Pfizer Gets Europe OK of Cibinqo in Adults With Atopic Dermatitis
10 Dezember 2021 - 4:19PM
Dow Jones News
By Colin Kellaher
Pfizer Inc. on Friday said the European Commission approved its
Cibinqo once-daily JAK inhibitor for adults with moderate-to-severe
atopic dermatitis, the most common form of the inflammatory skin
disease eczema.
The New York drugmaker said the commission approved 100- and
200-milligram doses of Cibinqo, a potential rival to the
blockbuster eczema treatment Dupixent from Regeneron
Pharmaceuticals Inc. and Sanofi SA, for adults who are candidates
for systemic therapy.
The company said the commission also approved a 50-milligram
dose for patients with moderate and severe renal kidney failure or
certain patients receiving treatment with inhibitors of cytochrome
P450 2C19.
Cibinqo, which received marketing approval in the U.K., Japan
and Korea earlier this year, is under review by the U.S. Food and
Drug Administration, which has delayed its decision and hasn't set
a new target action date as the agency looks into the safety of JAK
inhibitor drugs.
Write to Colin Kellaher at colin.kellaher@wsj.com
(END) Dow Jones Newswires
December 10, 2021 10:04 ET (15:04 GMT)
Copyright (c) 2021 Dow Jones & Company, Inc.
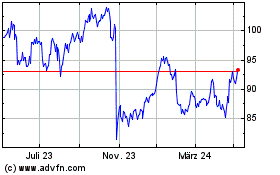
Sanofi (EU:SAN)
Historical Stock Chart
Von Mär 2024 bis Apr 2024
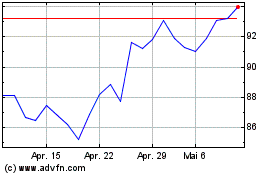
Sanofi (EU:SAN)
Historical Stock Chart
Von Apr 2023 bis Apr 2024