0001787400false00017874002024-01-082024-01-08
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): January 8, 2024
Nkarta, Inc.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
Delaware |
001-39370 |
47-4515206 |
(State or Other Jurisdiction of Incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
|
|
|
1150 Veterans Boulevard South San Francisco, CA |
|
94080 |
(Address of Principal Executive Offices) |
|
(Zip Code) |
Registrant’s Telephone Number, Including Area Code: (925) 407-1049
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
|
|
☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
Common Stock, $0.0001 par value per share |
|
NKTX |
|
The Nasdaq Stock Market LLC (Nasdaq Global Select Market) |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☒
Item. 7.01 Regulation FD Disclosure.
On January 8, 2024, Nkarta, Inc. (the “Company”) made available an updated corporate presentation to reflect certain business and strategic updates. The Company intends to use this presentation in meetings with analysts, investors, and others from time to time. A copy of the presentation is attached as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated by reference herein. The corporate presentation will also be available in the “Investors” section of the Company’s website at www.nkartatx.com. The Company’s website and any information contained on the Company’s website are not incorporated by reference into, and are not considered part of, this Current Report on Form 8-K.
The information in Item 7.01 of this Current Report on Form 8-K (including Exhibit 99.1) shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be, or be deemed, incorporated by reference in any filings under the Securities Act of 1933, as amended (the “Securities Act”), unless the Company specifically states that the information is to be considered “filed” under the Exchange Act or incorporates it by reference into a filing under the Securities Act or the Exchange Act.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
Nkarta, Inc. |
|
|
|
|
Date: January 8, 2024 |
|
By: |
/s/ Alicia Hager |
|
|
|
Alicia J. Hager, J.D., Ph.D. |
|
|
|
Chief Legal Officer |
JANUARY 2024 ENGINEERING Natural Killer Cells for next generation treatment of cancer and autoimmune diseases ON DEMAND Exhibit 99.1

Forward-looking statements This presentation contains forward‐looking statements, within the meaning of the Private Securities Litigation Reform Act of 1995, regarding future events and the future results of the company that are based on current expectations, estimates, forecasts, and projections about the industry in which the company operates and the future of our business, future plans and strategies, projections, anticipated trends and events, the economy, and other future conditions, and the beliefs and assumptions of the management of the company. Words such as “address,” “anticipate,” “believe,” “consider,” “continue,” “develop,” “estimate,” “expect,” “further,” “goal,” “intend,” “may,” “plan,” “potential,” “project,” “seek,” “should,” “target,” “will,” variations of such words, and similar expressions are intended to identify such forward-looking statements. Such statements reflect the current views of the company and its management with respect to future events and are subject to inherent risks, uncertainties, and changes in circumstances that are difficult to predict and may be outside our control. Therefore, you should not rely on any of these forward-looking statements. Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, the company's actual results, performance, or achievements could differ materially from the results expressed in, or implied by, these forward-looking statements. Please see section entitled “Risk Factors” in our annual, quarterly and other filings with the Securities and Exchange Commission for a description of these risks and uncertainties. This presentation has been prepared by the company based on information it has obtained from sources it believes to be reliable. Summaries of documents contained in this presentation may not be complete. The company does not represent that the information herein is complete. The information in this presentation is current only as of the date on the cover, and the company's business or financial condition and other information in this presentation may change after that date. The company undertakes no obligation to update any forward‐looking statements in order to reflect any event or circumstance occurring after the date of this presentation or currently unknown facts or conditions. Interim data from clinical trials are subject to the risk that one or more of the clinical outcomes may materially change as patient enrollment continues and more data on existing patients become available. The clinical trial programs are ongoing, and the final results may be materially different from those reflected in any interim data the company reports. Further, others, including regulatory agencies, may not accept or agree with the company’s assumptions, estimates, calculations, conclusions or analyses or may interpret or weigh the importance of data differently, which could impact the value of the particular program, the approvability or commercialization of the particular product candidate or product and the value of the company in general. In addition, the information the company chooses to publicly disclose regarding a particular study or clinical trial is typically a summary of extensive information, and you or others may not agree with what the company determines is the material or otherwise appropriate information to include in its disclosure, and any information the company determines not to disclose may ultimately be deemed significant with respect to future decisions, conclusions, views, activities or otherwise regarding a particular product, product candidate or business.
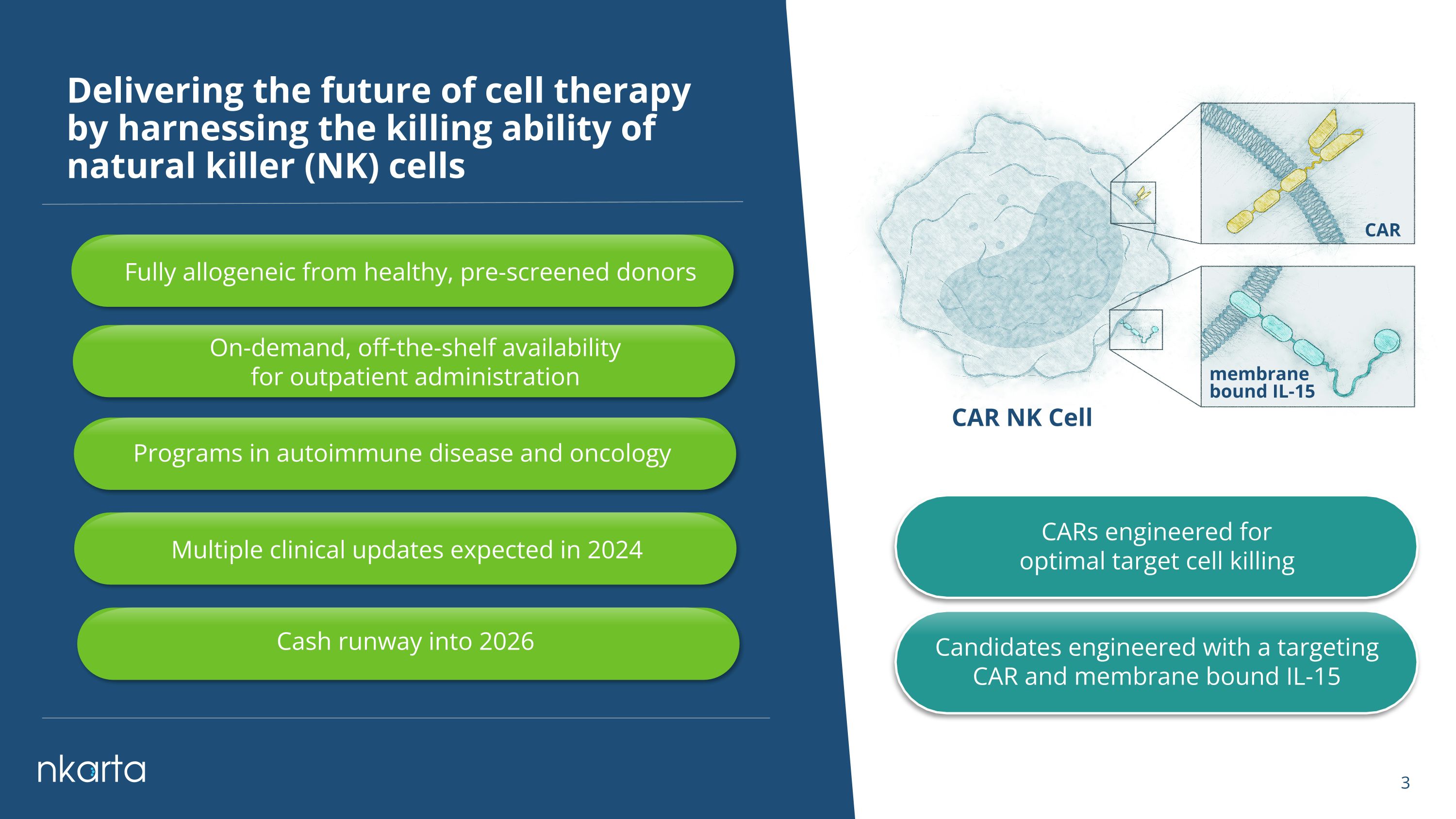
Delivering the future of cell therapy �by harnessing the killing ability of natural killer (NK) cells CARs engineered for optimal target cell killing CAR NK Cell CAR membrane �bound IL-15 Candidates engineered with a targeting CAR and membrane bound IL-15 Fully allogeneic from healthy, pre-screened donors Programs in autoimmune disease and oncology On-demand, off-the-shelf availability �for outpatient administration Multiple clinical updates expected in 2024 Cash runway into 2026
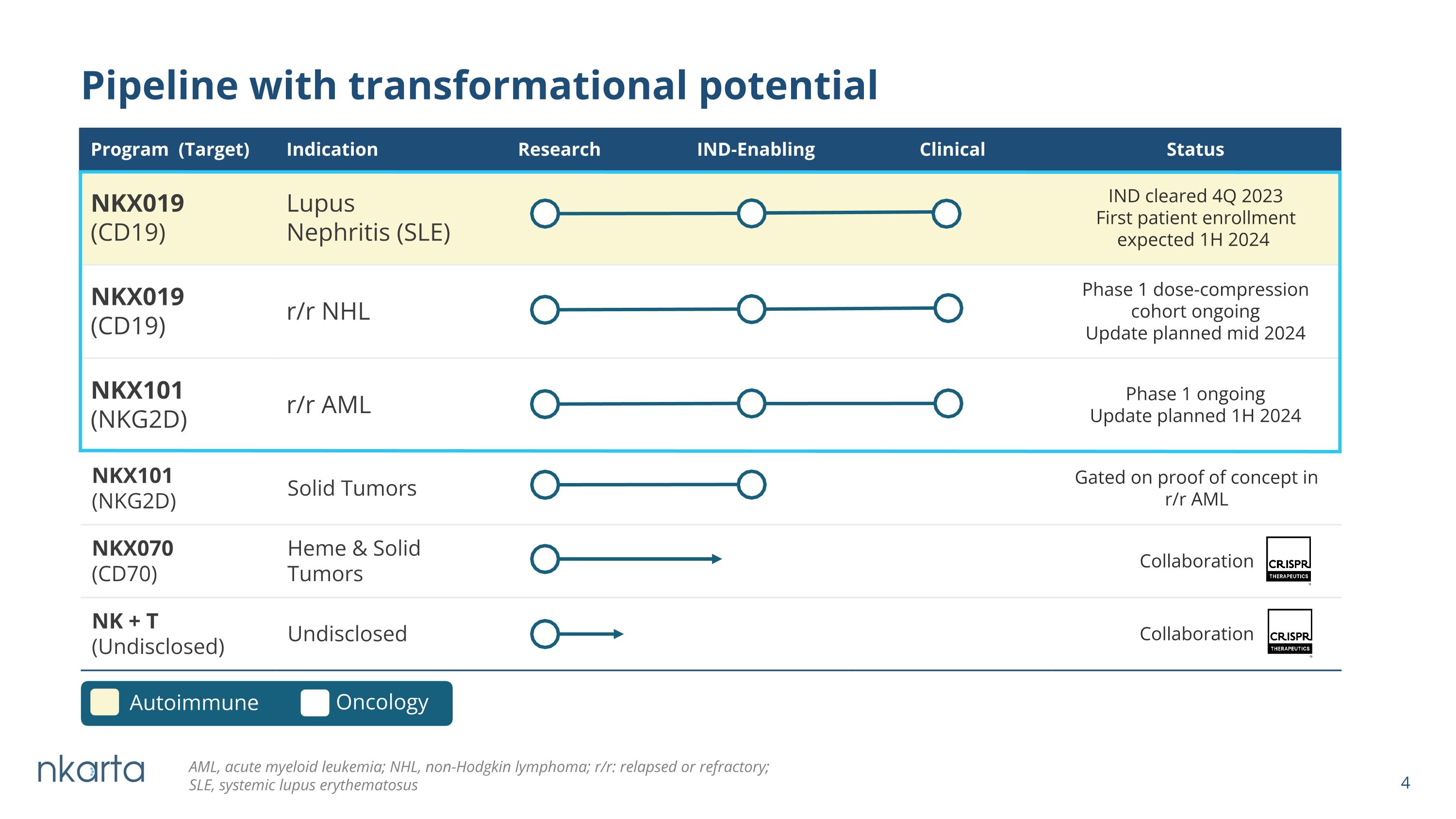
NKX101 (NKG2D) Solid Tumors Gated on proof of concept in r/r AML NKX070 (CD70) Heme & Solid Tumors Collaboration NK + T (Undisclosed) Undisclosed Collaboration Pipeline with transformational potential Program (Target) Indication Research IND-Enabling Clinical Status NKX019 (CD19) Lupus Nephritis (SLE) IND cleared 4Q 2023 First patient enrollment expected 1H 2024 NKX019 (CD19) r/r NHL Phase 1 dose-compression cohort ongoing Update planned mid 2024 NKX101 (NKG2D) r/r AML Phase 1 ongoing Update planned 1H 2024 Autoimmune Oncology AML, acute myeloid leukemia; NHL, non-Hodgkin lymphoma; r/r: relapsed or refractory; SLE, systemic lupus erythematosus

NKX019 in Autoimmune Disease
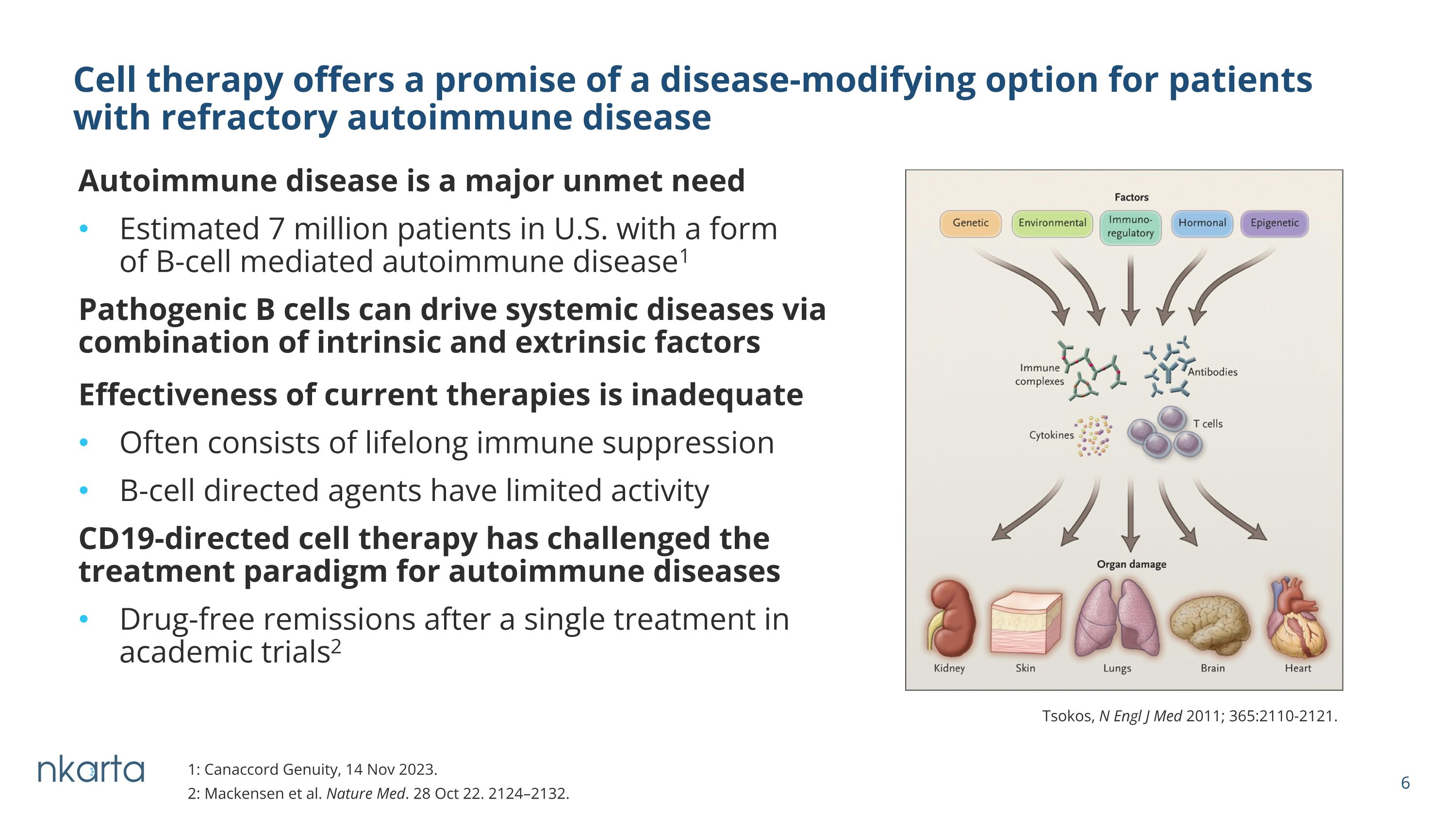
Effectiveness of current therapies is inadequate Often consists of lifelong immune suppression B-cell directed agents have limited activity CD19-directed cell therapy has challenged the treatment paradigm for autoimmune diseases Drug-free remissions after a single treatment in academic trials2 Autoimmune disease is a major unmet need Estimated 7 million patients in U.S. with a form of B-cell mediated autoimmune disease1 Pathogenic B cells can drive systemic diseases via combination of intrinsic and extrinsic factors Cell therapy offers a promise of a disease-modifying option for patients �with refractory autoimmune disease Tsokos, N Engl J Med 2011; 365:2110-2121. 1: Canaccord Genuity, 14 Nov 2023. 2: Mackensen et al. Nature Med. 28 Oct 22. 2124–2132.
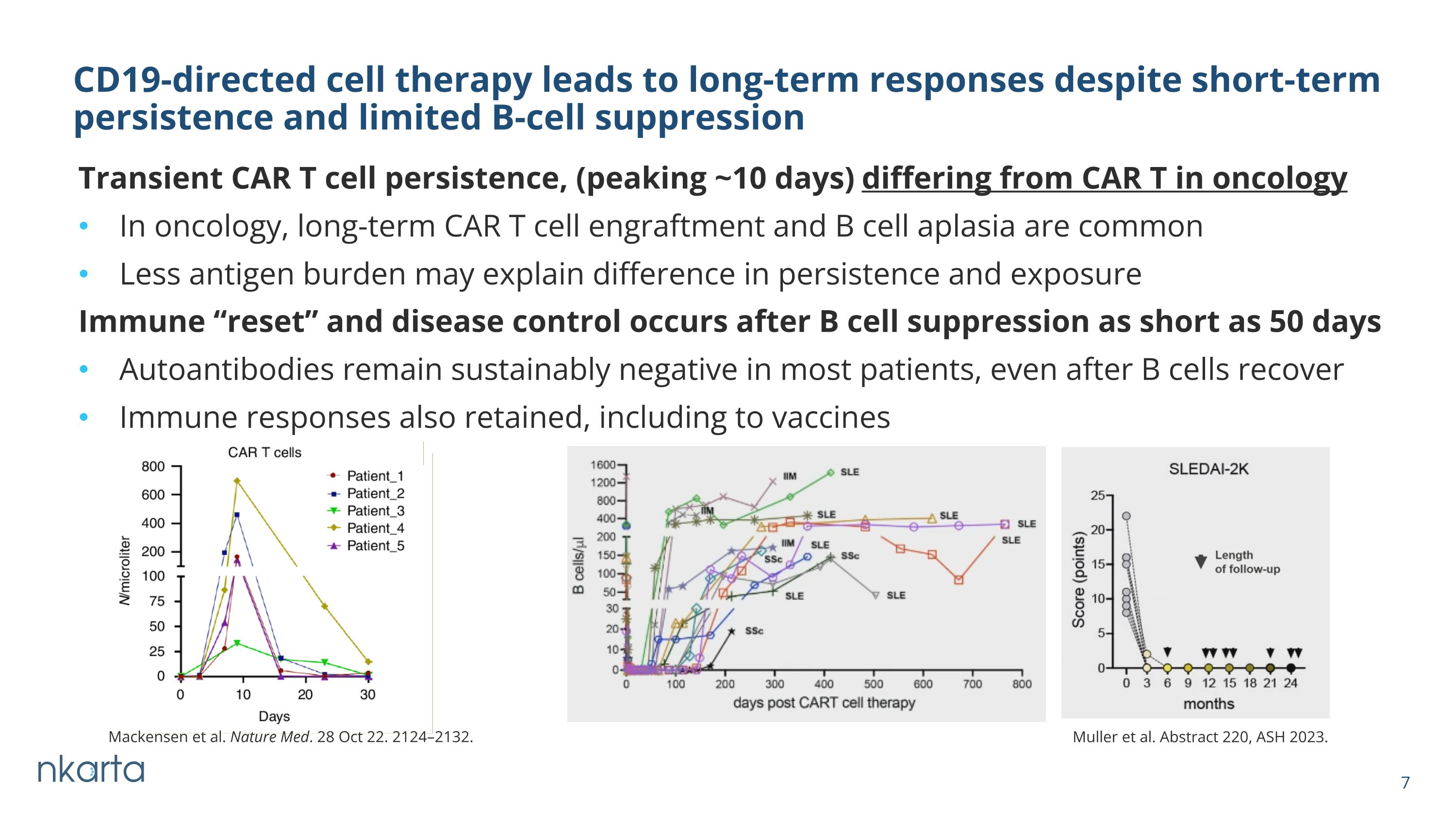
Transient CAR T cell persistence, (peaking ~10 days) differing from CAR T in oncology In oncology, long-term CAR T cell engraftment and B cell aplasia are common Less antigen burden may explain difference in persistence and exposure CD19-directed cell therapy leads to long-term responses despite short-term persistence and limited B-cell suppression Muller et al. Abstract 220, ASH 2023. Immune “reset” and disease control occurs after B cell suppression as short as 50 days Autoantibodies remain sustainably negative in most patients, even after B cells recover Immune responses also retained, including to vaccines Mackensen et al. Nature Med. 28 Oct 22. 2124–2132.
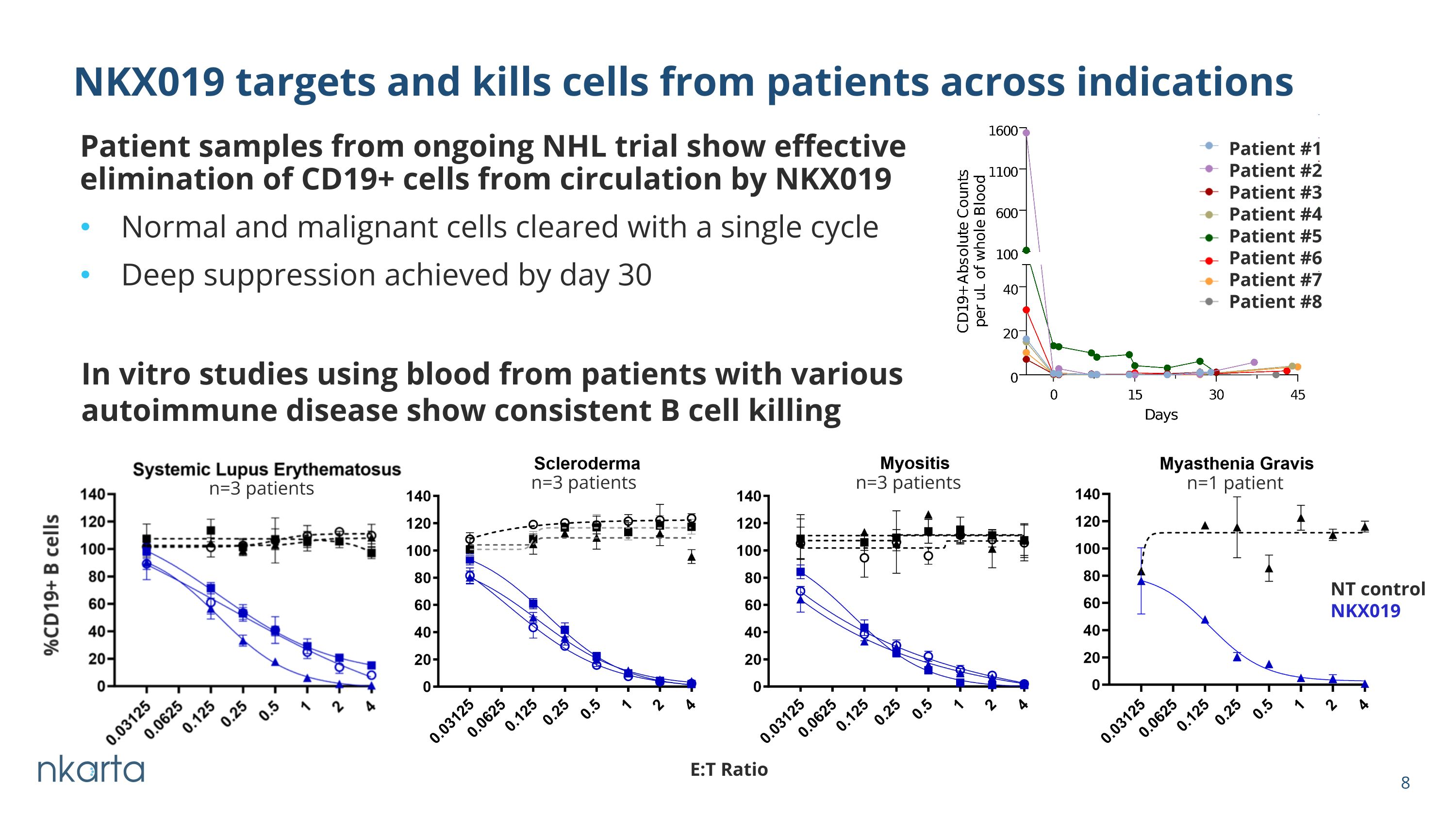
Patient samples from ongoing NHL trial show effective elimination of CD19+ cells from circulation by NKX019 Normal and malignant cells cleared with a single cycle Deep suppression achieved by day 30 NKX019 targets and kills cells from patients across indications E:T Ratio NT control NKX019 n=3 patients n=1 patient n=3 patients n=3 patients In vitro studies using blood from patients with various autoimmune disease show consistent B cell killing Patient #1 Patient #2 Patient #3 Patient #4 Patient #5 Patient #6 Patient #7 Patient #8
NK cells reach peak activity at infusion for rapid target exposure Allows maximal immediate effect without in vivo expansion or permanent engraftment T cells require expansion, which delays effects and requires lymphodepletion (LD) Opportunity to reduce chemotherapy exposure via disease-tailored LD Autocrine stimulation via mbIL-15 may reduce need for LD-induced cytokines Elimination of fludarabine limits risks of cytopenias, infection, and secondary MDS1 Allogeneic NK cells are cleared by host immunity Low risk of prolonged B-cell aplasia Long-lived CAR T cells have FDA-issued risk of T-cell malignancy2 Superior safety and accessibility in non-malignant setting On-demand availability without need for cumbersome infrastructure Low risk of expansion-related toxicity including CRS and ICANS CD19 CAR NK cells may be ideally suited for autoimmune disease 2: Nelson. Lancet. Vol 402. 2181. December 9, 2023 1: Fludarabine USPI
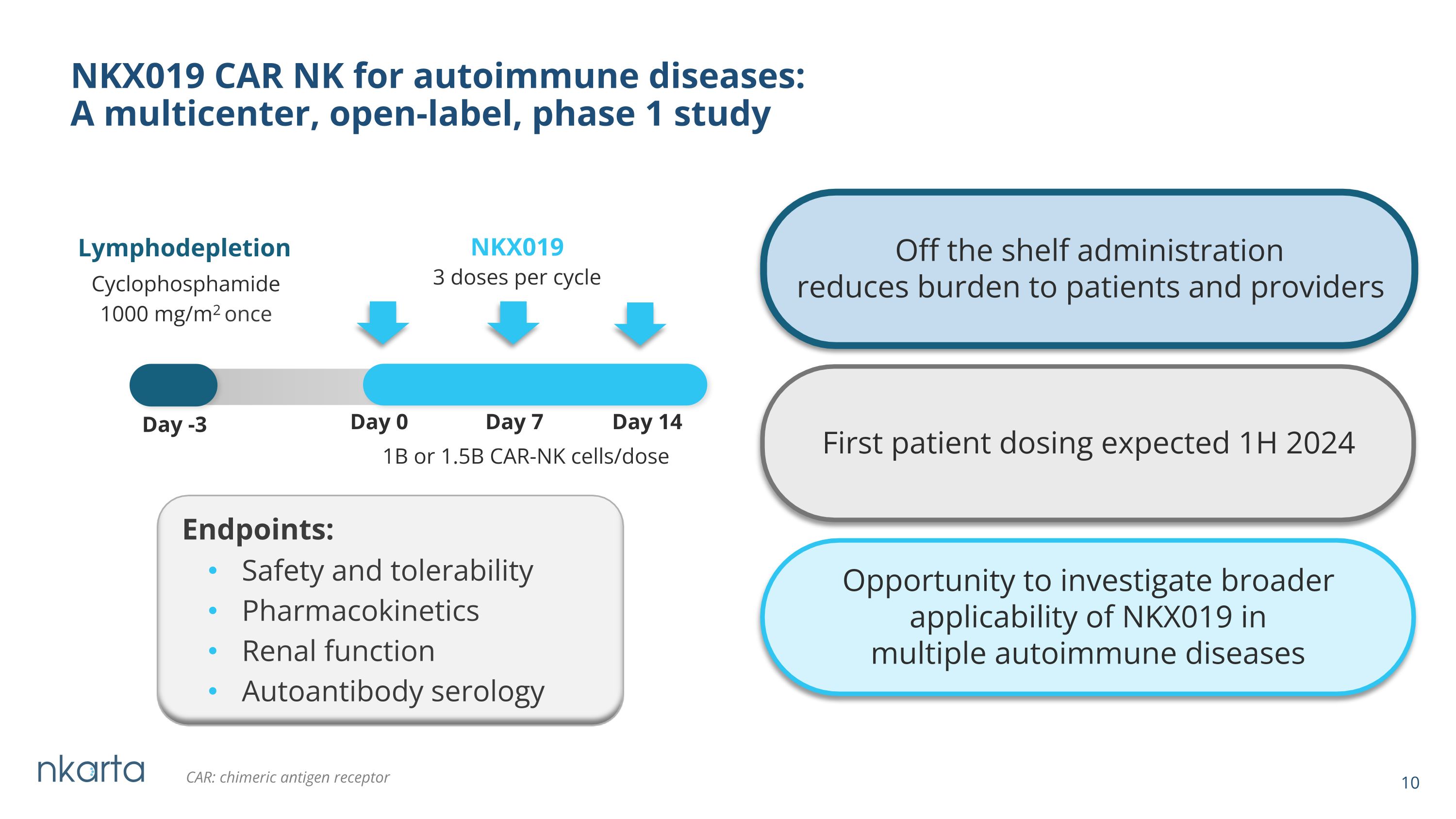
NKX019 CAR NK for autoimmune diseases: A multicenter, open-label, phase 1 study NKX019 1B or 1.5B CAR-NK cells/dose Lymphodepletion Cyclophosphamide 1000 mg/m2 once Day -3 Day 0 Day 7 Day 14 First patient dosing expected 1H 2024 CAR: chimeric antigen receptor Endpoints: 3 doses per cycle Opportunity to investigate broader �applicability of NKX019 in �multiple autoimmune diseases Off the shelf administration reduces burden to patients and providers Safety and tolerability Pharmacokinetics Renal function Autoantibody serology
NKX019 and NKX101 �in oncology

NKX019 CD19 CAR NK in r/r non-Hodgkin lymphoma
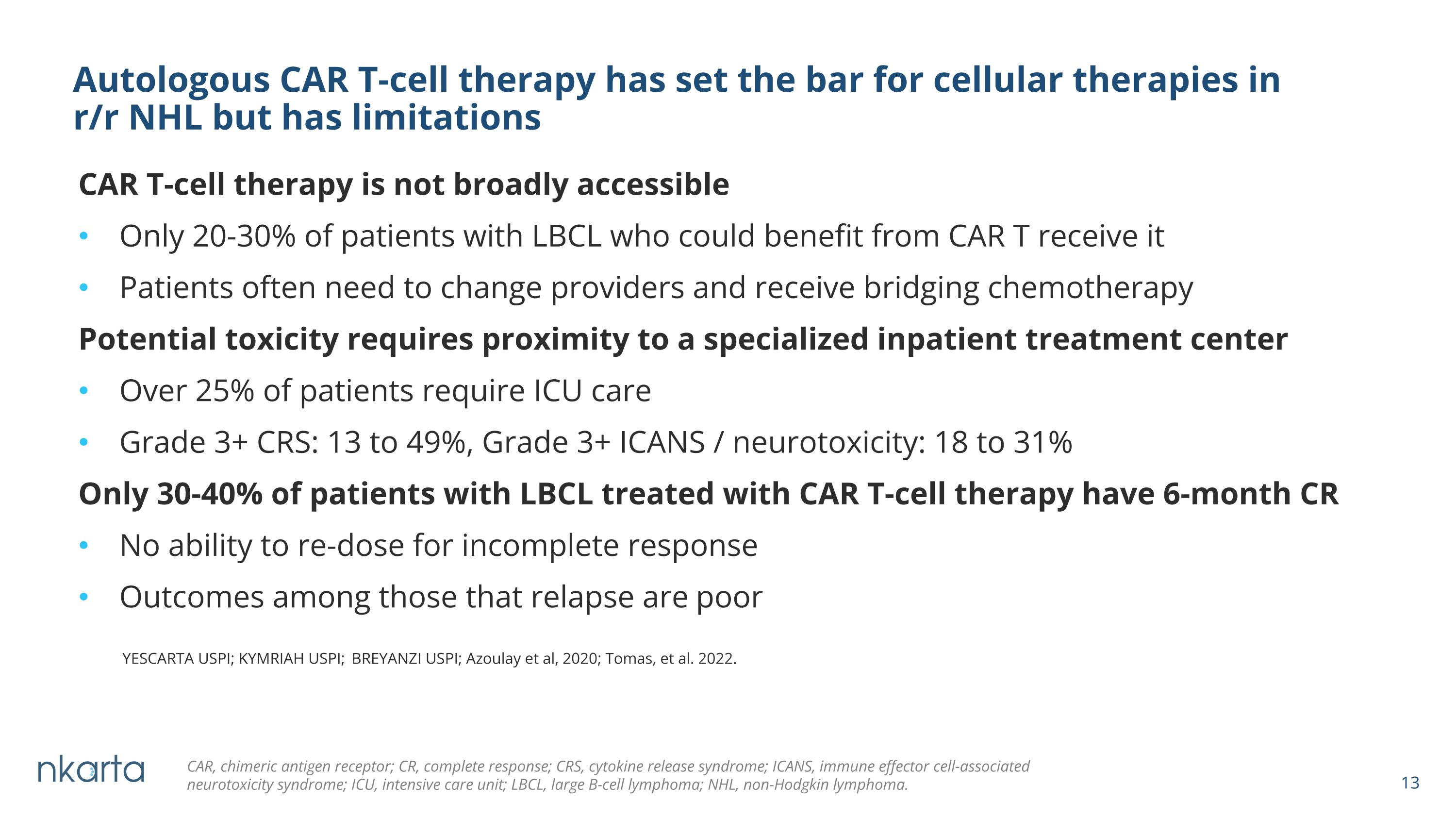
CAR T-cell therapy is not broadly accessible Only 20-30% of patients with LBCL who could benefit from CAR T receive it Patients often need to change providers and receive bridging chemotherapy Potential toxicity requires proximity to a specialized inpatient treatment center Over 25% of patients require ICU care Grade 3+ CRS: 13 to 49%, Grade 3+ ICANS / neurotoxicity: 18 to 31% Only 30-40% of patients with LBCL treated with CAR T-cell therapy have 6-month CR No ability to re-dose for incomplete response Outcomes among those that relapse are poor Autologous CAR T-cell therapy has set the bar for cellular therapies in �r/r NHL but has limitations YESCARTA USPI; KYMRIAH USPI; BREYANZI USPI; Azoulay et al, 2020; Tomas, et al. 2022. CAR, chimeric antigen receptor; CR, complete response; CRS, cytokine release syndrome; ICANS, immune effector cell-associated neurotoxicity syndrome; ICU, intensive care unit; LBCL, large B-cell lymphoma; NHL, non-Hodgkin lymphoma. NKX019
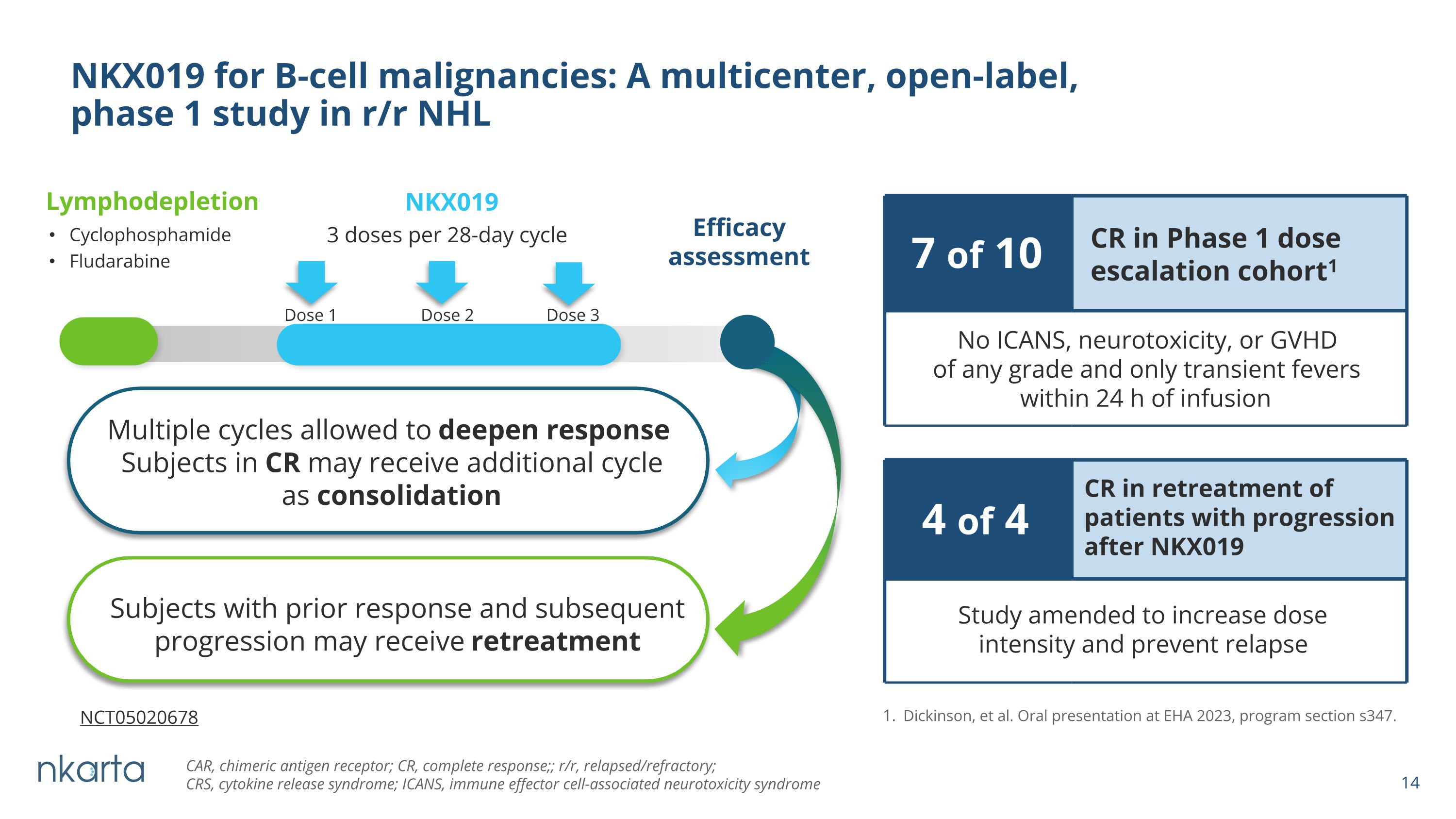
NKX019 for B-cell malignancies: A multicenter, open-label, �phase 1 study in r/r NHL NKX019 3 doses per 28-day cycle Lymphodepletion Cyclophosphamide Fludarabine Efficacy assessment Dose 1 Dose 2 Dose 3 Dickinson, et al. Oral presentation at EHA 2023, program section s347. 4 of 4 CR in retreatment of patients with progression after NKX019 Study amended to increase dose intensity and prevent relapse 7 of 10 CR in Phase 1 dose escalation cohort1 No ICANS, neurotoxicity, or GVHD of any grade and only transient fevers within 24 h of infusion Multiple cycles allowed to deepen response Subjects in CR may receive additional cycle as consolidation Subjects with prior response and subsequent progression may receive retreatment CAR, chimeric antigen receptor; CR, complete response;; r/r, relapsed/refractory; �CRS, cytokine release syndrome; ICANS, immune effector cell-associated neurotoxicity syndrome NCT05020678
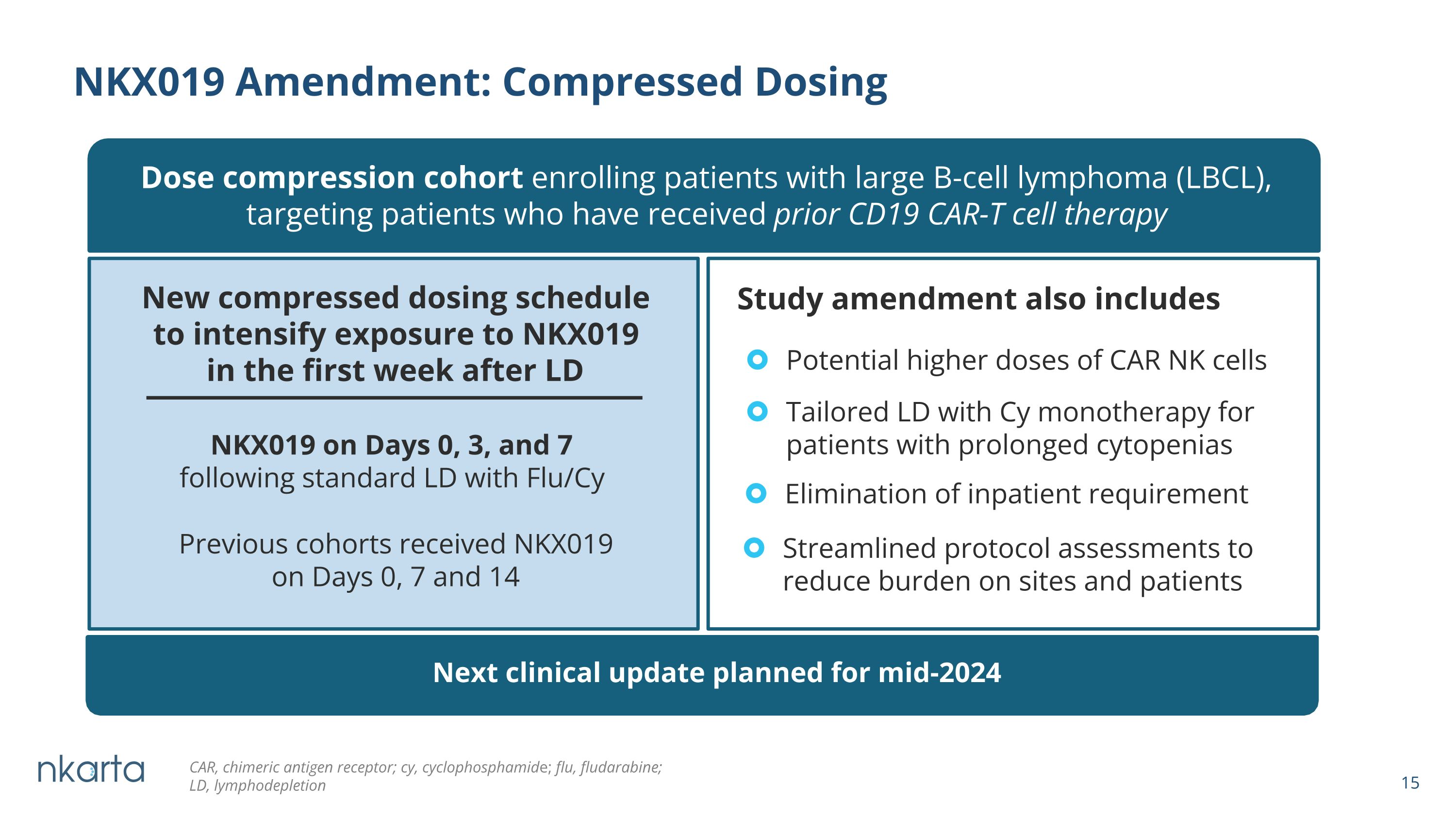
NKX019 Amendment: Compressed Dosing Dose compression cohort enrolling patients with large B-cell lymphoma (LBCL), targeting patients who have received prior CD19 CAR-T cell therapy New compressed dosing schedule to intensify exposure to NKX019 in the first week after LD Previous cohorts received NKX019 on Days 0, 7 and 14 NKX019 on Days 0, 3, and 7 following standard LD with Flu/Cy Potential higher doses of CAR NK cells Tailored LD with Cy monotherapy for patients with prolonged cytopenias Elimination of inpatient requirement Streamlined protocol assessments to reduce burden on sites and patients Study amendment also includes Next clinical update planned for mid-2024 CAR, chimeric antigen receptor; cy, cyclophosphamide; flu, fludarabine; �LD, lymphodepletion
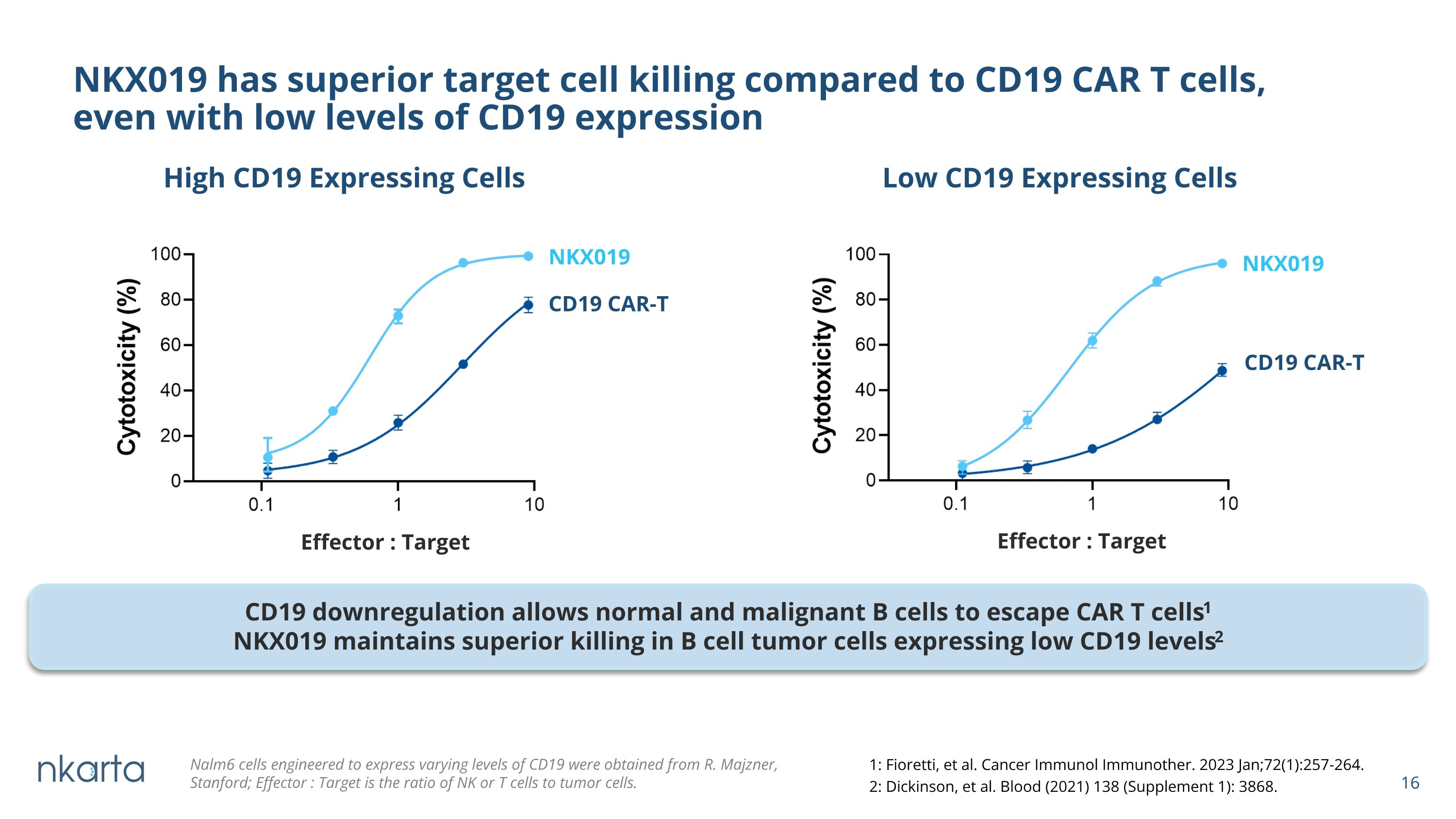
NKX019 has superior target cell killing compared to CD19 CAR T cells, �even with low levels of CD19 expression NKX019 Low CD19 Expressing Cells High CD19 Expressing Cells NKX019 CD19 CAR-T CD19 CAR-T Effector : Target Effector : Target Nalm6 cells engineered to express varying levels of CD19 were obtained from R. Majzner, Stanford; Effector : Target is the ratio of NK or T cells to tumor cells. CD19 downregulation allows normal and malignant B cells to escape CAR T cells1 NKX019 maintains superior killing in B cell tumor cells expressing low CD19 levels2 NKX019 1: Fioretti, et al. Cancer Immunol Immunother. 2023 Jan;72(1):257-264. 2: Dickinson, et al. Blood (2021) 138 (Supplement 1): 3868.

NKX101 NKG2D CAR NK in�r/r acute myeloid leukemia
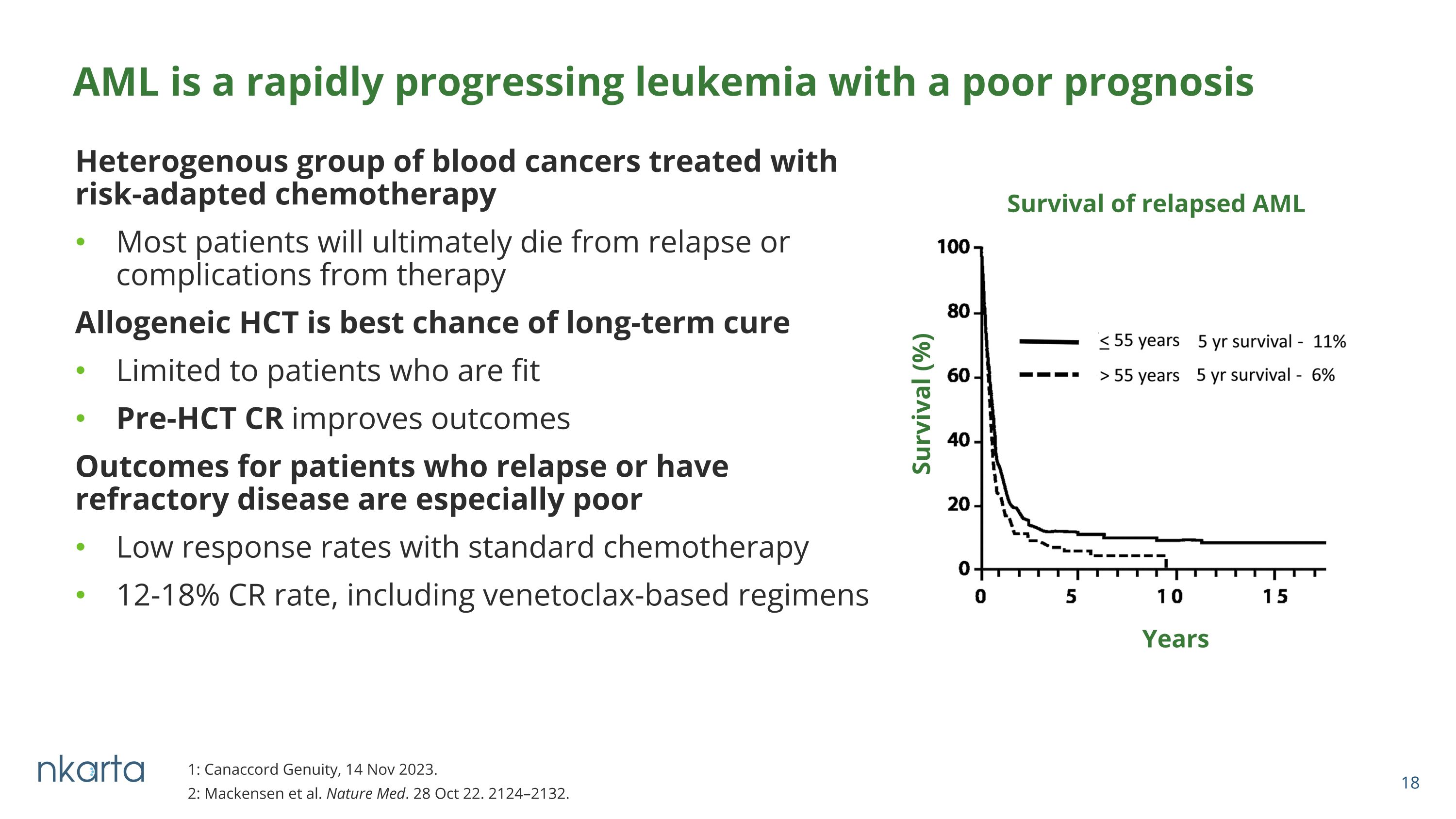
Heterogenous group of blood cancers treated with risk-adapted chemotherapy Most patients will ultimately die from relapse or complications from therapy Allogeneic HCT is best chance of long-term cure Limited to patients who are fit Pre-HCT CR improves outcomes Outcomes for patients who relapse or have refractory disease are especially poor Low response rates with standard chemotherapy 12-18% CR rate, including venetoclax-based regimens 1: Canaccord Genuity, 14 Nov 2023. 2: Mackensen et al. Nature Med. 28 Oct 22. 2124–2132. AML is a rapidly progressing leukemia with a poor prognosis Survival (%) Survival of relapsed AML Years
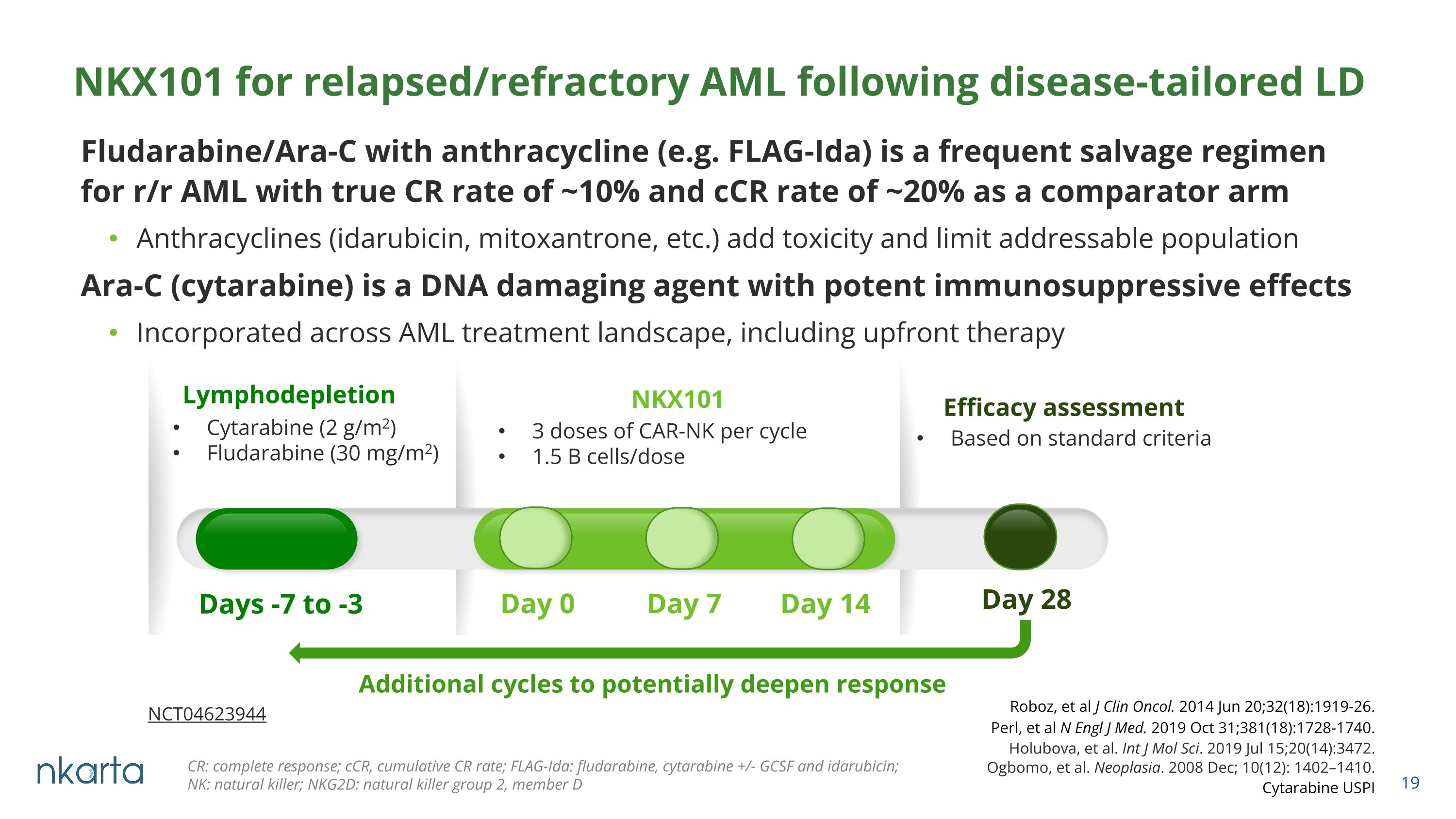
Fludarabine/Ara-C with anthracycline (e.g. FLAG-Ida) is a frequent salvage regimen for r/r AML with true CR rate of ~10% and cCR rate of ~20% as a comparator arm Anthracyclines (idarubicin, mitoxantrone, etc.) add toxicity and limit addressable population Ara-C (cytarabine) is a DNA damaging agent with potent immunosuppressive effects Incorporated across AML treatment landscape, including upfront therapy NKX101 for relapsed/refractory AML following disease-tailored LD Ogbomo, et al. Neoplasia. 2008 Dec; 10(12): 1402–1410. Holubova, et al. Int J Mol Sci. 2019 Jul 15;20(14):3472. Cytarabine USPI Perl, et al N Engl J Med. 2019 Oct 31;381(18):1728-1740. Roboz, et al J Clin Oncol. 2014 Jun 20;32(18):1919-26. CR: complete response; cCR, cumulative CR rate; FLAG-Ida: fludarabine, cytarabine +/- GCSF and idarubicin; �NK: natural killer; NKG2D: natural killer group 2, member D Lymphodepletion NKX101 3 doses of CAR-NK per cycle 1.5 B cells/dose Day 0 Day 7 Day 14 Cytarabine (2 g/m2) Fludarabine (30 mg/m2) Days -7 to -3 Additional cycles to potentially deepen response Efficacy assessment Day 28 Based on standard criteria NCT04623944
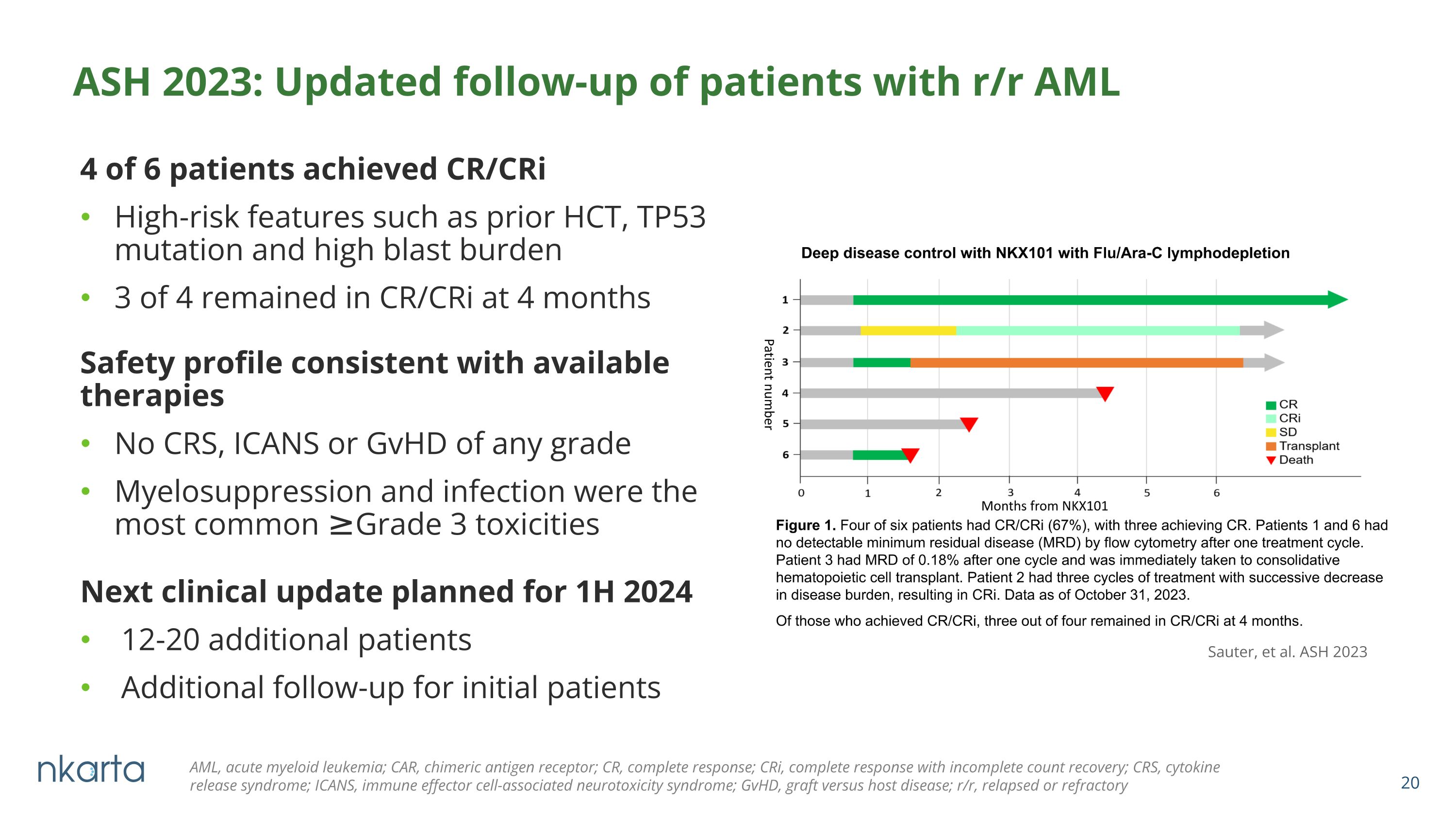
4 of 6 patients achieved CR/CRi High-risk features such as prior HCT, TP53 mutation and high blast burden 3 of 4 remained in CR/CRi at 4 months ASH 2023: Updated follow-up of patients with r/r AML Sauter, et al. ASH 2023 AML, acute myeloid leukemia; CAR, chimeric antigen receptor; CR, complete response; CRi, complete response with incomplete count recovery; CRS, cytokine release syndrome; ICANS, immune effector cell-associated neurotoxicity syndrome; GvHD, graft versus host disease; r/r, relapsed or refractory Safety profile consistent with available therapies No CRS, ICANS or GvHD of any grade Myelosuppression and infection were the most common ≥Grade 3 toxicities Next clinical update planned for 1H 2024 12-20 additional patients Additional follow-up for initial patients
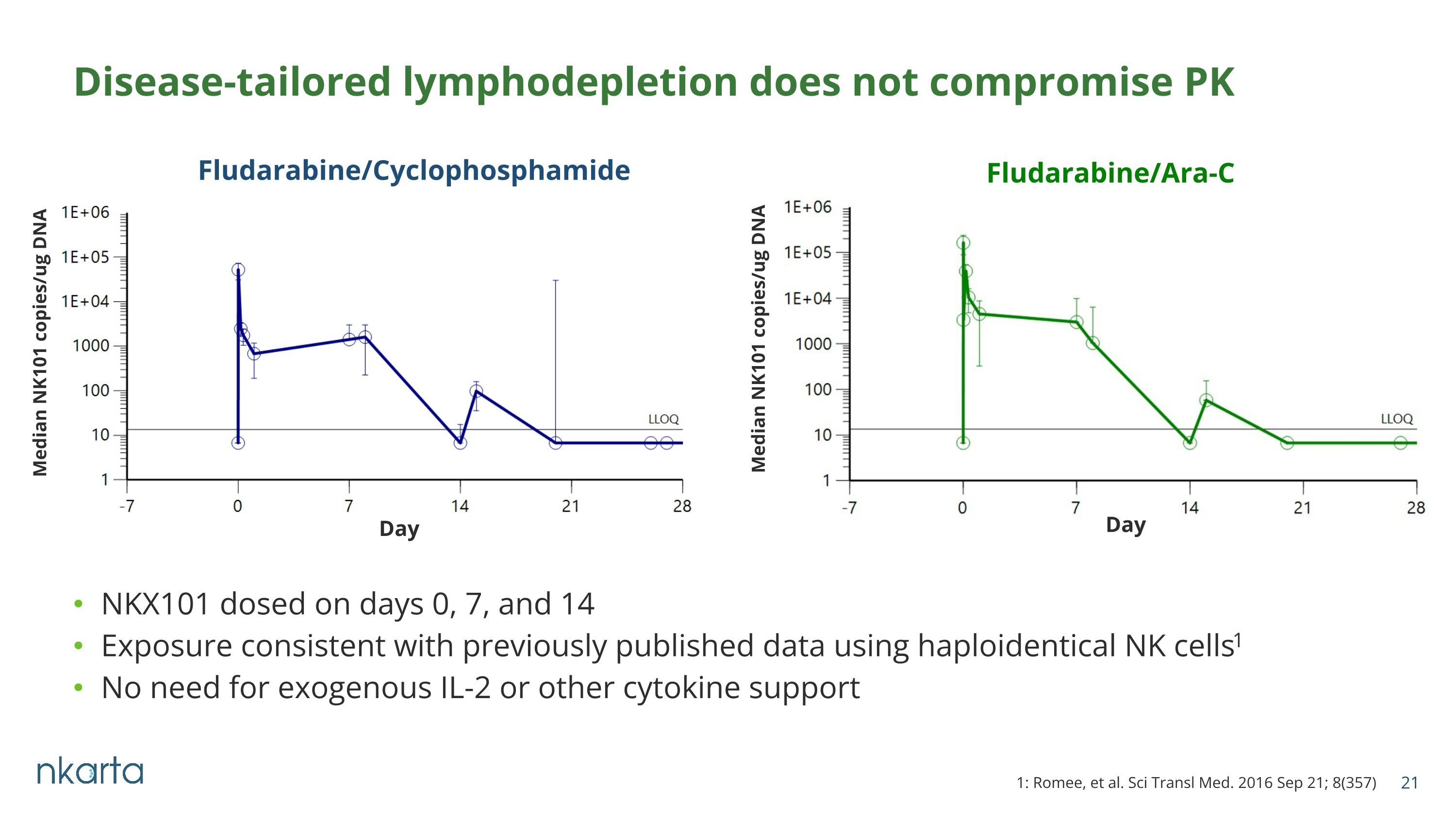
NKX101 dosed on days 0, 7, and 14 Exposure consistent with previously published data using haploidentical NK cells1 No need for exogenous IL-2 or other cytokine support Disease-tailored lymphodepletion does not compromise PK Fludarabine/Cyclophosphamide Fludarabine/Ara-C Median NK101 copies/ug DNA Median NK101 copies/ug DNA Day Day 1: Romee, et al. Sci Transl Med. 2016 Sep 21; 8(357)
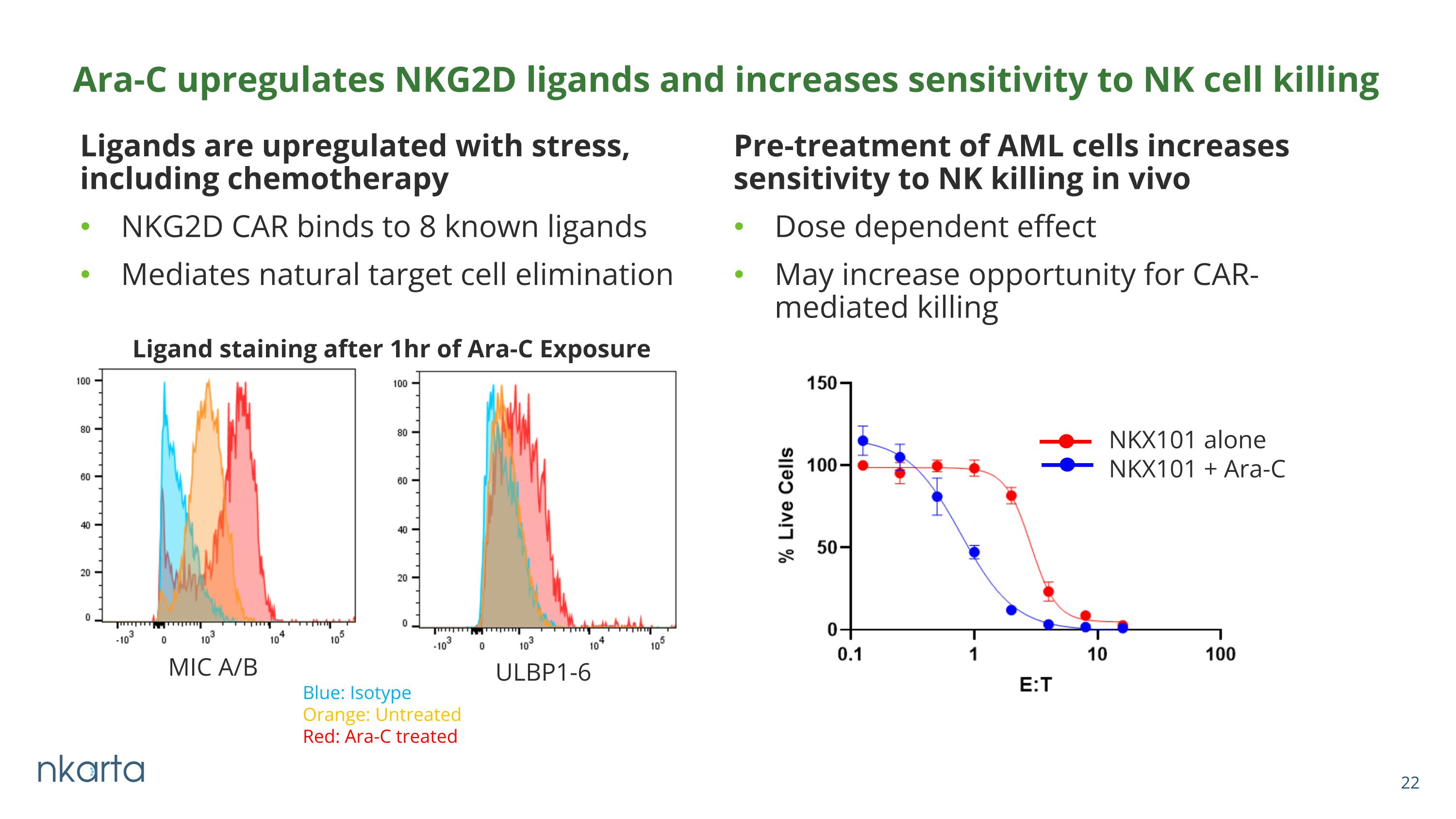
Ligands are upregulated with stress, including chemotherapy NKG2D CAR binds to 8 known ligands Mediates natural target cell elimination Pre-treatment of AML cells increases sensitivity to NK killing in vivo Dose dependent effect May increase opportunity for CAR-mediated killing Ara-C upregulates NKG2D ligands and increases sensitivity to NK cell killing MIC A/B ULBP1-6 Blue: Isotype Orange: Untreated Red: Ara-C treated Ligand staining after 1hr of Ara-C Exposure NKX101 alone NKX101 + Ara-C
Summary
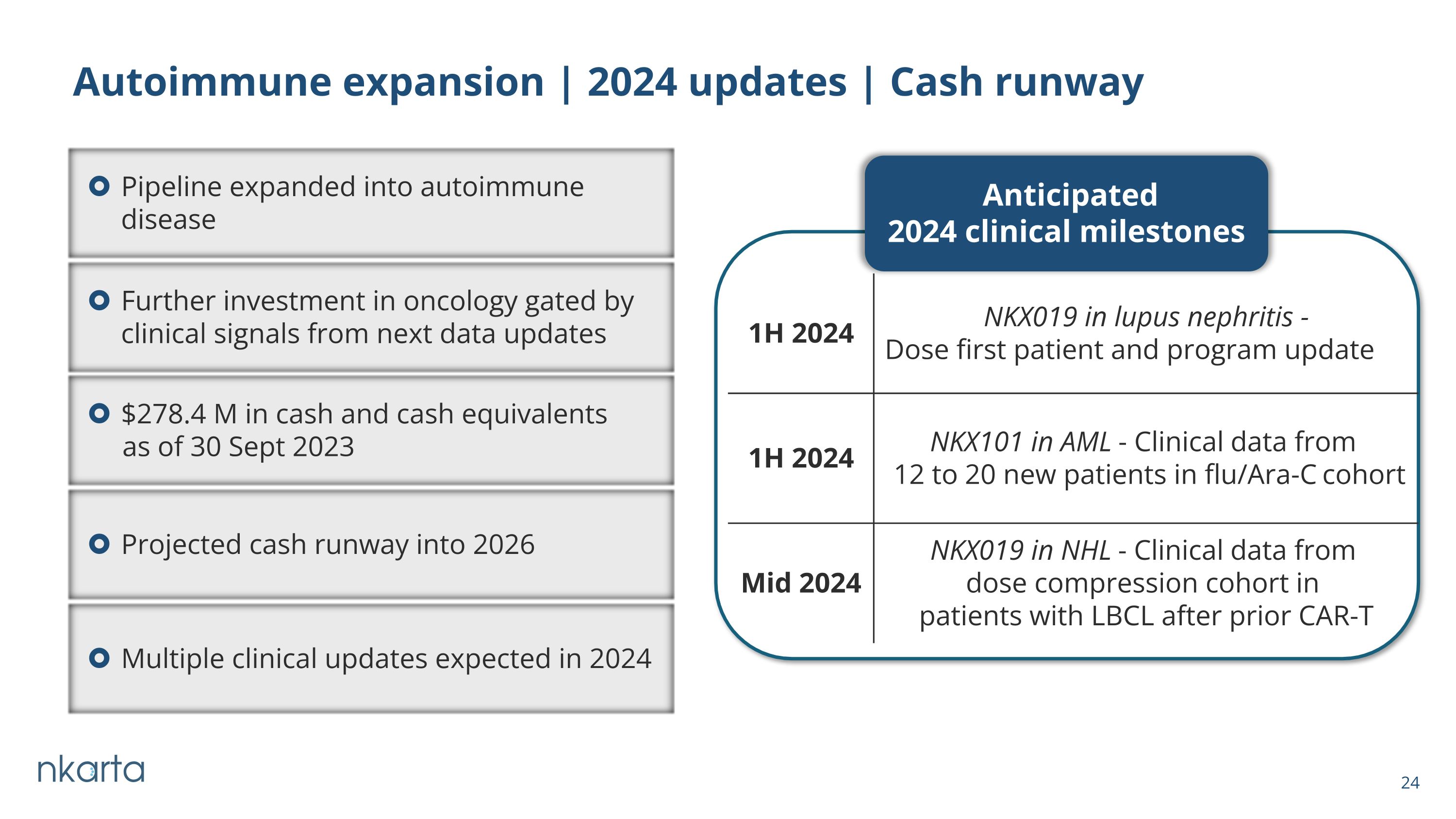
Autoimmune expansion | 2024 updates | Cash runway Anticipated 2024 clinical milestones 1H 2024 NKX019 in lupus nephritis - Dose first patient and program update 1H 2024 NKX101 in AML - Clinical data from 12 to 20 new patients in flu/Ara-C cohort Mid 2024 NKX019 in NHL - Clinical data from dose compression cohort in patients with LBCL after prior CAR-T Pipeline expanded into autoimmune disease Further investment in oncology gated by clinical signals from next data updates $278.4 M in cash and cash equivalents as of 30 Sept 2023 Projected cash runway into 2026 Multiple clinical updates expected in 2024
v3.23.4
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Nkarta (NASDAQ:NKTX)
Historical Stock Chart
Von Okt 2024 bis Nov 2024

Nkarta (NASDAQ:NKTX)
Historical Stock Chart
Von Nov 2023 bis Nov 2024
