net Revenue (YoY Growth): 3Q: $52.8M
(+14.7% reported; +14.5% CC) | YTD: $155.8M (+20.0% reported; +20.6% CC) U.S. Net Revenue (YoY Growth): 3Q: $44.6M (+11.5% reported) | YTD: $131.8M (+16.9% reported) International Net Revenue (YoY Growth): 3Q: $8.2M (+36.2% reported; +34.7% CC) |
YTD: $24.0M (+40.6% reported; +45.3% CC) 2023 Net Revenue Guidance: Reaffirming previous guidance of $214M – $218M (+19% reported / +20% CC at midpoint) U.S. producing sales representatives increased 17.9% YoY to 257 U.S. surgeon customers
increased 9.4% YoY to a record 2,061 54% YoY improvement in 3Q 2023 aEBITDA from ($2.7M) to ($1.2M); Expect positive annual aEBITDA in 2024 Announced $150M credit facility with Ares Capital; 3Q 2023 pro forma liquidity $147M, including $97M cash
Received IDE approval to commence feasibility study for configurations of the SMART Total Talus™ System used in conjunction with the Paragon 28® APEX 3D™ Total Ankle Replacement System Two product launches during 3Q 2023: BEAST
Cortical Fiber and JAWS Great White Staple Two limited market product releases in October: R3INFORCE™ Extraosseous Repair System and Bridgeline™ Adaptive Tape Launched second mobile surgeon training lab
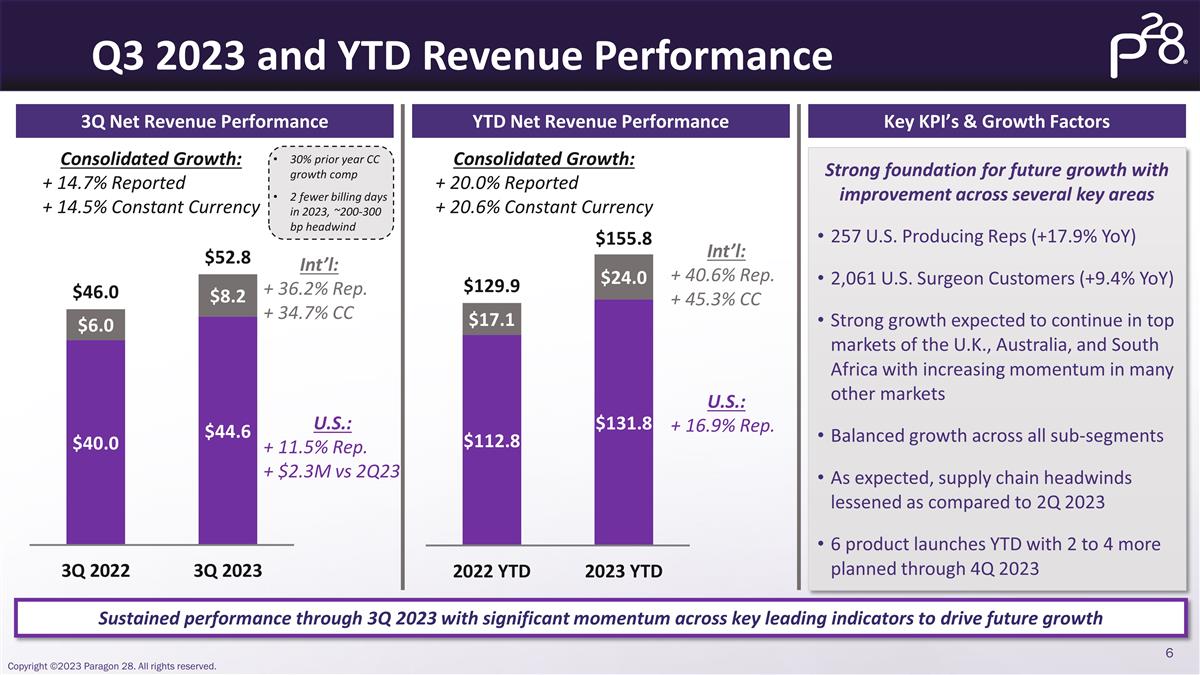
Q3 2023 and YTD Revenue Performance 3Q
Net Revenue Performance Sustained performance through 3Q 2023 with significant momentum across key leading indicators to drive future growth YTD Net Revenue Performance Key KPI’s & Growth Factors Strong foundation for future growth with
improvement across several key areas 257 U.S. Producing Reps (+17.9% YoY) 2,061 U.S. Surgeon Customers (+9.4% YoY) Strong growth expected to continue in top markets of the U.K., Australia, and South Africa with increasing momentum in many other
markets Balanced growth across all sub-segments As expected, supply chain headwinds lessened as compared to 2Q 2023 6 product launches YTD with 2 to 4 more planned through 4Q 2023 Consolidated Growth: + 14.7% Reported + 14.5% Constant Currency
Int’l: + 36.2% Rep. + 34.7% CC U.S.: + 11.5% Rep. + $2.3M vs 2Q23 30% prior year CC growth comp 2 fewer billing days in 2023, ~200-300 bp headwind Consolidated Growth: + 20.0% Reported + 20.6% Constant Currency Int’l: + 40.6% Rep. +
45.3% CC U.S.: + 16.9% Rep.
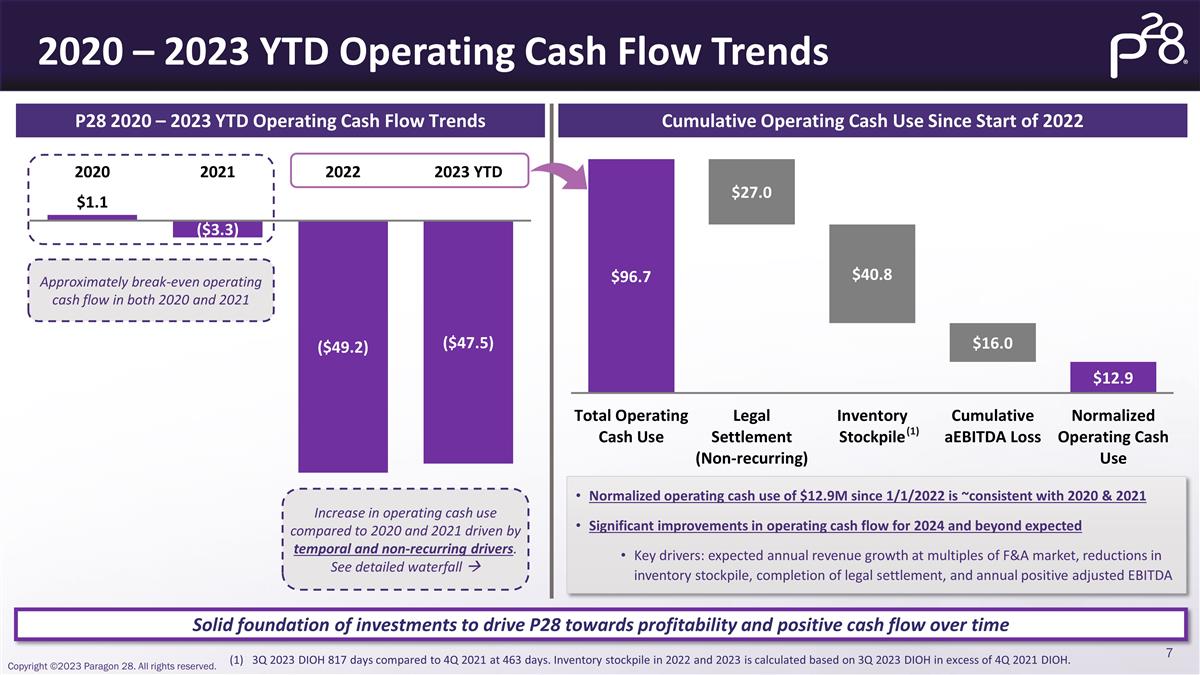
2020 – 2023 YTD Operating Cash
Flow Trends Solid foundation of investments to drive P28 towards profitability and positive cash flow over time Cumulative Operating Cash Use Since Start of 2022 3Q 2023 DIOH 817 days compared to 4Q 2021 at 463 days. Inventory stockpile in 2022 and
2023 is calculated based on 3Q 2023 DIOH in excess of 4Q 2021 DIOH. Normalized operating cash use of $12.9M since 1/1/2022 is ~consistent with 2020 & 2021 Significant improvements in operating cash flow for 2024 and beyond expected Key drivers:
expected annual revenue growth at multiples of F&A market, reductions in inventory stockpile, completion of legal settlement, and annual positive adjusted EBITDA P28 2020 – 2023 YTD Operating Cash Flow Trends Increase in operating cash use
compared to 2020 and 2021 driven by temporal and non-recurring drivers. See detailed waterfall à Approximately break-even operating cash flow in both 2020 and 2021 (1)
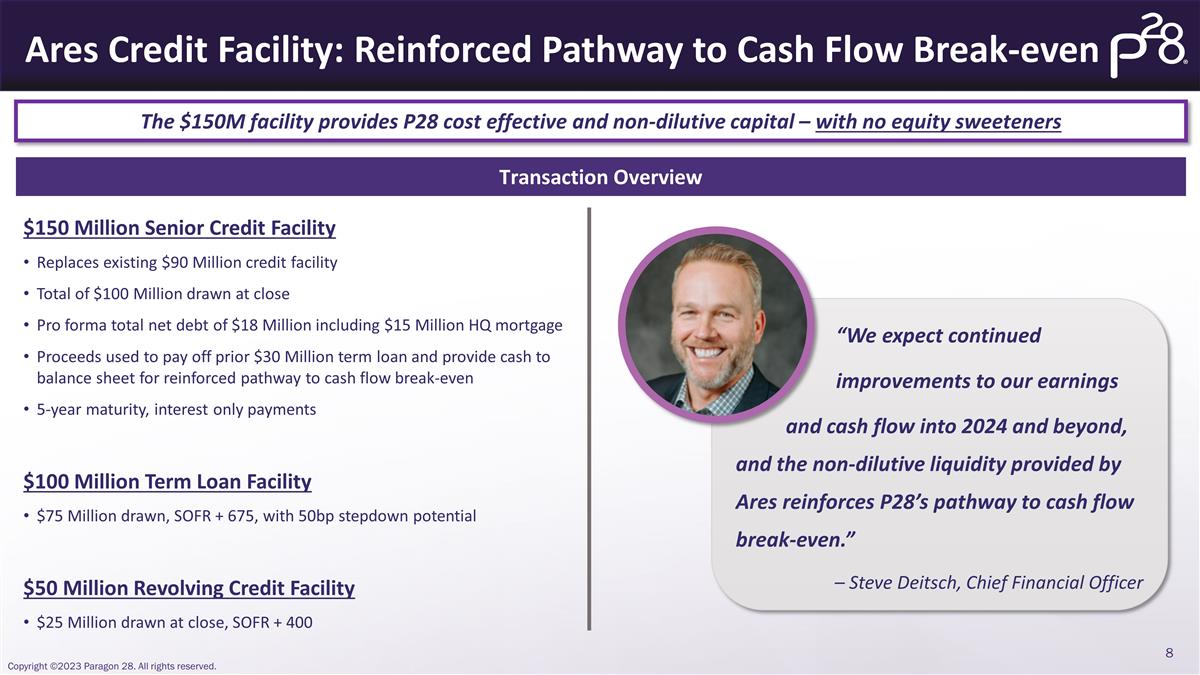
“We expect continued
improvements to our earnings and cash flow into 2024 and beyond, and the non-dilutive liquidity provided by Ares reinforces P28’s pathway to cash flow break-even.” – Steve Deitsch, Chief Financial Officer Ares Credit Facility:
Reinforced Pathway to Cash Flow Break-even The $150M facility provides P28 cost effective and non-dilutive capital – with no equity sweeteners Transaction Overview $150 Million Senior Credit Facility Replaces existing $90 Million credit
facility Total of $100 Million drawn at close Pro forma total net debt of $18 Million including $15 Million HQ mortgage Proceeds used to pay off prior $30 Million term loan and provide cash to balance sheet for reinforced pathway to cash flow
break-even 5-year maturity, interest only payments $100 Million Term Loan Facility $75 Million drawn, SOFR + 675, with 50bp stepdown potential $50 Million Revolving Credit Facility $25 Million drawn at close, SOFR + 400
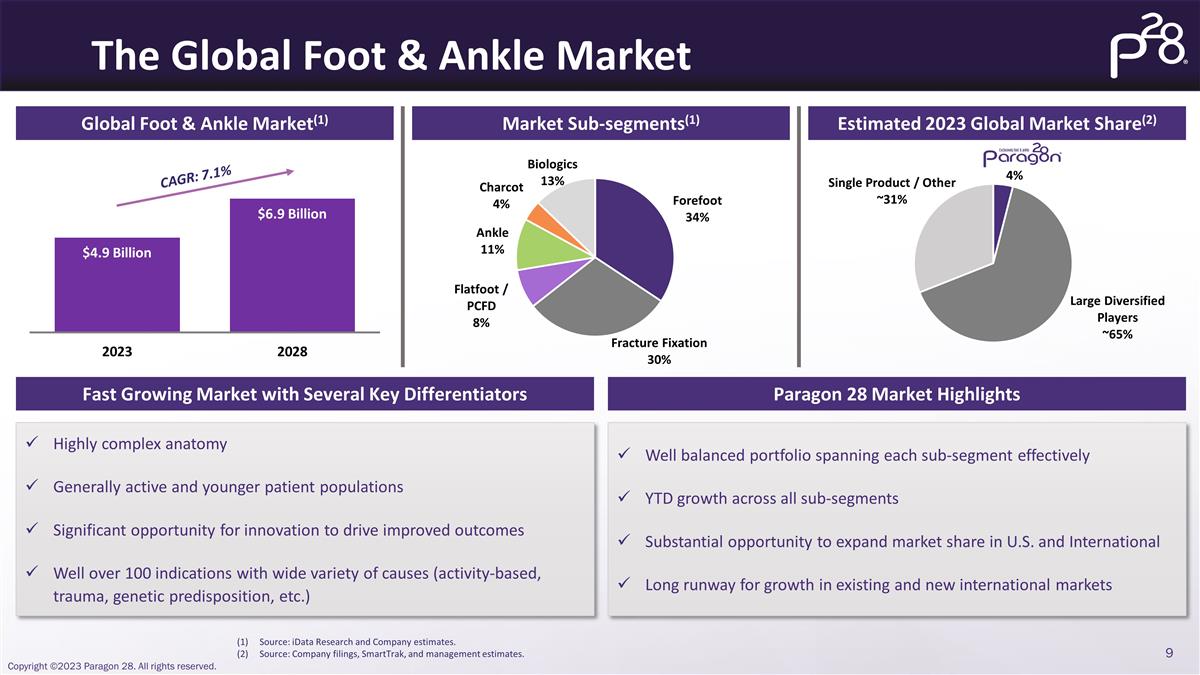
The Global Foot & Ankle Market
Global Foot & Ankle Market(1) Market Sub-segments(1) Estimated 2023 Global Market Share(2) CAGR: 7.1% Source: iData Research and Company estimates. Source: Company filings, SmartTrak, and management estimates. Fast Growing Market with Several
Key Differentiators Paragon 28 Market Highlights Highly complex anatomy Generally active and younger patient populations Significant opportunity for innovation to drive improved outcomes Well over 100 indications with wide variety of causes
(activity-based, trauma, genetic predisposition, etc.) Well balanced portfolio spanning each sub-segment effectively YTD growth across all sub-segments Substantial opportunity to expand market share in U.S. and International Long runway for growth
in existing and new international markets
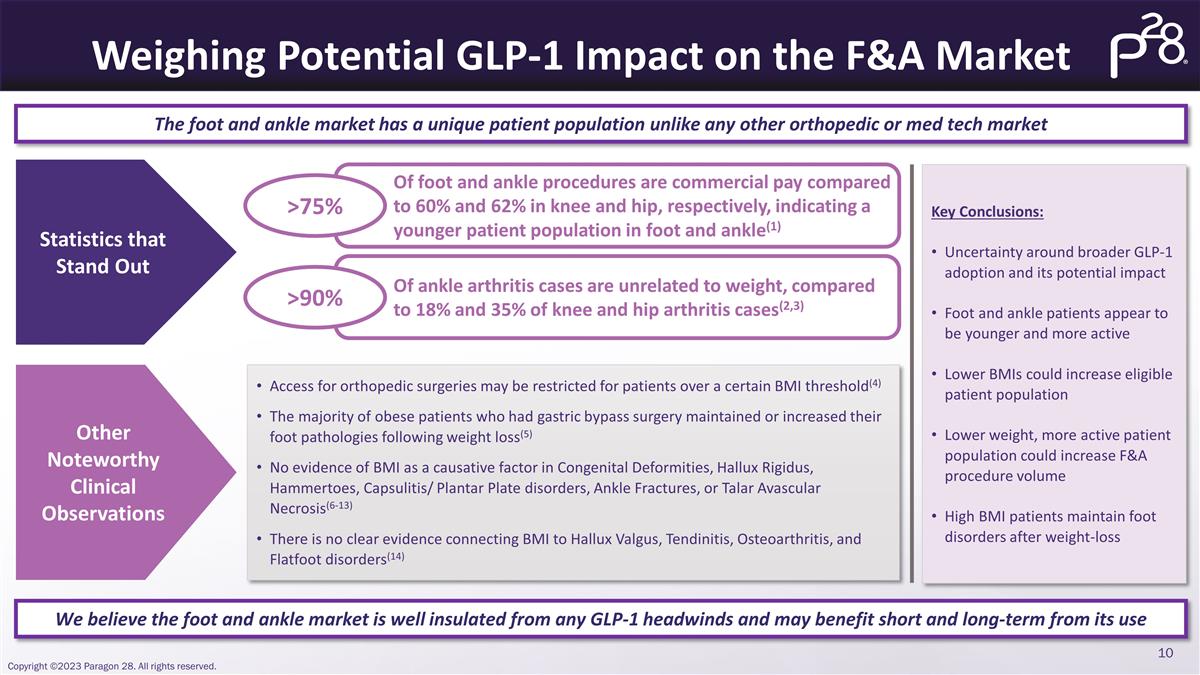
Weighing Potential GLP-1 Impact on
the F&A Market The foot and ankle market has a unique patient population unlike any other orthopedic or med tech market We believe the foot and ankle market is well insulated from any GLP-1 headwinds and may benefit short and long-term from its
use Statistics that Stand Out Other Noteworthy Clinical Observations Of foot and ankle procedures are commercial pay compared to 60% and 62% in knee and hip, respectively, indicating a younger patient population in foot and ankle(1) >75% Of ankle
arthritis cases are unrelated to weight, compared to 18% and 35% of knee and hip arthritis cases(2,3) >90% Access for orthopedic surgeries may be restricted for patients over a certain BMI threshold(4) The majority of obese patients who had
gastric bypass surgery maintained or increased their foot pathologies following weight loss(5) No evidence of BMI as a causative factor in Congenital Deformities, Hallux Rigidus, Hammertoes, Capsulitis/ Plantar Plate disorders, Ankle Fractures, or
Talar Avascular Necrosis(6-13) There is no clear evidence connecting BMI to Hallux Valgus, Tendinitis, Osteoarthritis, and Flatfoot disorders(14) Key Conclusions: Uncertainty around broader GLP-1 adoption and its potential impact Foot and ankle
patients appear to be younger and more active Lower BMIs could increase eligible patient population Lower weight, more active patient population could increase F&A procedure volume High BMI patients maintain foot disorders after
weight-loss
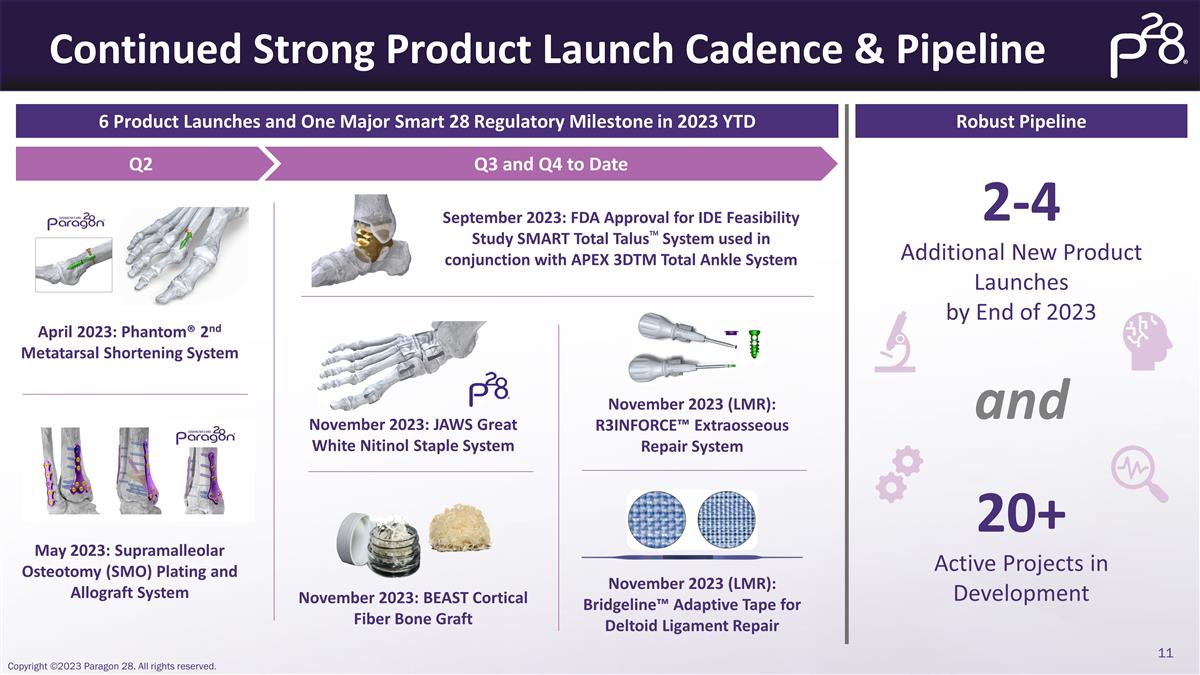
Continued Strong Product Launch
Cadence & Pipeline 6 Product Launches and One Major Smart 28 Regulatory Milestone in 2023 YTD Robust Pipeline 20+ Active Projects in Development 2-4 Additional New Product Launches by End of 2023 Q2 Q3 and Q4 to Date April 2023: Phantom®
2nd Metatarsal Shortening System May 2023: Supramalleolar Osteotomy (SMO) Plating and Allograft System September 2023: FDA Approval for IDE Feasibility Study SMART Total Talus™ System used in conjunction with APEX 3DTM Total Ankle System
November 2023: JAWS Great White Nitinol Staple System November 2023: BEAST Cortical Fiber Bone Graft November 2023 (LMR): R3INFORCE™ Extraosseous Repair System November 2023 (LMR): Bridgeline™ Adaptive Tape for Deltoid Ligament Repair
and

Appendix

GLP-1 Cited Sources Source: 2021
payor data per MedScout. Medicare Advantage included in commercial figure. Saltzman, Charles L., et al. "Epidemiology of ankle arthritis: report of a consecutive series of 639 patients from a tertiary orthopaedic center." The Iowa orthopaedic
journal 25 (2005): 44. Valderrabano, Victor, et al. "Etiology of ankle osteoarthritis." Clinical Orthopaedics and Related Research® 467.7 (2009): 1800-1806 Stewart, Matthew. “Obesity in Elective Foot and Ankle Surgery.” The
Orthopedic clinics of North America 49.3 (2018). Pico, Ana Maria et al. "Quality of LIfe, Pedobarographic Parameters, and Foot Disorders in Patients with Extreme Obesity: Preliminary Results on Changes After Bariatric Surgery with Gastric Bypass."
Obesity Surgery (2023) https://doi.org/10.1007/s11695-023-06843-5 Zammit, Gerard V., Hylton B. Menz, and Shannon E. Munteanu. "Structural factors associated with hallux limitus/rigidus: a systematic review of case
control studies." journal of orthopaedic & sports physical therapy 39.10 (2009): 733-742. Lam, Aaron, et al. "Hallux rigidus: how do I approach it?." World Journal of Orthopedics 8.5 (2017): 364. Malhota,
Karan et al. "The pathology and management of lesser toe deformities." Effort Open Reviews 1 (2016) 409-419 Park, Chul Hyun, et al. "Forefoot disorders and conservative treatment." Yeungnam Univ J Med 36 (2019) 92-98 Scheer, Ryan C, et al.
"Ankle Fracture Epidemiology in the United States: Patient-Related Trends and Mechanisms of Injury." The Journal of Foot & Ankle Surgery 59 (2020) 479-483 Rydberg, Emilia Moller, et al. "Epidemiology of more than 50,000 ankle fractures in
the Swedish Fracture Register during a period of 10 years." Journal of Orthopaedic Surgery and Research 18:79 (2023) 1-12 Zhang, Hanci, et al. "Avascular Osteonecrosis of the Talus: Current Treatment Strategies." Foot & Ankle
International 43 (2022) 291-302 Gross, Christopher E, et al. "Treatments for Avascular Necrosis of the Talus A Systemic Review." Foot & Ankle Specialist 7 (2014) 387-397 Butterworth, PA et al. "The association between body mass index and
musculoskeletal foot disorders: a systematic review." Obes Rev. 13 (2012) 630-642
v3.23.3
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Paragon 28 (NYSE:FNA)
Historical Stock Chart
Von Mär 2024 bis Apr 2024
Paragon 28 (NYSE:FNA)
Historical Stock Chart
Von Apr 2023 bis Apr 2024