Jazz Pharmaceuticals plc false 0001232524 0001232524 2025-01-13 2025-01-13
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported) January 13, 2025
JAZZ PHARMACEUTICALS PUBLIC LIMITED COMPANY
(Exact name of registrant as specified in its charter)
|
|
|
|
|
| Ireland |
|
001-33500 |
|
98-1032470 |
| (State or Other Jurisdiction of Incorporation) |
|
(Commission File No.) |
|
(IRS Employer
Identification No.) |
Fifth Floor, Waterloo Exchange, Waterloo Road, Dublin 4, Ireland
D04 E5W7
(Address of principal executive offices, including zip code)
011-353-1-634-7800
(Registrant’s telephone number, including area code)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
| Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
| Ordinary shares, nominal value $0.0001 per share |
|
JAZZ |
|
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 2.02. |
Results of Operations and Financial Condition. |
On January 14, 2025, Jazz Pharmaceuticals plc (the “Company”) will present a corporate overview and financial update at the J.P. Morgan Healthcare Conference in San Francisco California, which presentation includes the Company’s expectations that it will meet its previously announced total, neuroscience and oncology revenue guidance ranges for the year ended December 31, 2024. A copy of the presentation is attached hereto as Exhibit 99.1.
The information contained in this Item 2.02 of this Current Report on Form 8-K shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liabilities of that Section or Sections 11 and 12(a)(2) of the Securities Act of 1933, as amended. The information contained in this Item 2.02 of this Current Report on Form 8-K shall not be incorporated by reference into any filing with the U.S. Securities and Exchange Commission made by the Company, whether made before or after the date hereof, regardless of any general incorporation language in such filing.
| Item 9.01. |
Financial Statements and Exhibits. |
(d) Exhibits
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
JAZZ PHARMACEUTICALS PUBLIC LIMITED COMPANY
|
|
|
|
|
| By: |
|
/s/ Philip L. Johnson |
| Name: |
|
Philip L. Johnson |
| Title: |
|
Executive Vice President and Chief Financial Officer |
Date: January 13, 2025

Exhibit 99.1 January 2025 rd 43 Annual J.P. Morgan Healthcare Conference
Innovating to Transform the Lives of Patients and Their Families Markella ® EPIDIOLEX patient diagnosed with Dravet syndrome January 2025

Transforming Lives. Redefining Possibilities. Caution Concerning
Forward-Looking Statements This presentation contains forward-looking statements and financial targets, including, but not limited to, statements related to: the Company’s growth prospects and future financial and operating results, including
the ability of the Company’s portfolio to drive long-term shareholder value; expectations with respect to indication expansion opportunities; 2024 total, neuroscience and oncology revenue guidance and the Company’s expectations related
thereto; the Company’s ability to drive significant cash flow generation; the Company’s commercial expectations, including with respect to revenue diversification and its expectations for significant growth; the Company’s
expectations with respect to the commercial potential of its products and product candidates, including the blockbuster potential for Epidiolex, the peak potential of zanidatamab, growth opportunities for Rylaze, Epidiolex/Epidyolex, Xywav and
Ziihera and Zepzelca’s potential approval as a first line therapy, and the potential regulatory paths related thereto; the value and growth potential of its products; the Company’s net product sales and goals for net product sales from
new and acquired products; the Company’s views and expectations relating to its patent portfolio, including with respect to expected patent protection; planned or anticipated clinical trial events, including with respect to initiations,
enrollment and data read-outs, and the anticipated timing thereof, and planned or anticipated regulatory submissions and filings and other regulatory matters, including potential approvals, including the timing thereof; and other statements that are
not historical facts. These forward-looking statements are based on the Company’s current plans, objectives, estimates, expectations and intentions and inherently involve significant risks and uncertainties. Actual results and the timing of
events could differ materially from those anticipated in such forward-looking statements as a result of these risks and uncertainties, which include, without limitation, risks and uncertainties associated with: maintaining or increasing sales of and
revenue from Xywav, Rylaze, Zepzelca, Epidiolex / Epidyolex, Ziihera and other key marketed products; effectively launching and commercializing the Company’s other products and product candidates; the successful completion of development and
regulatory activities with respect to the Company's product candidates; obtaining and maintaining adequate coverage and reimbursement for the Company’s products; the time-consuming and uncertain regulatory approval process, including the risk
that the Company’s current and/or planned regulatory submissions may not be submitted, accepted or approved by applicable regulatory authorities in a timely manner or at all; the costly and time-consuming pharmaceutical product development and
the uncertainty of clinical success, including risks related to failure or delays in successfully initiating or completing clinical trials and assessing patients such as those experienced, and expected to be experienced, by the Company; regulatory
initiatives and changes in tax laws; market volatility; protecting and enhancing the Company’s intellectual property rights and the Company’s commercial success being dependent upon its obtaining, maintaining and defending intellectual
property protection for its products and product candidates; delays or problems in the supply or manufacture of the Company’s products and product candidates; complying with applicable U.S. and non-U.S. regulatory requirements, including those
governing the research, development, manufacturing and distribution of controlled substances; government investigations, legal proceedings and other actions; identifying and consummating corporate development transactions, financing these
transactions and successfully integrating acquired product candidates, products and businesses; the Company’s ability to realize the anticipated benefits of its collaborations and license agreements with third parties; the sufficiency of the
Company’s cash flows and capital resources; the Company’s ability to achieve targeted or expected future financial performance and results and the uncertainty of future tax, accounting and other provisions and estimates; the Company's
ability to meet its projected long-term goals and objectives, in the time periods that the Company anticipates, or at all, and the inherent uncertainty and significant judgments and assumptions underlying the Company's long-term goals and
objectives; the completion of financial closing procedures, final audit adjustments and other developments that may arise that would cause the Company’s expectations with respect to the Company’s 2024 revenue guidance to differ, perhaps
materially, from the financial results that will be reflected in the Company’s audited consolidated financial statements for the fiscal year ended December 31, 2024; and other risks and uncertainties affecting the Company, including those
described from time to time under the caption “Risk Factors” and elsewhere in the Company’s Securities and Exchange Commission filings and reports, including the Company’s Annual Report on Form 10-K for the year ended
December 31, 2023 as supplemented by the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2024, and its future filings and reports. Other risks and uncertainties of which the Company is not currently aware may also
affect its forward-looking statements and may cause actual results and the timing of events to differ materially from those anticipated. The forward-looking statements made in this presentation are made only as of the date hereof or as of the dates
indicated in the forward-looking statements, even if they are subsequently made available by the Company on its website or otherwise. The Company undertakes no obligation to update or supplement any forward-looking statements to reflect actual
results, new information, future events, changes in its expectations or other circumstances that exist after the date as of which the forward-looking statements were made. January 2025 2

Our Purpose is to innovate to transform the lives of patients and their
families. Who We Are We are focused on developing life-changing medicines for people with serious diseases, often with limited or no therapeutic options, so they can live their lives more fully. Jennie Caroline Xywav patient living with IH Rylaze
patient diagnosed with ALL / LBL ALL/LBL = acute lymphoblastic leukemia / lymphoblastic lymphoma; IH = idiopathic hypersomnia. January 2025 3
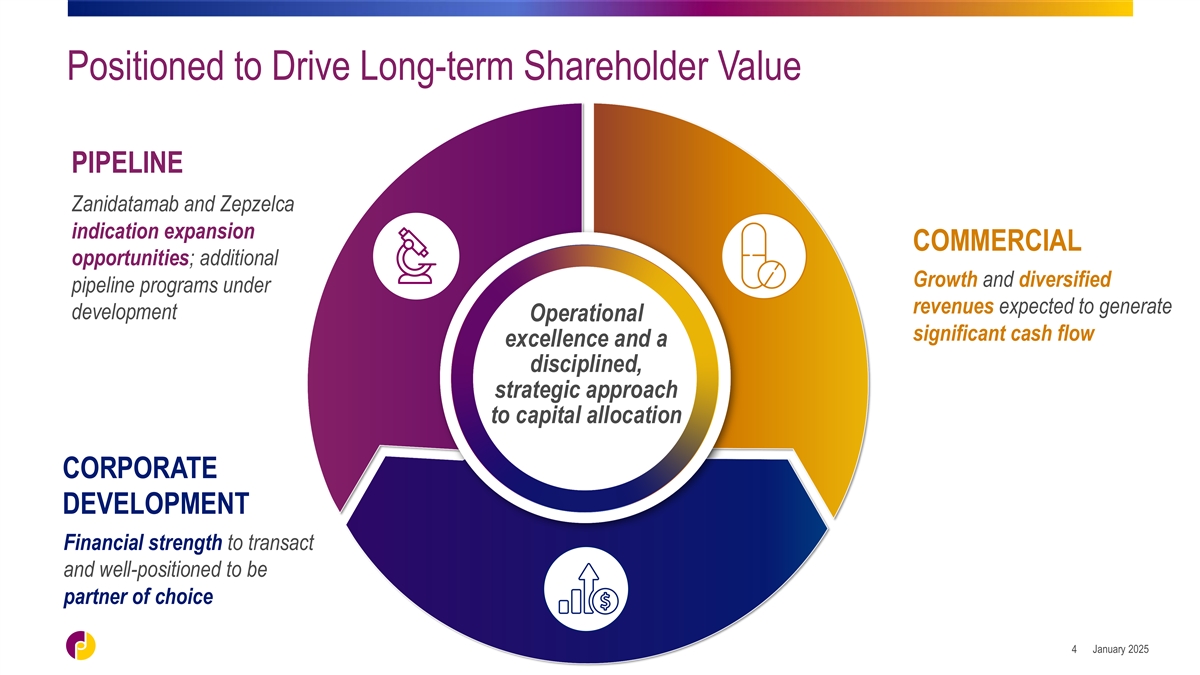
Positioned to Drive Long-term Shareholder Value PIPELINE Zanidatamab and
Zepzelca indication expansion COMMERCIAL opportunities; additional Growth and diversified pipeline programs under revenues expected to generate development Operational significant cash flow excellence and a disciplined, strategic approach to capital
allocation CORPORATE DEVELOPMENT Financial strength to transact and well-positioned to be partner of choice January 2025 4
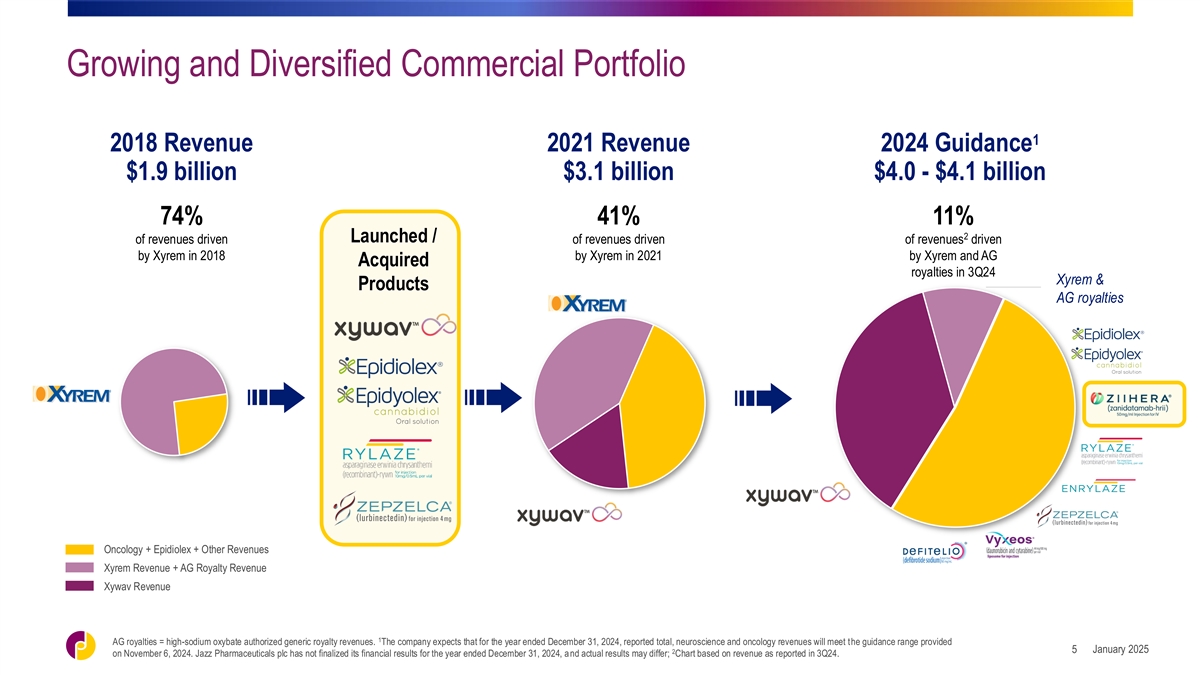
Growing and Diversified Commercial Portfolio 1 2018 Revenue 2021 Revenue
2024 Guidance $1.9 billion $3.1 billion $4.0 - $4.1 billion 74% 41% 11% 2 Launched / of revenues driven of revenues driven of revenues driven by Xyrem in 2018 by Xyrem in 2021 by Xyrem and AG Acquired royalties in 3Q24 Xyrem & Products AG
royalties Oncology + Epidiolex + Other Revenues Xyrem Revenue + AG Royalty Revenue Xywav Revenue 1 AG royalties = high-sodium oxybate authorized generic royalty revenues. The company expects that for the year ended December 31, 2024, reported total,
neuroscience and oncology revenues will meet the guidance range provided January 2025 5 2 on November 6, 2024. Jazz Pharmaceuticals plc has not finalized its financial results for the year ended December 31, 2024, and actual results may differ;
Chart based on revenue as reported in 3Q24.
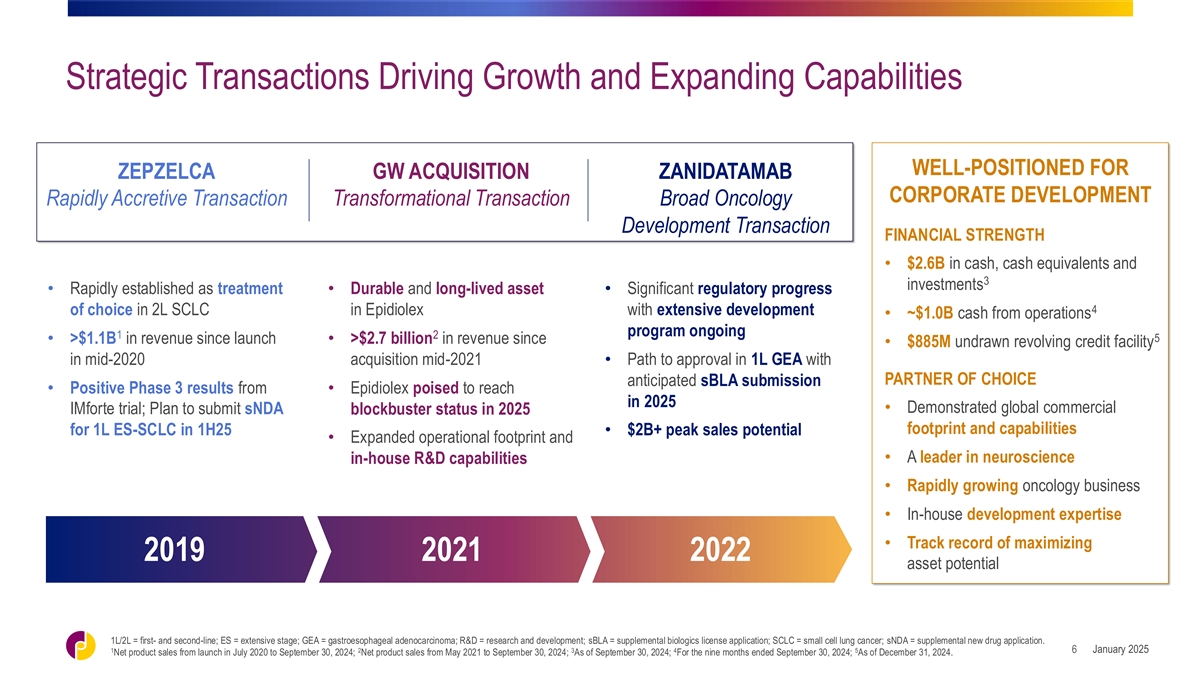
Strategic Transactions Driving Growth and Expanding Capabilities
WELL-POSITIONED FOR ZEPZELCA GW ACQUISITION ZANIDATAMAB CORPORATE DEVELOPMENT Rapidly Accretive Transaction Transformational Transaction Broad Oncology Development Transaction FINANCIAL STRENGTH • $2.6B in cash, cash equivalents and 3
investments • Rapidly established as treatment • Durable and long-lived asset • Significant regulatory progress 4 of choice in 2L SCLC in Epidiolex with extensive development • ~$1.0B cash from operations program ongoing 1 2
5 • >$1.1B in revenue since launch • >$2.7 billion in revenue since • $885M undrawn revolving credit facility in mid-2020 acquisition mid-2021• Path to approval in 1L GEA with PARTNER OF CHOICE anticipated sBLA
submission • Positive Phase 3 results from • Epidiolex poised to reach in 2025 • Demonstrated global commercial IMforte trial; Plan to submit sNDA blockbuster status in 2025 footprint and capabilities for 1L ES-SCLC in 1H25•
$2B+ peak sales potential • Expanded operational footprint and • A leader in neuroscience in-house R&D capabilities • Rapidly growing oncology business • In-house development expertise • Track record of maximizing
2019 2021 2022 asset potential 1L/2L = first- and second-line; ES = extensive stage; GEA = gastroesophageal adenocarcinoma; R&D = research and development; sBLA = supplemental biologics license application; SCLC = small cell lung cancer; sNDA =
supplemental new drug application. 1 2 3 4 5 January 2025 6 Net product sales from launch in July 2020 to September 30, 2024; Net product sales from May 2021 to September 30, 2024; As of September 30, 2024; For the nine months ended September 30,
2024; As of December 31, 2024.
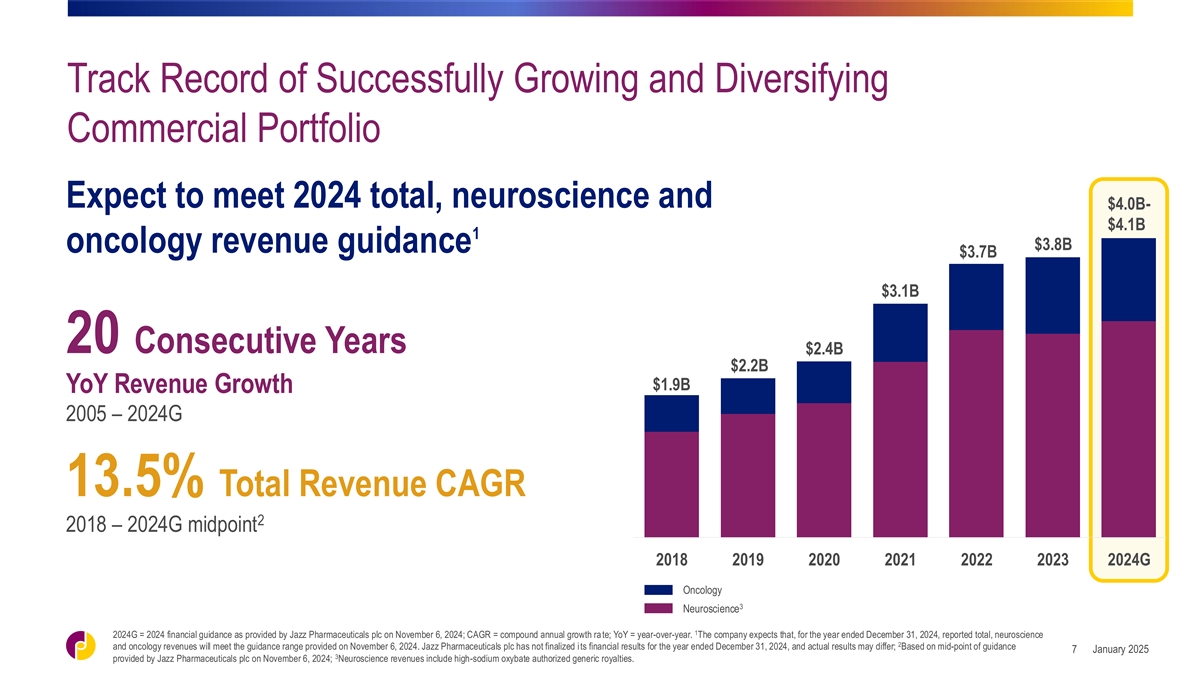
Track Record of Successfully Growing and Diversifying Commercial
Portfolio Expect to meet 2024 total, neuroscience and $4.0B- $4.1B 1 $3.8B oncology revenue guidance $3.7B $3.1B 20 Consecutive Years $2.4B $2.2B $1.9B YoY Revenue Growth 2005 – 2024G 13.5% Total Revenue CAGR 2 2018 – 2024G midpoint 2018
2019 2020 2021 2022 2023 2024G Oncology 3 Neuroscience 1 2024G = 2024 financial guidance as provided by Jazz Pharmaceuticals plc on November 6, 2024; CAGR = compound annual growth ra te; YoY = year-over-year. The company expects that, for the year
ended December 31, 2024, reported total, neuroscience 2 and oncology revenues will meet the guidance range provided on November 6, 2024. Jazz Pharmaceuticals plc has not finalized its financial results for the year ended December 31, 2024, and
actual results may differ; Based on mid-point of guidance January 2025 7 3 provided by Jazz Pharmaceuticals plc on November 6, 2024; Neuroscience revenues include high-sodium oxybate authorized generic royalties.

Pipeline Focused Investments in Promising R&D Portfolio January 2025
8
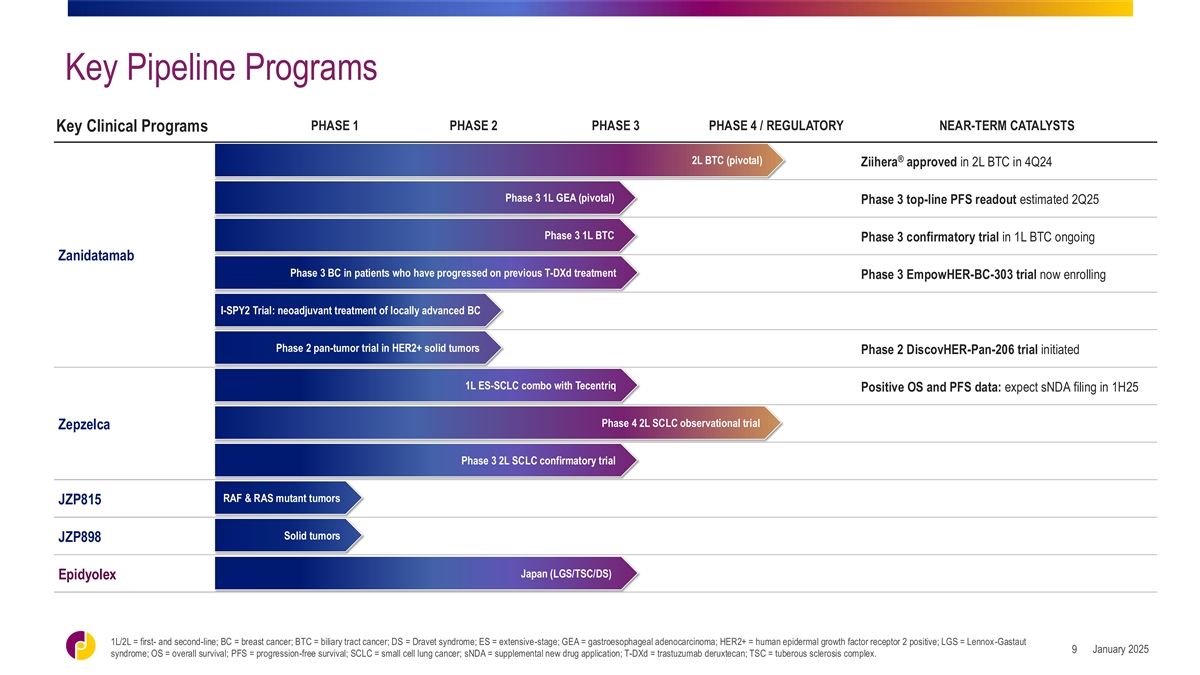
Key Pipeline Programs PHASE 1 PHASE 2 PHASE 3 PHASE 4 / REGULATORY
NEAR-TERM CATALYSTS Key Clinical Programs ® 2L BTC (pivotal) Ziihera approved in 2L BTC in 4Q24 Phase 3 1L GEA (pivotal) Phase 3 top-line PFS readout estimated 2Q25 Phase 3 1L BTC Phase 3 confirmatory trial in 1L BTC ongoing Zanidatamab Phase 3
BC in patients who have progressed on previous T-DXd treatment Phase 3 EmpowHER-BC-303 trial now enrolling I-SPY2 Trial: neoadjuvant treatment of locally advanced BC Phase 2 pan-tumor trial in HER2+ solid tumors Phase 2 DiscovHER-Pan-206 trial
initiated 1L ES-SCLC combo with Tecentriq Positive OS and PFS data: expect sNDA filing in 1H25 Phase 4 2L SCLC observational trial Zepzelca Phase 3 2L SCLC confirmatory trial RAF & RAS mutant tumors JZP815 Solid tumors JZP898 Japan (LGS/TSC/DS)
Epidyolex 1L/2L = first- and second-line; BC = breast cancer; BTC = biliary tract cancer; DS = Dravet syndrome; ES = extensive-stage; GEA = gastroesophageal adenocarcinoma; HER2+ = human epidermal growth factor receptor 2 positive; LGS =
Lennox-Gastaut January 2025 9 syndrome; OS = overall survival; PFS = progression-free survival; SCLC = small cell lung cancer; sNDA = supplemental new drug application; T-DXd = trastuzumab deruxtecan; TSC = tuberous sclerosis complex.
Zanidatamab Has the Potential to Transform HER2-Targeted Therapies
Zanidatamab is a highly active, differentiated HER2-targeted bispecific mAb with compelling and durable survival data Best-in-Class $2B+ Novel and Compelling Profile Commercial Differentiated Clinical Data in Addresses Opportunity MOA Multiple Unmet
Need Indications HER2 = human epidermal growth factor receptor 2; mAb = monoclonal antibody; MOA = mechanism of action. January 2025 10
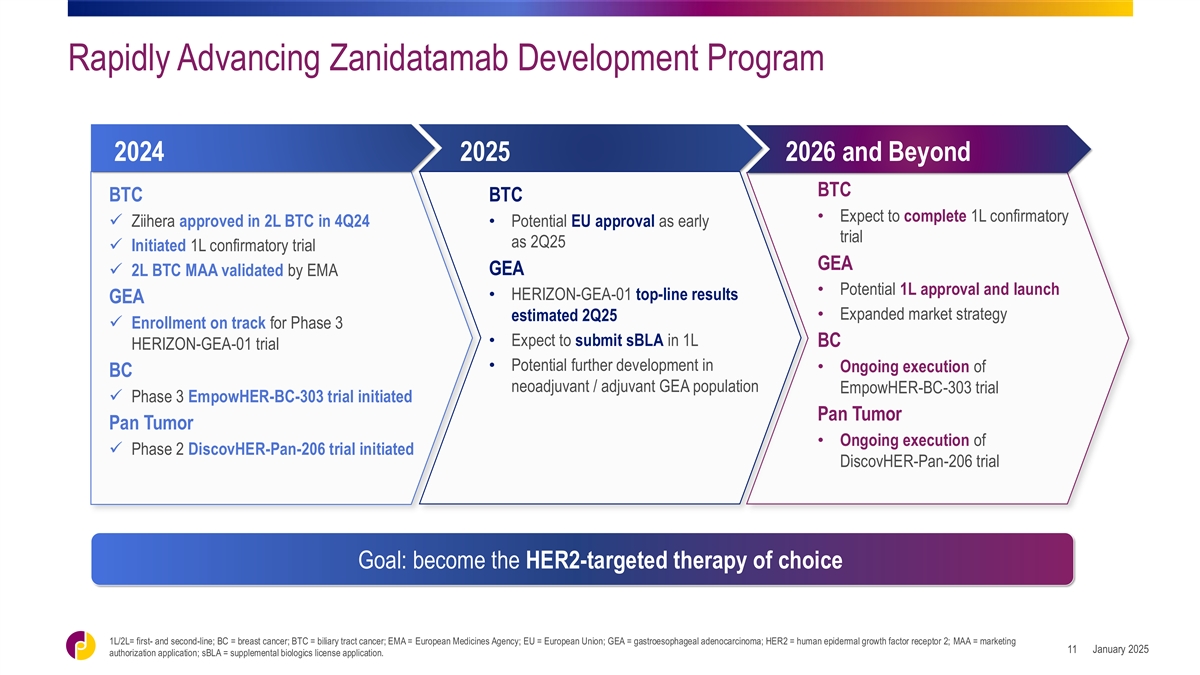
Rapidly Advancing Zanidatamab Development Program 2024 2025 2026 and
Beyond BTC BTC BTC • Expect to complete 1L confirmatory ✓ Ziihera approved in 2L BTC in 4Q24• Potential EU approval as early trial as 2Q25 ✓ Initiated 1L confirmatory trial GEA GEA ✓ 2L BTC MAA validated by EMA
• Potential 1L approval and launch • HERIZON-GEA-01 top-line results GEA • Expanded market strategy estimated 2Q25 ✓ Enrollment on track for Phase 3 • Expect to submit sBLA in 1L BC HERIZON-GEA-01 trial •
Potential further development in • Ongoing execution of BC neoadjuvant / adjuvant GEA population EmpowHER-BC-303 trial ✓ Phase 3 EmpowHER-BC-303 trial initiated Pan Tumor Pan Tumor • Ongoing execution of ✓ Phase 2
DiscovHER-Pan-206 trial initiated DiscovHER-Pan-206 trial Goal: become the HER2-targeted therapy of choice 1L/2L= first- and second-line; BC = breast cancer; BTC = biliary tract cancer; EMA = European Medicines Agency; EU = European Union; GEA =
gastroesophageal adenocarcinoma; HER2 = human epidermal growth factor receptor 2; MAA = marketing January 2025 11 authorization application; sBLA = supplemental biologics license application.
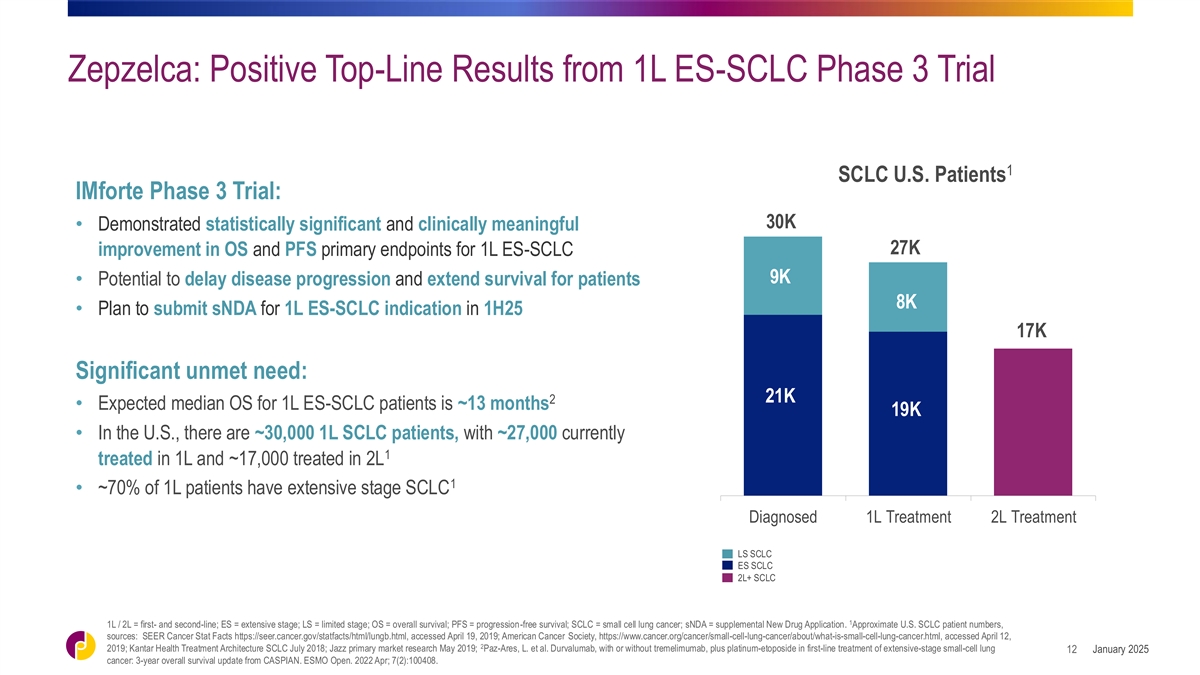
Zepzelca: Positive Top-Line Results from 1L ES-SCLC Phase 3 Trial 1
SCLC U.S. Patients IMforte Phase 3 Trial: 30K • Demonstrated statistically significant and clinically meaningful 27K improvement in OS and PFS primary endpoints for 1L ES-SCLC 9K • Potential to delay disease progression and extend
survival for patients 8K • Plan to submit sNDA for 1L ES-SCLC indication in 1H25 17K Significant unmet need: 21K 2 • Expected median OS for 1L ES-SCLC patients is ~13 months 19K • In the U.S., there are ~30,000 1L SCLC patients,
with ~27,000 currently 1 treated in 1L and ~17,000 treated in 2L 1 • ~70% of 1L patients have extensive stage SCLC Diagnosed 1L Treatment 2L Treatment LS SCLC ES SCLC 2L+ SCLC 1 1L / 2L = first- and second-line; ES = extensive stage; LS =
limited stage; OS = overall survival; PFS = progression-free survival; SCLC = small cell lung cancer; sNDA = supplemental New Drug Application. Approximate U.S. SCLC patient numbers, sources: SEER Cancer Stat Facts
https://seer.cancer.gov/statfacts/html/lungb.html, accessed April 19, 2019; American Cancer Society, https://www.cancer.org/cancer/small-cell-lung-cancer/about/what-is-small-cell-lung-cancer.html, accessed April 12, 2 2019; Kantar Health Treatment
Architecture SCLC July 2018; Jazz primary market research May 2019; Paz-Ares, L. et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide in first-line treatment of extensive-stage small-cell lung January 2025 12 cancer: 3-year
overall survival update from CASPIAN. ESMO Open. 2022 Apr; 7(2):100408.

Key Commercial Products Highly Differentiated Therapies Poised for
Growth January 2025 13
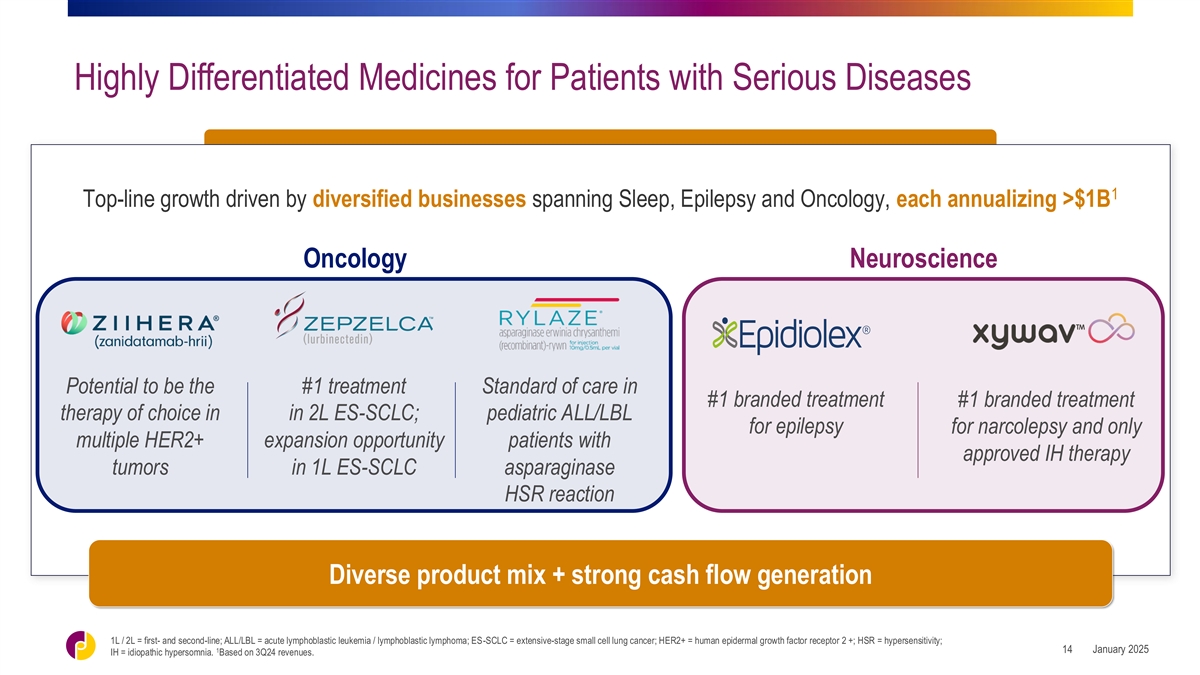
Highly Differentiated Medicines for Patients with Serious Diseases 1
Top-line growth driven by diversified businesses spanning Sleep, Epilepsy and Oncology, each annualizing >$1B Oncology Neuroscience Potential to be the #1 treatment Standard of care in #1 branded treatment #1 branded treatment therapy of choice
in in 2L ES-SCLC; pediatric ALL/LBL for epilepsy for narcolepsy and only multiple HER2+ expansion opportunity patients with approved IH therapy tumors in 1L ES-SCLC asparaginase HSR reaction Diverse product mix + strong cash flow generation 1L / 2L
= first- and second-line; ALL/LBL = acute lymphoblastic leukemia / lymphoblastic lymphoma; ES-SCLC = extensive-stage small cell lung cancer; HER2+ = human epidermal growth factor receptor 2 +; HSR = hypersensitivity; 1 January 2025 14 IH =
idiopathic hypersomnia. Based on 3Q24 revenues.
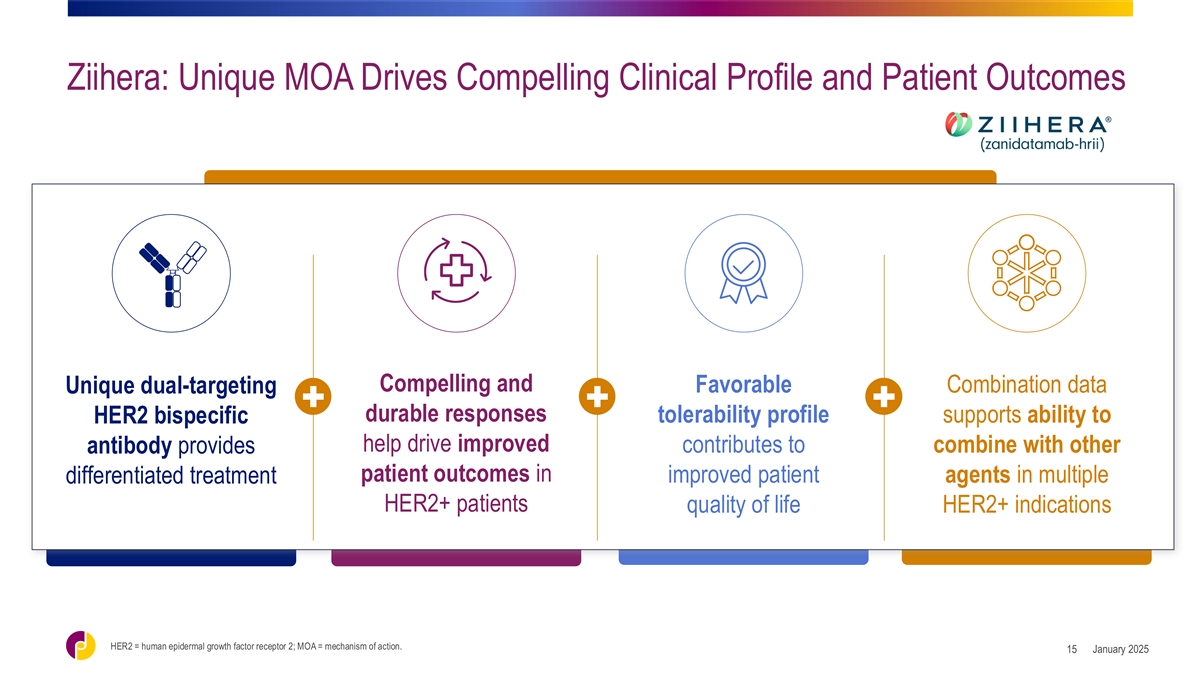
Ziihera: Unique MOA Drives Compelling Clinical Profile and Patient
Outcomes Compelling and Favorable Combination data Unique dual-targeting durable responses tolerability profile supports ability to HER2 bispecific help drive improved contributes to combine with other antibody provides patient outcomes in improved
patient agents in multiple differentiated treatment HER2+ patients quality of life HER2+ indications HER2 = human epidermal growth factor receptor 2; MOA = mechanism of action. January 2025 15
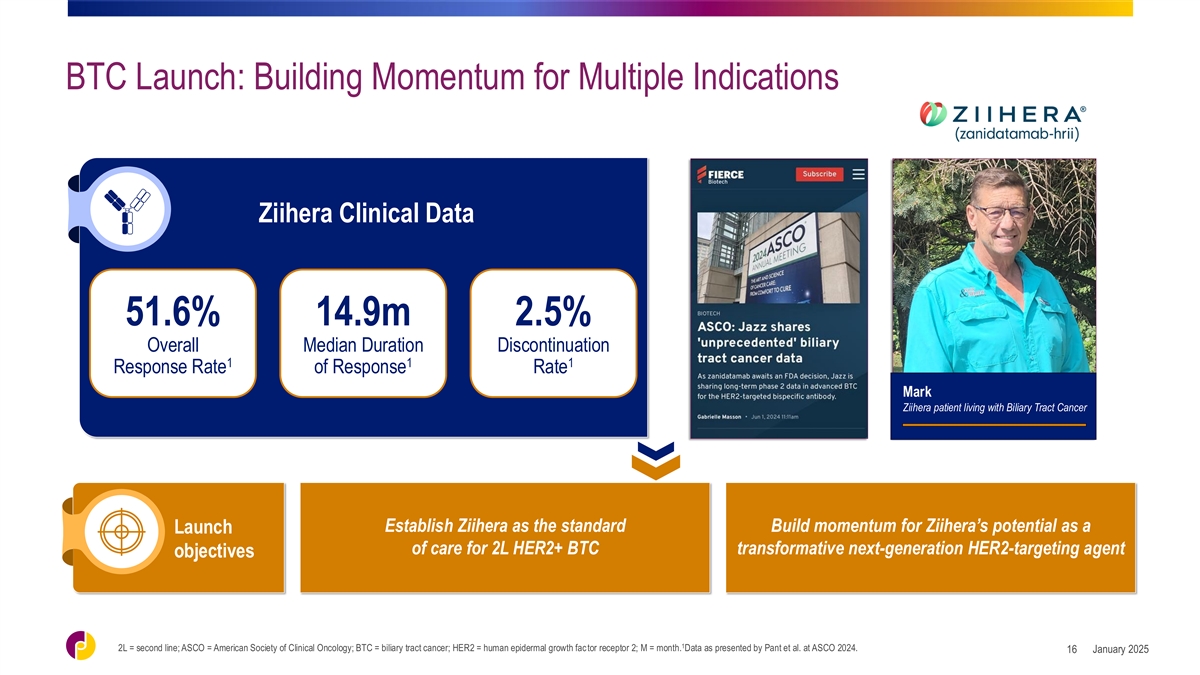
BTC Launch: Building Momentum for Multiple Indications Ziihera Clinical
Data 51.6% 14.9m 2.5% Overall Median Duration Discontinuation 1 1 1 Response Rate of Response Rate Mark Ziihera patient living with Biliary Tract Cancer Establish Ziihera as the standard Build momentum for Ziihera’s potential as a Launch of
care for 2L HER2+ BTC transformative next-generation HER2-targeting agent objectives 1 2L = second line; ASCO = American Society of Clinical Oncology; BTC = biliary tract cancer; HER2 = human epidermal growth factor receptor 2; M = month. Data as
presented by Pant et al. at ASCO 2024. January 2025 16
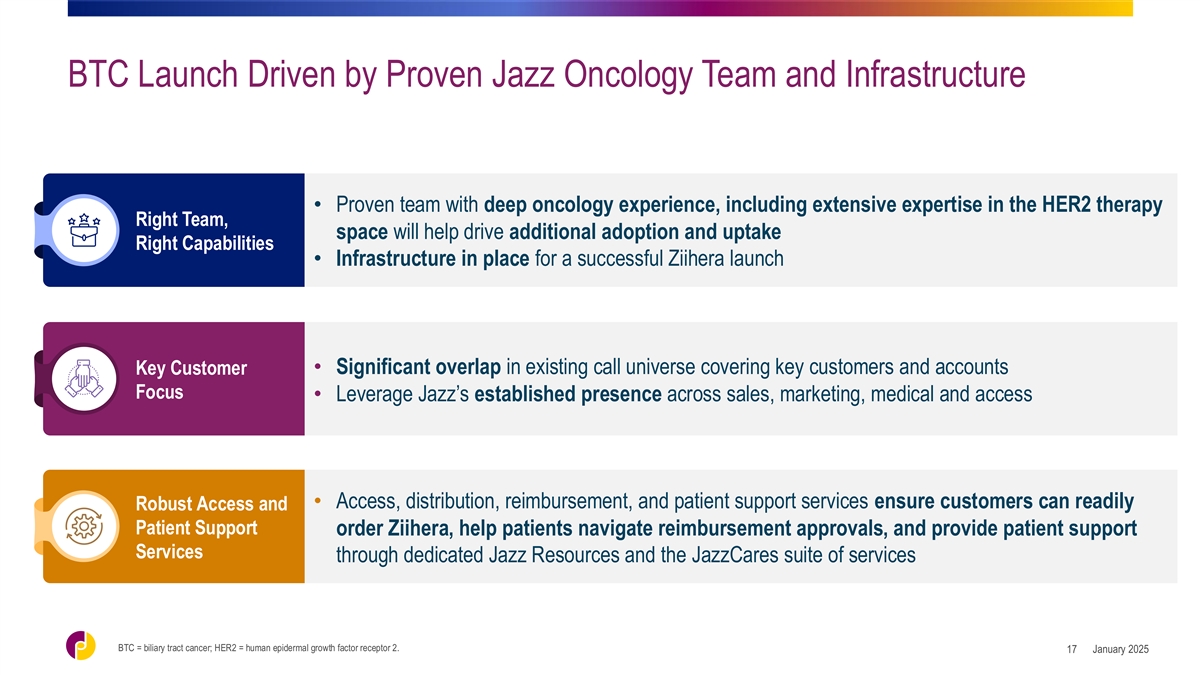
BTC Launch Driven by Proven Jazz Oncology Team and Infrastructure
• Proven team with deep oncology experience, including extensive expertise in the HER2 therapy Right Team, space will help drive additional adoption and uptake Right Capabilities • Infrastructure in place for a successful Ziihera launch
• Significant overlap in existing call universe covering key customers and accounts Key Customer Focus • Leverage Jazz’s established presence across sales, marketing, medical and access • Access, distribution, reimbursement,
and patient support services ensure customers can readily Robust Access and Patient Support order Ziihera, help patients navigate reimbursement approvals, and provide patient support Services through dedicated Jazz Resources and the JazzCares suite
of services BTC = biliary tract cancer; HER2 = human epidermal growth factor receptor 2. January 2025 17
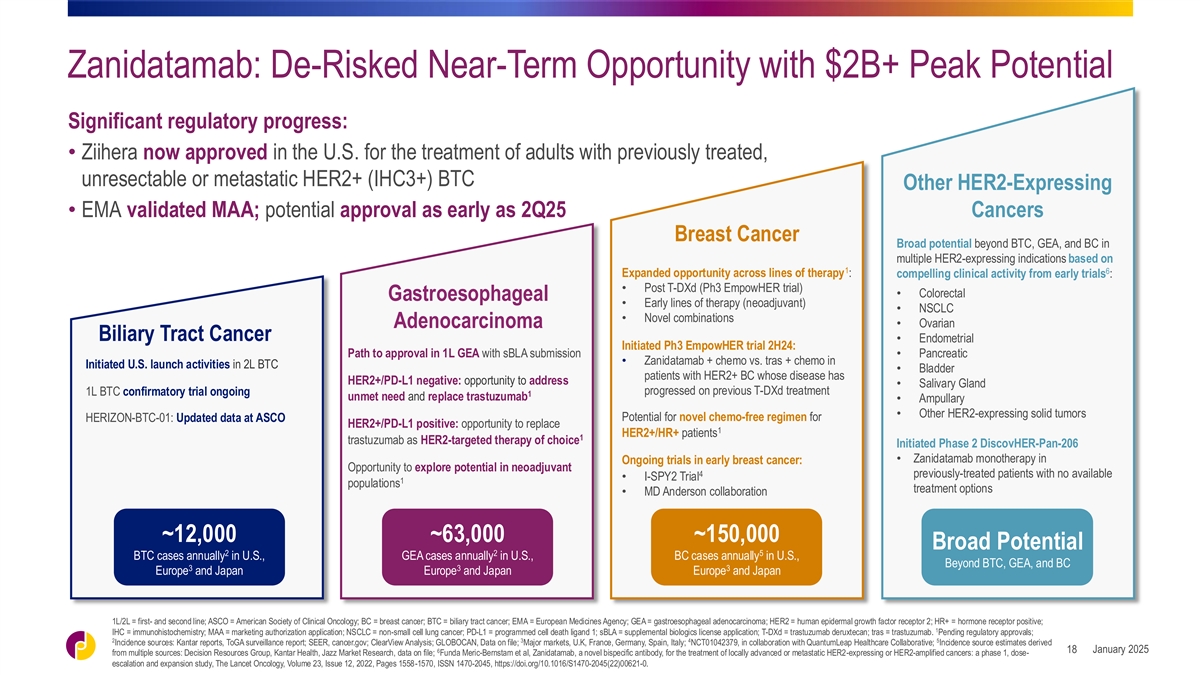
Zanidatamab: De-Risked Near-Term Opportunity with $2B+ Peak Potential
Significant regulatory progress: • Ziihera now approved in the U.S. for the treatment of adults with previously treated, unresectable or metastatic HER2+ (IHC3+) BTC Other HER2-Expressing • EMA validated MAA; potential approval as early
as 2Q25 Cancers Breast Cancer Broad potential beyond BTC, GEA, and BC in multiple HER2-expressing indications based on 1 6 Expanded opportunity across lines of therapy : compelling clinical activity from early trials : • Post T-DXd (Ph3
EmpowHER trial) • Colorectal Gastroesophageal • Early lines of therapy (neoadjuvant) • NSCLC • Novel combinations • Ovarian Adenocarcinoma Biliary Tract Cancer • Endometrial Initiated Ph3 EmpowHER trial 2H24: Path
to approval in 1L GEA with sBLA submission • Pancreatic • Zanidatamab + chemo vs. tras + chemo in Initiated U.S. launch activities in 2L BTC • Bladder patients with HER2+ BC whose disease has HER2+/PD-L1 negative: opportunity to
address • Salivary Gland progressed on previous T-DXd treatment 1L BTC confirmatory trial ongoing 1 unmet need and replace trastuzumab • Ampullary • Other HER2-expressing solid tumors Potential for novel chemo-free regimen for
HERIZON-BTC-01: Updated data at ASCO HER2+/PD-L1 positive: opportunity to replace 1 HER2+/HR+ patients 1 trastuzumab as HER2-targeted therapy of choice Initiated Phase 2 DiscovHER-Pan-206 • Zanidatamab monotherapy in Ongoing trials in early
breast cancer: Opportunity to explore potential in neoadjuvant 4 previously-treated patients with no available • I-SPY2 Trial 1 populations treatment options • MD Anderson collaboration ~12,000 ~63,000 ~150,000 Broad Potential 2 2 5 BTC
cases annually in U.S., GEA cases annually in U.S., BC cases annually in U.S., Beyond BTC, GEA, and BC 3 3 3 Europe and Japan Europe and Japan Europe and Japan 1L/2L = first- and second line; ASCO = American Society of Clinical Oncology; BC = breast
cancer; BTC = biliary tract cancer; EMA = European Medicines Agency; GEA = gastroesophageal adenocarcinoma; HER2 = human epidermal growth factor receptor 2; HR+ = hormone receptor positive; 1 IHC = immunohistochemistry; MAA = marketing authorization
application; NSCLC = non-small cell lung cancer; PD-L1 = programmed cell death ligand 1; sBLA = supplemental biologics license application; T-DXd = trastuzumab deruxtecan; tras = trastuzumab. Pending regulatory approvals; 2 3 4 5 Incidence sources:
Kantar reports, ToGA surveillance report; SEER, cancer.gov; ClearView Analysis; GLOBOCAN, Data on file; Major markets, U.K, France, Germany, Spain, Italy; NCT01042379, in collaboration with QuantumLeap Healthcare Collaborative; Incidence source
estimates derived 6 January 2025 18 from multiple sources: Decision Resources Group, Kantar Health, Jazz Market Research, data on file; Funda Meric-Bernstam et al, Zanidatamab, a novel bispecific antibody, for the treatment of locally advanced or
metastatic HER2-expressing or HER2-amplified cancers: a phase 1, dose- escalation and expansion study, The Lancet Oncology, Volume 23, Issue 12, 2022, Pages 1558-1570, ISSN 1470-2045, https://doi.org/10.1016/S1470-2045(22)00621-0.
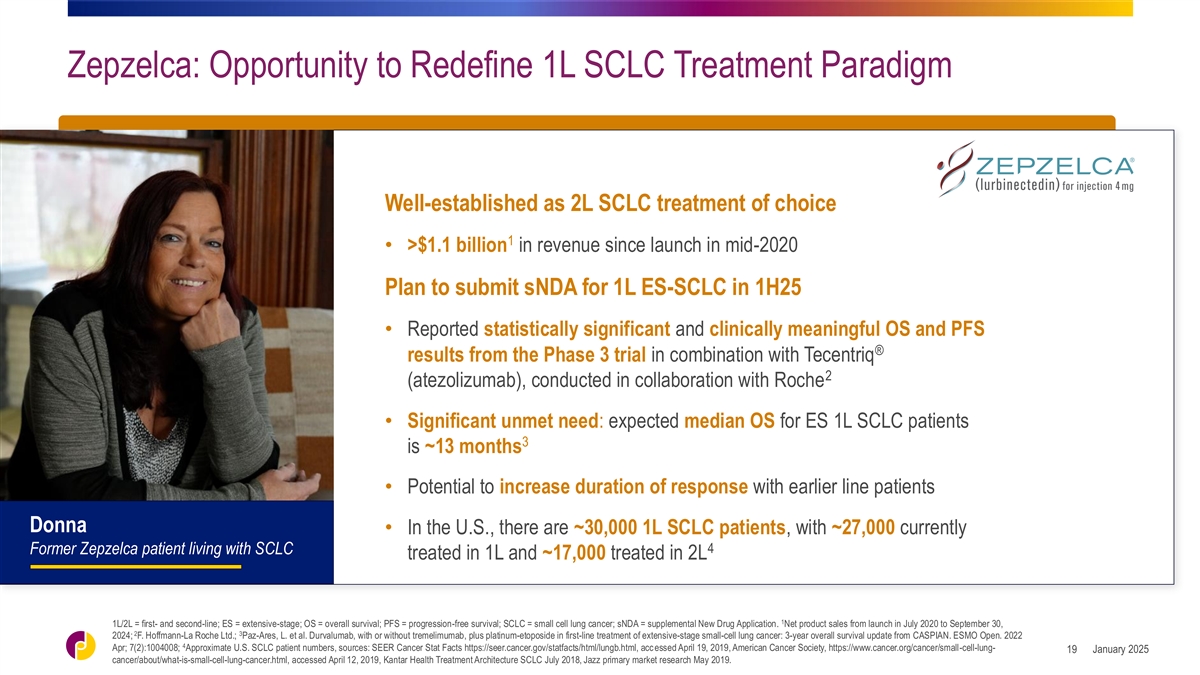
Zepzelca: Opportunity to Redefine 1L SCLC Treatment Paradigm
Well-established as 2L SCLC treatment of choice 1 • >$1.1 billion in revenue since launch in mid-2020 Plan to submit sNDA for 1L ES-SCLC in 1H25 • Reported statistically significant and clinically meaningful OS and PFS ® results
from the Phase 3 trial in combination with Tecentriq 2 (atezolizumab), conducted in collaboration with Roche • Significant unmet need: expected median OS for ES 1L SCLC patients 3 is ~13 months • Potential to increase duration of
response with earlier line patients Donna • In the U.S., there are ~30,000 1L SCLC patients, with ~27,000 currently 4 Former Zepzelca patient living with SCLC treated in 1L and ~17,000 treated in 2L 1 1L/2L = first- and second-line; ES =
extensive-stage; OS = overall survival; PFS = progression-free survival; SCLC = small cell lung cancer; sNDA = supplemental New Drug Application. Net product sales from launch in July 2020 to September 30, 2 3 2024; F. Hoffmann-La Roche Ltd.;
Paz-Ares, L. et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer: 3-year overall survival update from CASPIAN. ESMO Open. 2022 4 Apr; 7(2):1004008; Approximate
U.S. SCLC patient numbers, sources: SEER Cancer Stat Facts https://seer.cancer.gov/statfacts/html/lungb.html, acc essed April 19, 2019, American Cancer Society, https://www.cancer.org/cancer/small-cell-lung- January 2025 19
cancer/about/what-is-small-cell-lung-cancer.html, accessed April 12, 2019, Kantar Health Treatment Architecture SCLC July 2018, Jazz primary market research May 2019.
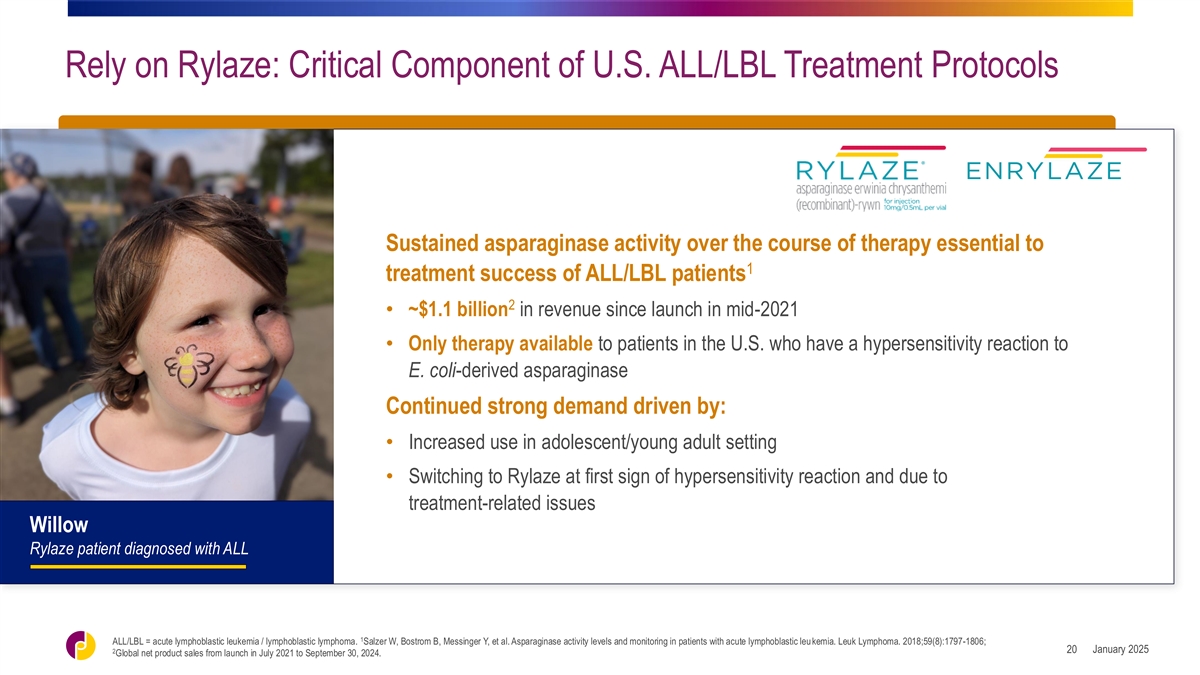
Rely on Rylaze: Critical Component of U.S. ALL/LBL Treatment Protocols
Sustained asparaginase activity over the course of therapy essential to 1 treatment success of ALL/LBL patients 2 • ~$1.1 billion in revenue since launch in mid-2021 • Only therapy available to patients in the U.S. who have a
hypersensitivity reaction to E. coli-derived asparaginase Continued strong demand driven by: • Increased use in adolescent/young adult setting • Switching to Rylaze at first sign of hypersensitivity reaction and due to treatment-related
issues Willow Rylaze patient diagnosed with ALL 1 ALL/LBL = acute lymphoblastic leukemia / lymphoblastic lymphoma. Salzer W, Bostrom B, Messinger Y, et al. Asparaginase activity levels and monitoring in patients with acute lymphoblastic leu kemia.
Leuk Lymphoma. 2018;59(8):1797-1806; January 2025 2 20 Global net product sales from launch in July 2021 to September 30, 2024.
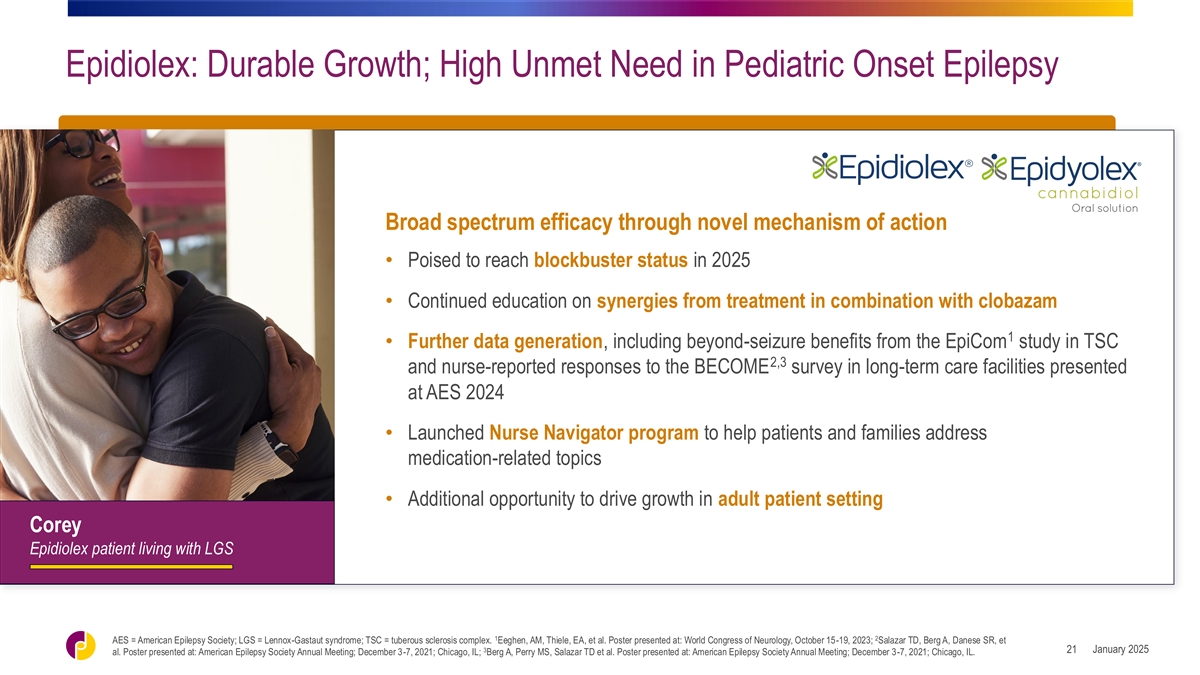
Epidiolex: Durable Growth; High Unmet Need in Pediatric Onset Epilepsy
Broad spectrum efficacy through novel mechanism of action • Poised to reach blockbuster status in 2025 • Continued education on synergies from treatment in combination with clobazam 1 • Further data generation, including
beyond-seizure benefits from the EpiCom study in TSC 2,3 and nurse-reported responses to the BECOME survey in long-term care facilities presented at AES 2024 • Launched Nurse Navigator program to help patients and families address
medication-related topics • Additional opportunity to drive growth in adult patient setting Corey Epidiolex patient living with LGS 1 2 AES = American Epilepsy Society; LGS = Lennox-Gastaut syndrome; TSC = tuberous sclerosis complex. Eeghen,
AM, Thiele, EA, et al. Poster presented at: World Congress of Neurology, October 15-19, 2023; Salazar TD, Berg A, Danese SR, et 3 January 2025 21 al. Poster presented at: American Epilepsy Society Annual Meeting; December 3-7, 2021; Chicago, IL;
Berg A, Perry MS, Salazar TD et al. Poster presented at: American Epilepsy Society Annual Meeting; December 3-7, 2021; Chicago, IL.

Xywav: Differentiated by Low Sodium; IH Provides Growth Opportunity 1
• Annualizing over $1.5 billion as of 3Q24 • Xywav remains #1 branded treatment for narcolepsy • Xywav is the only approved oxybate therapy that doesn’t carry a warning and precaution related to high sodium intake • FDA
published its summary of clinical superiority findings stating Xywav is clinically superior to Xyrem by means of greater safety • Positive impact from Field Nurse Educator program supporting both narcolepsy and IH • See most opportunity
for growth in IH as the only approved therapy to treat IH and no near-term competition Cindy Xywav patient living with IH 1 FDA = Food and Drug Administration; IH = idiopathic hypersomnia. Based net product sales reported for quarter ended September
30, 2024. January 2025 22

Well-Positioned to Deliver Long-Term Value Operational Excellence and
Commercial Execution January 2025 23
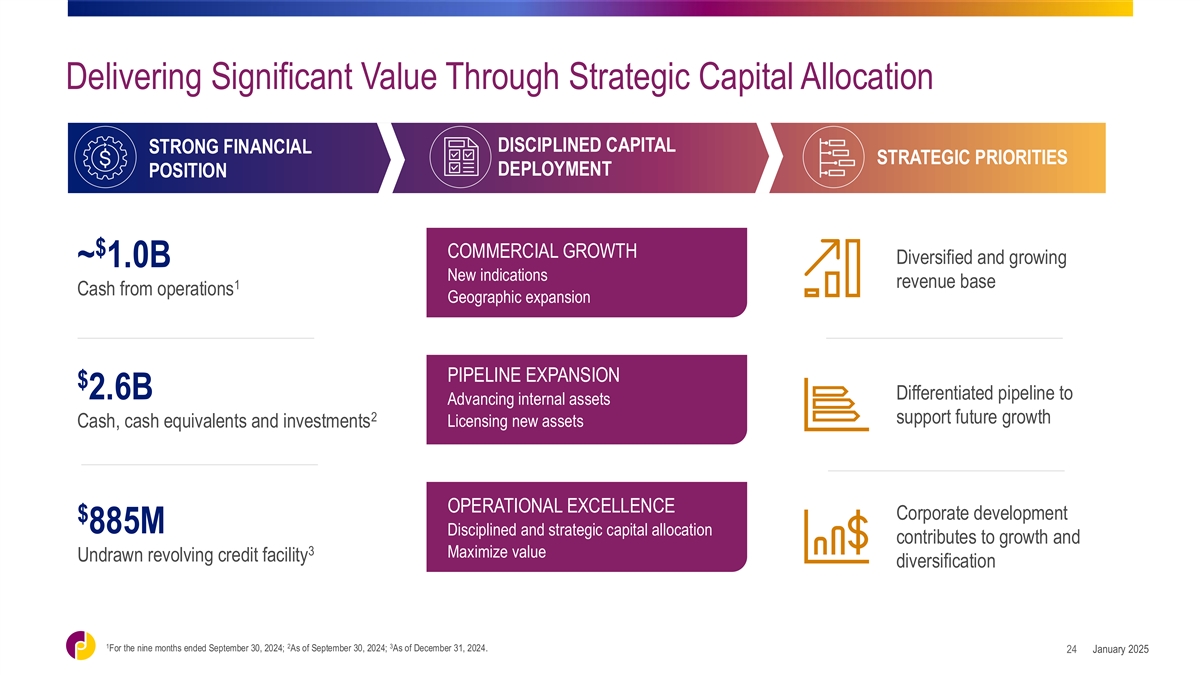
Delivering Significant Value Through Strategic Capital Allocation
DISCIPLINED CAPITAL STRONG FINANCIAL STRATEGIC PRIORITIES DEPLOYMENT POSITION $ COMMERCIAL GROWTH Diversified and growing ~ 1.0B New indications revenue base 1 Cash from operations Geographic expansion PIPELINE EXPANSION $ 2.6B Differentiated
pipeline to Advancing internal assets 2 support future growth Licensing new assets Cash, cash equivalents and investments OPERATIONAL EXCELLENCE Corporate development $ 885M Disciplined and strategic capital allocation contributes to growth and 3
Maximize value Undrawn revolving credit facility diversification 1 2 3 For the nine months ended September 30, 2024; As of September 30, 2024; As of December 31, 2024. January 2025 24
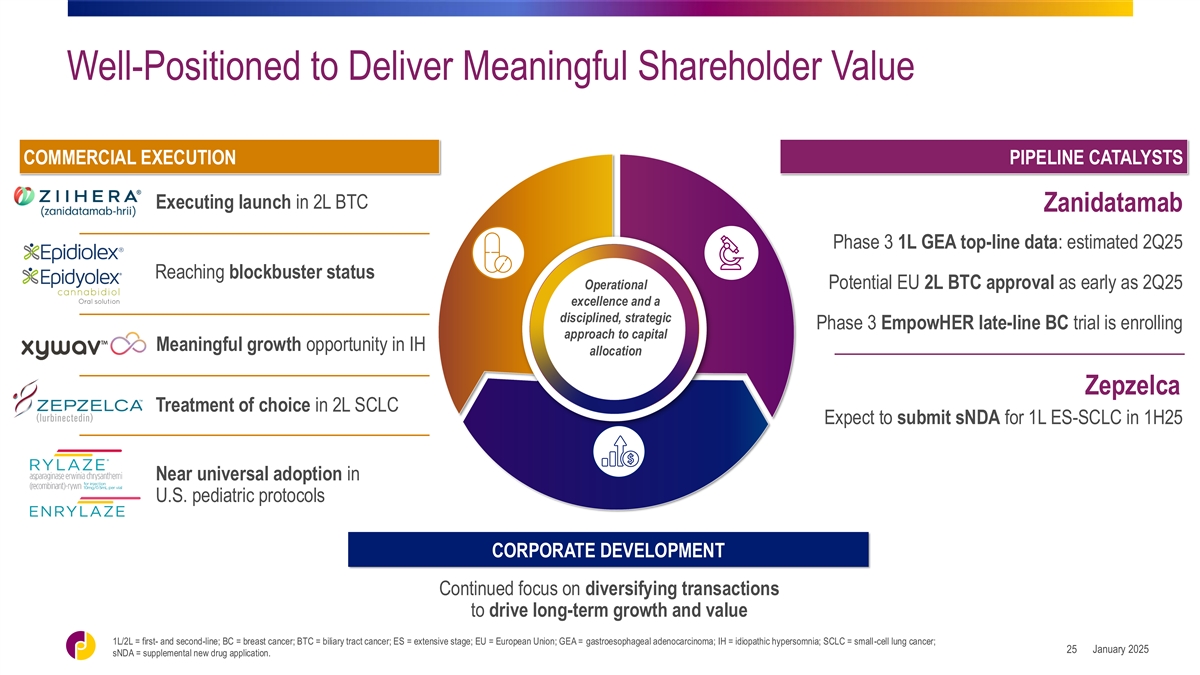
Well-Positioned to Deliver Meaningful Shareholder Value COMMERCIAL
EXECUTION PIPELINE CATALYSTS Executing launch in 2L BTC Zanidatamab Phase 3 1L GEA top-line data: estimated 2Q25 Reaching blockbuster status Potential EU 2L BTC approval as early as 2Q25 Operational excellence and a disciplined, strategic Phase 3
EmpowHER late-line BC trial is enrolling approach to capital Meaningful growth opportunity in IH allocation Zepzelca Treatment of choice in 2L SCLC Expect to submit sNDA for 1L ES-SCLC in 1H25 Near universal adoption in U.S. pediatric protocols
CORPORATE DEVELOPMENT Continued focus on diversifying transactions to drive long-term growth and value 1L/2L = first- and second-line; BC = breast cancer; BTC = biliary tract cancer; ES = extensive stage; EU = European Union; GEA = gastroesophageal
adenocarcinoma; IH = idiopathic hypersomnia; SCLC = small-cell lung cancer; January 2025 25 sNDA = supplemental new drug application.

Q&A January 2025 26

Thank You January 2025 27
v3.24.4
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 3 such as an Office Park
| Name: |
dei_EntityAddressAddressLine3 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionISO 3166-1 alpha-2 country code.
| Name: |
dei_EntityAddressCountry |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:countryCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14a
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Jazz Pharmaceuticals (NASDAQ:JAZZ)
Historical Stock Chart
Von Dez 2024 bis Jan 2025

Jazz Pharmaceuticals (NASDAQ:JAZZ)
Historical Stock Chart
Von Jan 2024 bis Jan 2025
