Sanofi Gets U.S. FDA Nod on Use of Dupixent in Children With Atopic Dermatitis
08 Juni 2022 - 8:04AM
Dow Jones News
By Cecilia Butini
Sanofi SA said late Tuesday that the U.S. Food and Drug
Administration has approved its blockbuster drug Dupixent for use
in children aged 6 months to 5 years with moderate-to-severe atopic
dermatitis.
The French pharma company said the approval is directed at those
children whose disease isn't adequately controlled with topical
prescription therapies, or medicines that are applied directly on
the skin.
Sanofi also said that a regulatory filing for this age group is
under review by the European Medicines Agency, and that submissions
to regulatory authorities in other countries are underway.
Write to Cecilia Butini at cecilia.butini@wsj.com
(END) Dow Jones Newswires
June 08, 2022 01:49 ET (05:49 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
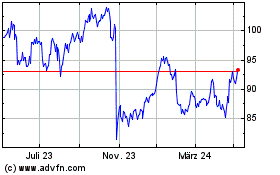
Sanofi (EU:SAN)
Historical Stock Chart
Von Mär 2024 bis Apr 2024
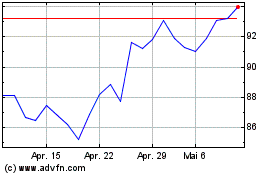
Sanofi (EU:SAN)
Historical Stock Chart
Von Apr 2023 bis Apr 2024