Altimmune Added to Nasdaq Biotechnology Index
19 Dezember 2024 - 1:30PM

Altimmune, Inc. (Nasdaq: ALT), a clinical-stage biopharmaceutical
company, today announced that it will be added to the Nasdaq
Biotechnology Index (Nasdaq: NBI) effective prior to the market
open on Monday, December 23, 2024.
“Our addition to the NBI is an important
recognition to conclude what has been a year of major progress for
the Company,” said Vipin K. Garg, Ph.D., President and Chief
Executive Officer of Altimmune. “With a number of significant
inflection points coming in 2025, including the topline data from
the Phase 2b IMPACT trial of pemvidutide in MASH in the second
quarter, we believe that we are well positioned heading into the
new year.”
Established in 1993, the Nasdaq Biotechnology
Index is designed to track the performance of a set of
Nasdaq-listed securities that are classified as either
Biotechnology or Pharmaceutical according to the Industry
Classification Benchmark (ICB). Companies in the NBI must meet
certain eligibility requirements, including market capitalization,
average daily trading volume and seasoning. The Index is evaluated
annually and serves as the basis for the iShares NASDAQ
Biotechnology Index Fund (Nasdaq: IBB).
For more information about the NASDAQ Biotechnology Index,
visit: https://indexes.nasdaqomx.com/Index/Overview/NBI.
About Pemvidutide
Pemvidutide is a novel, investigational,
peptide-based GLP-1/glucagon dual receptor agonist in development
for the treatment of obesity and MASH. Activation of the GLP-1 and
glucagon receptors is believed to mimic the complementary effects
of diet and exercise on weight loss, with GLP-1 suppressing
appetite and glucagon increasing energy expenditure. Glucagon is
also recognized as having direct effects on hepatic fat metabolism,
which is believed to lead to rapid reductions in levels of liver
fat and serum lipids. In clinical trials to date, once-weekly
pemvidutide has demonstrated compelling weight loss with
class-leading lean mass preservation, and robust reductions in
triglycerides, LDL cholesterol, liver fat content and blood
pressure. The U.S. FDA has granted Fast Track designation
to pemvidutide for the treatment of MASH. Pemvidutide recently
completed the MOMENTUM Phase 2 obesity trial and is being studied
in the ongoing IMPACT Phase 2b MASH trial.
About Altimmune
Altimmune is a clinical-stage biopharmaceutical
company focused on developing innovative next-generation
peptide-based therapeutics. The Company is developing pemvidutide,
a GLP-1/glucagon dual receptor agonist for the treatment of obesity
and MASH. For more information, please visit www.altimmune.com.
Follow @Altimmune, Inc. on
LinkedInFollow @AltimmuneInc on
Twitter
Company Contact:Greg WeaverChief Financial
OfficerPhone: 240-654-1450ir@altimmune.com
Investor Contact:Lee RothBurns McClellanPhone:
646-382-3403lroth@burnsmc.com
Media Contact:Danielle CanteyInizio Evoke,
BiotechPhone: 619-826-4657Danielle.cantey@inizioevoke.com
This press release was published by a CLEAR® Verified
individual.
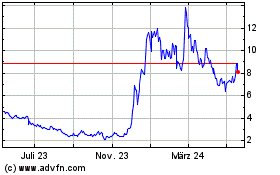
Altimmune (NASDAQ:ALT)
Historical Stock Chart
Von Dez 2024 bis Jan 2025
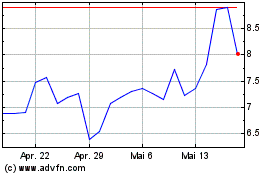
Altimmune (NASDAQ:ALT)
Historical Stock Chart
Von Jan 2024 bis Jan 2025