Aurora Spine Corporation Announces Newly Published Paper on First 6-month Clinical Evaluation of its ZIP™ Fusion Implant
04 Dezember 2023 - 1:00PM

Aurora Spine Corporation ("Aurora Spine" or the "Company") (TSXV:
ASG) (OTCQB: ASAPF), a designer and manufacturer of innovative
medical devices that improve spinal surgery outcomes, today
announced the publication of the first 6-month clinical evaluation
of its ZIP™ fusion implant in the Journal of Pain Research.
Lumbar interlaminar decompression with
interspinous fixation is an established safe and effective
treatment for spinal stenosis. Early maintenance of improvements in
pain intensity and function are critical for durability of symptom
relief. The purpose of this study is to investigate the efficacy of
minimally invasive treatments for low back pain during the early
period after treatment and their utility in setting the course for
longer term success.
The scientific paper titled “Early Functional
Outcomes in Low Back Pain Subjects with a Novel Interspinous Fusion
Device: REFINE Study 6-Month Results”, is a pivotal multi-center
study of its ZIP™ Interspinous Fixation device for patients
suffering from back pain due to symptomatic degenerative disc
disease and spinal stenosis.
The REFINE Screwless™ ZIP Study utilized patient
evaluations at 3- and 6-months following treatment and is part of
an actively enrolling, institutional review board (IRB) approved,
single-arm, multicenter, prospective, open-label 12-month study.
Clinical efficacy was assessed primarily using the change from
baseline in Oswestry Disability Index (ODI), Visual Analog Scale
(VAS) of the back and leg pain during walking and standing, and
Zurich Claudication Questionnaire (ZCQ), and secondarily using the
Patient Global Impression of Change (PGIC) and Patient-Reported
Outcomes Measurement Information System (PROMIS).
This 6-month interim analysis at 42% enrollment of
patients was conducted to determine prolonged safety and efficacy
of the ZIP interspinous fusion device. Our analysis showed a
sustained improvement in clinical efficacy, and safety endpoints,
when compared to the 3-months evaluations, across both
interventional pain and neurosurgery specialties.
The ZIP™ device is an alternative to more invasive
traditional spinal surgery. The ZIP device's minimally invasive
surgical technique works through a single, small surgical cut. Once
in place, the device acts as a fusion support column to open the
passageways that contain the spinal cord and nerve roots. This
procedure may reduce the compression on the nerves, resulting in
potential pain relief in the leg, groin, and buttocks, and then
return to a more active lifestyle.
"We appreciate all the support from the remarkable
group of physicians from the neurosurgery and interventional pain
management specialties across the country to bring the REFINE ZIP
multicenter study to success," said Trent J. Northcutt, President
and Chief Executive Officer of Aurora Spine.
Dr. Sebastian Koga, a neurosurgeon from Covington,
LA emphasized, “Our study demonstrates that the ZIP device provides
a stable platform for posterior fusion. The patients go home the
same day as the surgery and use less medications for chronic pain.
I am happy to contribute objective clinical evidence to this area
of spine surgery. In my practice I use the device as an adjuvant to
most single-level decompressions.”
According to a functional neurosurgeon Dr. Steven
Falowski, who enrolled the first patient for the REFINE study,
"Many of my patients suffering from lower back or leg pain could
benefit from the ZIP minimally invasive outpatient treatment option
that replaces traditional more invasive spinal decompression
surgery commonly done with pedicle screws. I am very excited with
these interim prospective 6 months results showing extremely
positive outcomes demonstrating the strong future of the ZIP
Screwless procedure. I am proud to participate as one of the
Principal Investigators in this groundbreaking multicenter study.
The purpose of this study is to develop the highest level of
scientific evidence and bring this landmark therapy to the
forefront."
Dr. Jason E. Pope, one of the Principal
Investigators in the study, a pain physician and anesthesiologist,
highlighted that “the ZIP device procedure operationalizes the
ability to perform a posterior interspinous fusion for those
specially trained pain physicians, offering an additional minimally
invasive option to remove pain and improve function in select
patients suffering from degenerative disc disease accompanied by
neurogenic claudication.”
The ZIP System was 510(k) cleared by the FDA in
2013 and has been commercially available in multiple sizes in the
US since 2014. With thousands of procedures already completed
worldwide, the ZIP device is safe and effective in an outpatient
setting. The ZIP device provides physicians the ability to remove
targeted ligament, bone, and facet capsule material. This quick
decompression involves minimal collateral tissue disruption and can
also work under local anesthesia. A previously published
retrospective study has also demonstrated the ZIP device’s safety
and effectiveness in the hands of interventional pain
physicians.
About Aurora Spine Aurora Spine
is focused on bringing new solutions to the spinal implant market
through a series of innovative, minimally invasive, regenerative
spinal implant technologies. Additional information can be accessed
at www.aurora-spine.com or www.aurorapaincare.com.
Neither TSX Venture Exchange nor its Regulation
Services Provider (as that term is defined in the policies of the
TSX Venture Exchange) accepts responsibility for the adequacy or
accuracy of this release.
Forward-Looking Statements This
news release contains forward-looking information that involves
substantial known and unknown risks and uncertainties, most of
which are beyond the control of Aurora Spine, including, without
limitation, those listed under "Risk Factors" and "Cautionary
Statement Regarding Forward-Looking Information" in Aurora Spine's
final prospectus (collectively, "forward-looking information").
Forward-looking information in this news release includes
information concerning the proposed use and success of the
Company's products in surgical procedures. Aurora Spine cautions
investors of Aurora Spine's securities about important factors that
could cause Aurora Spine's actual results to differ materially from
those projected in any forward-looking statements included in this
news release. Any statements that express, or involve discussions
as to, expectations, beliefs, plans, objectives, assumptions or
future events or performance are not historical facts and may be
forward-looking and may involve estimates, assumptions and
uncertainties which could cause actual results or outcomes to
differ unilaterally from those expressed in such forward-looking
statements. No assurance can be given that the expectations set out
herein will prove to be correct and, accordingly, prospective
investors should not place undue reliance on these forward-looking
statements. These statements speak only as of the date of this
press release, and Aurora Spine does not assume any obligation to
update or revise them to reflect new events or circumstances.
Contact: Aurora Spine Corporation
Trent Northcutt President and Chief Executive Officer (760)
424-2004
Chad Clouse Chief Financial Officer (760) 424-2004
www.aurora-spine.com
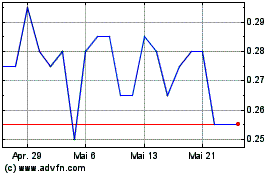
Aurora Spine (TSXV:ASG)
Historical Stock Chart
Von Apr 2024 bis Mai 2024
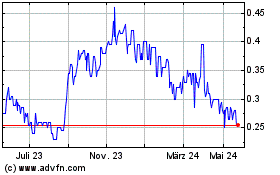
Aurora Spine (TSXV:ASG)
Historical Stock Chart
Von Mai 2023 bis Mai 2024