Kazia Therapeutics Shares Higher Premarket on Key FDA Designation
06 Juli 2023 - 1:51PM
Dow Jones News
By Colin Kellaher
U.S.-listed shares of Kazia Therapeutics jumped in premarket
trading Thursday after the Australian biotechnology company said
the U.S. Food and Drug Administration granted a second fast-track
designation to its lead program, paxalisib, in certain brain-cancer
patients.
The Sydney-based company said the latest designation covers
paxalisib for the treatment of solid tumor brain metastases
harboring PI3K pathway mutations in combination with radiation
therapy.
The FDA's fast-track program is designed to facilitate the
development and expedite the review of treatments for serious or
potentially life-threatening illnesses with high unmet medical
needs.
Kazia previously won FDA fast-track designation for paxalisib in
glioblastoma, an aggressive type of brain tumor, in August
2020.
Kazia's U.S.-listed shares, which closed Wednesday at $1.17,
were recently up nearly 26% to $1.47 in premarket trading.
Write to Colin Kellaher at colin.kellaher@wsj.com
(END) Dow Jones Newswires
July 06, 2023 07:36 ET (11:36 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
Kazia Therapeutics (ASX:KZA)
Historical Stock Chart
Von Nov 2024 bis Dez 2024
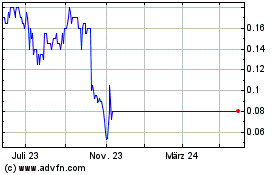
Kazia Therapeutics (ASX:KZA)
Historical Stock Chart
Von Dez 2023 bis Dez 2024
Echtzeit-Nachrichten über Kazia Therapeutics Limited (Australische Börse): 0 Nachrichtenartikel
Weitere Kazia Therapeutics Limited News-Artikel