false 0001878313 0001878313 2024-06-04 2024-06-04
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
Current Report
Pursuant to Section 13 or 15(d)
of The Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): June 4, 2024
MAIA Biotechnology, Inc.
(Exact name of registrant as specified in its charter)
|
|
|
|
|
| Delaware |
|
001-41455 |
|
83-1495913 |
| (State or other jurisdiction of incorporation) |
|
(Commission File Number) |
|
(IRS Employer Identification No.) |
|
|
|
| 444 West Lake Street, Suite 1700 |
|
|
| Chicago, IL |
|
60606 |
| (Address of principal executive offices) |
|
(Zip Code) |
(312) 416-8592
(Registrant’s telephone number, including area code)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
| Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
| Common Stock |
|
MAIA |
|
NYSE American |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (17 CFR §230.405) or Rule 12b-2 of the Securities Exchange Act of 1934 (17 CFR §240.12b-2).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01 |
Regulation FD Disclosure. |
1. MAIA Biotechnology, Inc. (the “Company”) has made available a presentation (the “Presentation”) about the Company’s business which was posted to the Company’s website on June 4, 2024, a copy of which is filed as Exhibit 99.1 to this Current Report on Form 8-K (this “Report”) and is hereby incorporated by reference.
2. The Company has made available a summary (“Summary”) highlighting certain aspects of the Company’s business, clinical programs and partnership with Regeneron which was posted to the Company’s website on June 4, 2024, a copy of which is filed as Exhibit 99.2 to this Current Report on Form 8-K (this “Report”) and is hereby incorporated by reference.
The information contained in each of the Presentation and the Summary is summary information that should be considered in the context of the Company’s filings with the Securities and Exchange Commission and other public announcements the Company may make by press release or otherwise from time to time. Each of the poster and the summary speaks as of the date of this Report. While the Company may elect to update the Presentation and/or the Summary in the future to reflect events and circumstances occurring or existing after the date of this Report, the Company specifically disclaims any obligation to do so.
Each of the Presentation and the Summary contains forward-looking statements, and as a result, investors should not place undue reliance on these forward-looking statements.
The information set forth in this Report, including, without limitation, the Presentation and the Summary, is not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that Section, nor shall it be incorporated by reference into a filing under the Securities Act of 1933, as amended, or the Exchange Act, regardless of any general incorporation language in such filing, except as shall be expressly set forth by specific reference in such a filing. This Report (including the exhibits hereto) will not be deemed an admission as to the materiality of any information required to be disclosed solely to satisfy the requirements of Regulation FD.
On June 4, 2024, the Company issued a press release announcing New Clinical Data Showing THIO’s Strong Efficacy in Non-Small Cell Lung Cancer.
A copy of the press release is attached hereto as Exhibit 99.3 and is incorporated herein by reference.
Forward-looking Statements
The Company cautions that all statements, other than statements of historical facts, contained in this Current Report on Form 8-K, or furnished herewith, are forward-looking statements. Forward-looking statements are subject to known and unknown risks, uncertainties, and other factors that may cause our or our industry’s actual results, levels or activity, performance or achievements to be materially different from those anticipated by such statements. The use of words such as “may,” “might,” “will,” “should,” “could,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “project,” “intend,” “future,” “potential,” or “continue,” and other similar expressions are intended to identify forward looking statements. However, the absence of these words does not mean that statements are not forward-looking. All forward-looking statements are based on current estimates, assumptions and expectations by our management that, although we believe to be reasonable, are inherently uncertain. Any forward-looking statement expressing an expectation or belief as to future events is expressed in good faith and believed to be reasonable at the time such forward-looking statement is made. However, these statements are not guarantees of future events and are subject to risks and uncertainties and other factors beyond our control that may cause actual results to differ materially from those expressed in any forward-looking statement, including, but not limited to: (i) the initiation, timing, cost, progress and results of our preclinical and clinical studies and our research and development programs, (ii) our ability to advance product candidates into, and successfully complete, clinical studies, (iii) the timing or likelihood of regulatory filings and approvals, (iv) our ability to develop, manufacture and commercialize our product candidates and to improve the manufacturing process, (v) the rate and degree of market acceptance of our product candidates, (vi) the
2
size and growth potential of the markets for our product candidates and our ability to serve those markets, and (vii) our expectations regarding our ability to obtain and maintain intellectual property protection for our product candidates. Any forward-looking statement speaks only as of the date on which it was made. The Company undertakes no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise, except as required by law.
| Item 9.01 |
Financial Statements and Exhibits. |
(d) Exhibits.
3
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
Dated: June 4, 2024
|
|
|
| MAIA BIOTECHNOLOGY, INC. |
|
|
| By: |
|
/s/ Vlad Vitoc |
| Name: |
|
Vlad Vitoc |
| Title: |
|
Chief Executive Officer |
4

Exhibit 99.1 TELOMERE TARGETING IMMUNOTHERAPIES FOR CANCER NYSE
AMERICAN: MAIA June 2024

FORWARD-LOOKING STATEMENTS NYSE American: MAIA All statements in this
presentation, other than those relating to historical facts, are forward-looking statements. These forward-looking statements may include, but are not limited to, statements relating to our objectives, plans, and strategies; statements that contain
projections of results of operations or of financial condition; statements relating to the industry and government policies and regulations relating to our industry; and all statements (other than statements of historical facts) that address
activities, events, or developments that we intend, expect, project, believe, or anticipate will or may occur in the future. Forward-looking statements are not guarantees of future performance and are subject to risks and uncertainties. We have
based these forward-looking statements on assumptions and assessments made by our management in light of their experience and their perception of historical trends, current conditions, expected future developments, and other factors they believe to
be appropriate. Important factors that could cause actual results, developments, and business decisions to differ materially from those anticipated in these forward-looking statements include, among other things: the overall global economic
environment; general market, political, and economic conditions in the countries in which we operate: projected capital expenditures and liquidity; changes in our strategy; government regulations and approvals; the application of certain service
license; and litigation and regulatory proceedings. Factors that may cause such differences also include, but are not limited to, those discussed under Risk Factors set forth in our Annual Report on Form 10-K for the year ended December 31, 2023 and
other periodic reports filed by the Company from time to time with the Securities and Exchange Commission. You may get these documents for free by visiting EDGAR on the Commission's website at www.sec.gov. We caution you that forward-looking
statements are not guarantees of future performance and that our actual results of operations, financial condition and liquidity, and the development of the industry in which we operate may differ materially from the forward-looking statements
contained in this presentation as a result of, among other factors, the factors referenced in the Risk Factors” section of our Annual Report on Form 10-K for the year ended December 31, 2023. In addition, even if our results of operations,
financial condition and liquidity, and the development of the industry in which we operate are consistent with the forward-looking statements contained in this presentation, they may not be predictive of results or developments in future periods.
This presentation shall not constitute an offer to sell or the solicitation of an offer to sell or the solicitation of an offer to buy any of our securities nor shall there be any sale of securities in any jurisdiction in which such offer,
solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction. Any offering of securities can only be made in compliance with applicable securities laws. You should read carefully
the factors described in the “Risk Factors section of our Annual Report on Form 10-K for the year ended December 31, 2023 to better understand the risks and uncertainties inherent in our business and underlying any forward-looking statements.
These statements are only current predictions and are subject to known and unknown risks, uncertainties, and other factors that may cause our or our industry's actual results, levels of activity, performance, or achievements to be materially
different from those anticipated by the forward-looking statements. You should not rely upon forward-looking statements as predictions of future events. Although we believe that the expectations reflected in the forward-looking statements are
reasonable, we cannot guarantee future results, levels of activity, performance, or achievements. Except as required by law, we are under no duty to update or revise any of the forward-looking statements, whether as a result of new information,
future events or otherwise, after the date of this prospectus. These forward-looking statements speak only as of the date of this presentation, and we assume no obligation to update or revise these forward-looking statements for any reason.
2
INVESTMENT PROFILE NYSE American: MAIA New science for cancer therapy:
dual MOA telomere targeting and immunogenicity. • Lead molecule THIO in clinic; 2nd generation compounds in R&D Phase 2 trial THIO-101 nearing completion: THIO sequenced with CPI in NSCLC. • Unprecedented disease control, response,
post-therapy patient benefit • Clinical supply agreement with Regeneron (Libtayo®) Key targeted clinical milestones within reach. • THIO-101 topline data in mid-2024; long-term data in 2nd half of 2024 • Multiple potential
pathways to FDA commercial approval Significant market opportunity in hard-to-treat cancers with unmet need. • NSCLC: largest tumor type globally, $34B annual sales • 3 FDA Orphan Drug Designations: liver (HCC), lung (SCLC) and brain
(malignant gliomas) THIO trials planned for additional cancer indications. • THIO-102 — colorectal cancer (CRC), HCC, SCLC, solid tumors • THIO-103 — SCLC, NSCLC 4 3
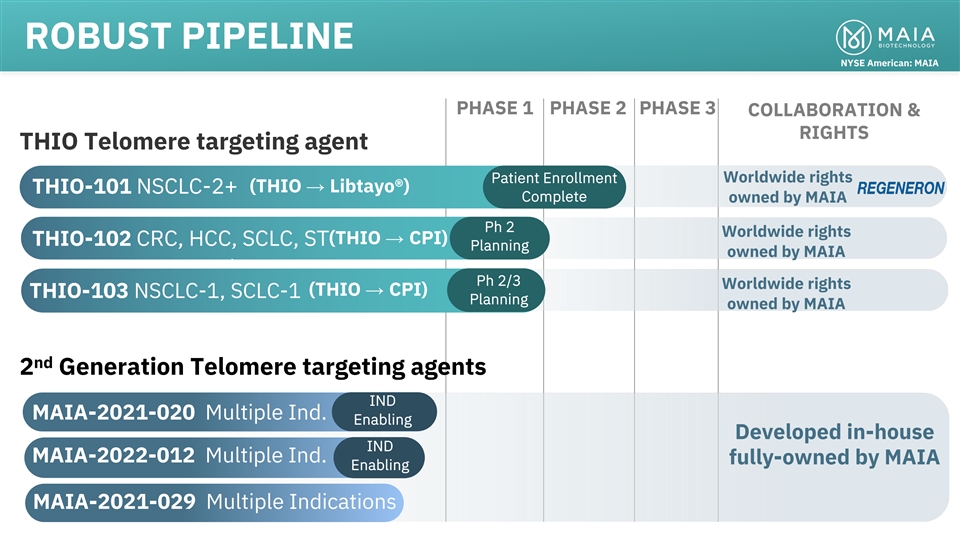
ROBUST PIPELINE NYSE American: MAIA PHASE 1 PHASE 2 PHASE 3
COLLABORATION & RIGHTS THIO Telomere targeting agent Patient Enrollment Worldwide rights (THIO → Libtayo®) THIO-101 NSCLC-2+ Complete owned by MAIA Ph 2 Worldwide rights (THIO → CPI) CRC, HCC, SCLC, ST THIO-102 Planning owned by
MAIA Ph 2/3 Worldwide rights (THIO → CPI) THIO-103 NSCLC-1, SCLC-1 Planning owned by MAIA nd 2 Generation Telomere targeting agents IND MAIA-2021-020 Multiple Ind. Enabling Developed in-house IND MAIA-2022-012 Multiple Ind. fully-owned by MAIA
Enabling MAIA-2021-029 Multiple Indications 4

NYSE American: MAIA MISSION AND APPROACH 5 5
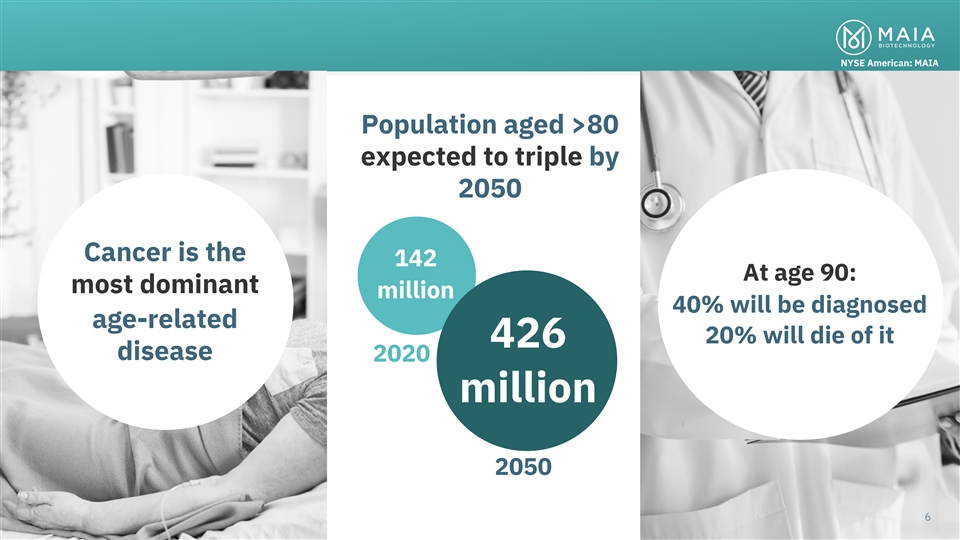
NYSE American: MAIA Population aged >80 expected to triple by 2050
Cancer is the 142 At age 90: most dominant million 40% will be diagnosed age-related 20% will die of it 426 disease 2020 million Cancer is the most dominant of the age-related disease categories and has life altering impacts in the lives of 2050
patients and their close ones 66

NYSE American: MAIA THIO is the only direct telomere targeting
anticancer agent in clinical development 77
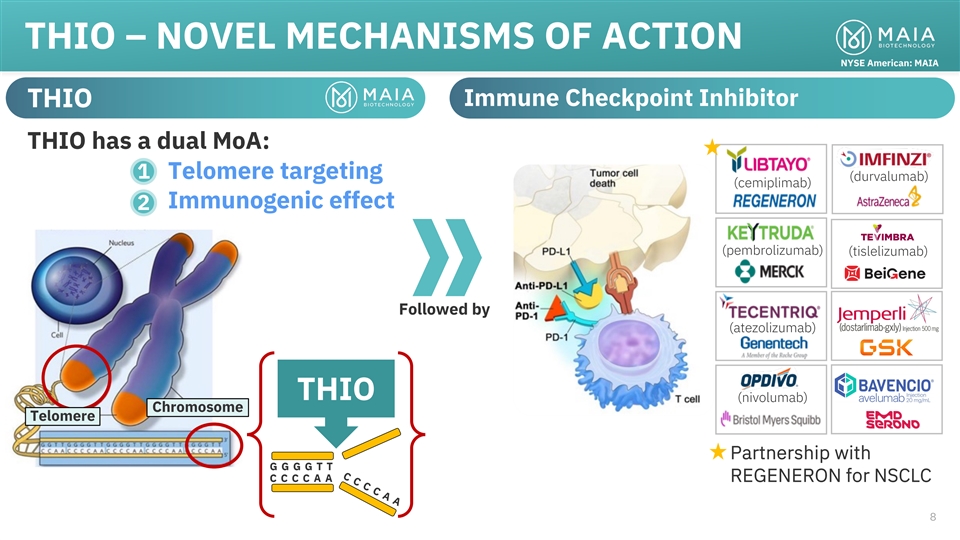
CCCCA A THIO – NOVEL MECHANISMS OF ACTION NYSE American: MAIA
Immune Checkpoint Inhibitor THIO THIO has a dual MoA: 1 • Telomere targeting (durvalumab) (cemiplimab) • Immunogenic effect 2 (pembrolizumab) (tislelizumab) Followed by (atezolizumab) THIO (nivolumab) Chromosome Telomere Partnership with
GGGGT T CCCCA A REGENERON for NSCLC 8
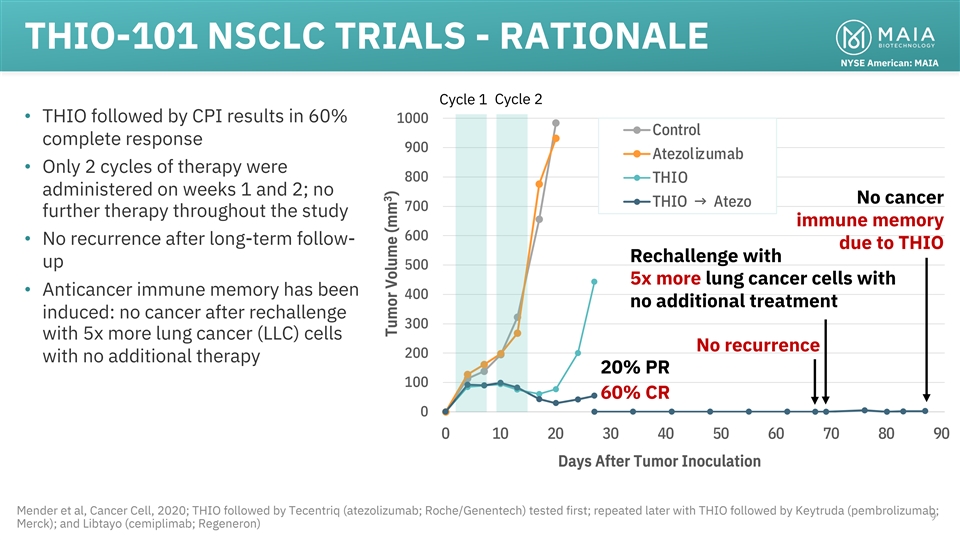
THIO-101 NSCLC TRIALS - RATIONALE NYSE American: MAIA Cycle 1 Cycle 2
• THIO followed by CPI results in 60% 1000 Control complete response 900 Atezolizumab • Only 2 cycles of therapy were 800 THIO administered on weeks 1 and 2; no No cancer THIO → Atezo 700 further therapy throughout the study immune
memory 600 • No recurrence after long-term follow- due to THIO Rechallenge with up 500 5x more lung cancer cells with • Anticancer immune memory has been 400 no additional treatment induced: no cancer after rechallenge 300 with 5x more
lung cancer (LLC) cells No recurrence 200 with no additional therapy 20% PR 100 60% CR 0 0 10 20 30 40 50 60 70 80 90 Days After Tumor Inoculation Mender et al, Cancer Cell, 2020; THIO followed by Tecentriq (atezolizumab; Roche/Genentech) tested
first; repeated later with THIO followed by Keytruda (pembrolizumab; 9 Merck); and Libtayo (cemiplimab; Regeneron) 3 Tumor Volume (mm )

NYSE American: MAIA THIO-101 TRIAL NON-SMALL CELL LUNG CANCER 10
10

REGENERON AGREEMENT NYSE American: MAIA & 11
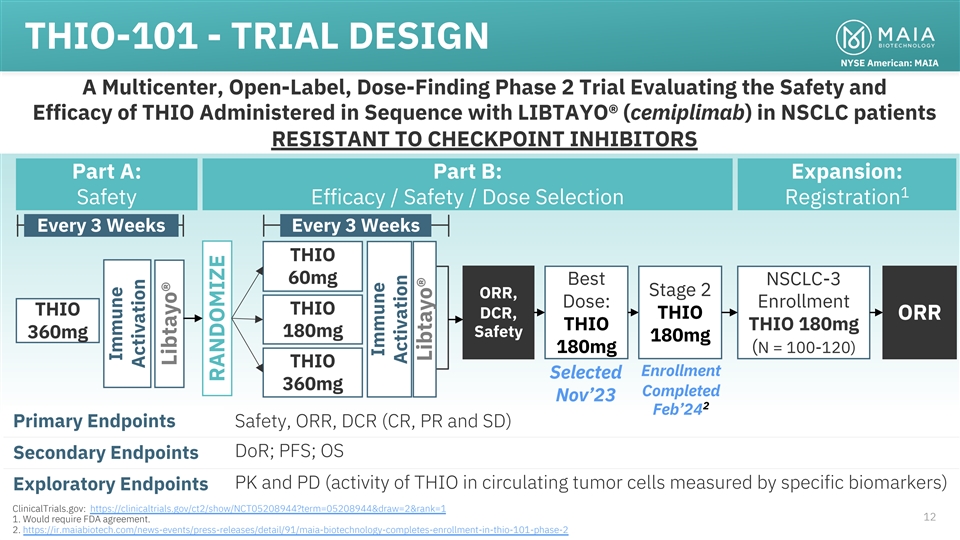
THIO-101 - TRIAL DESIGN NYSE American: MAIA A Multicenter, Open-Label,
Dose-Finding Phase 2 Trial Evaluating the Safety and Efficacy of THIO Administered in Sequence with LIBTAYO® (cemiplimab) in NSCLC patients RESISTANT TO CHECKPOINT INHIBITORS Part A: Part B: Expansion: 1 Safety Efficacy / Safety / Dose
Selection Registration Every 3 Weeks Every 3 Weeks THIO 60mg Best NSCLC-3 Stage 2 ORR, Dose: Enrollment THIO THIO DCR, THIO ORR THIO THIO 180mg 180mg Safety 360mg 180mg 180mg (N = 100-120) THIO Enrollment Selected 360mg Completed Nov’23 2
Feb’24 Primary Endpoints Safety, ORR, DCR (CR, PR and SD) DoR; PFS; OS Secondary Endpoints PK and PD (activity of THIO in circulating tumor cells measured by specific biomarkers) Exploratory Endpoints ClinicalTrials.gov:
https://clinicaltrials.gov/ct2/show/NCT05208944?term=05208944&draw=2&rank=1 12 1. Would require FDA agreement. 2. https://ir.maiabiotech.com/news-events/press-releases/detail/91/maia-biotechnology-completes-enrollment-in-thio-101-phase-2
Immune Activation Libtayo® RANDOMIZE Immune Activation Libtayo®
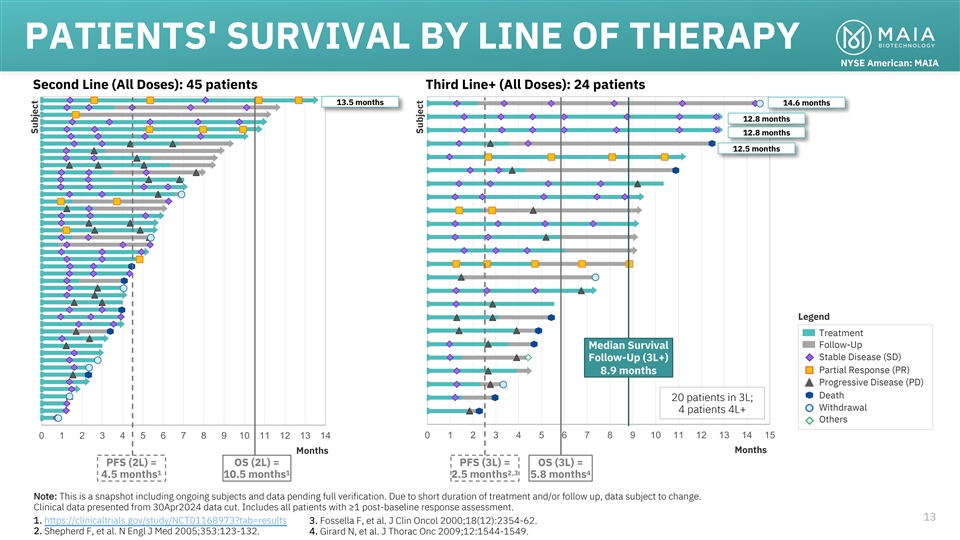
PATIENTS' SURVIVAL BY LINE OF THERAPY NYSE American: MAIA Second Line
(All Doses): 45 patients Third Line+ (All Doses): 24 patients 13.5 months 14.6 months 12.8 months 12.8 months 12.5 months Legend Treatment Follow-Up Median Survival Stable Disease (SD) Follow-Up (3L+) Partial Response (PR) 8.9 months Progressive
Disease (PD) Death 20 patients in 3L; Withdrawal 4 patients 4L+ Others 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Months Months PFS (2L) = OS (2L) = PFS (3L) = OS (3L) = 1 1 2,3 4 4.5 months 10.5 months 2.5 months 5.8
months Note: This is a snapshot including ongoing subjects and data pending full verification. Due to short duration of treatment and/or follow up, data subject to change. Clinical data presented from 30Apr2024 data cut. Includes all patients with
≥1 post-baseline response assessment. 13 1. https://clinicaltrials.gov/study/NCT01168973?tab=results 3. Fossella F, et al. J Clin Oncol 2000;18(12):2354-62. 2. Shepherd F, et al. N Engl J Med 2005;353:123-132. 4. Girard N, et al. J Thorac Onc
2009;12:1544-1549. Subject Subject
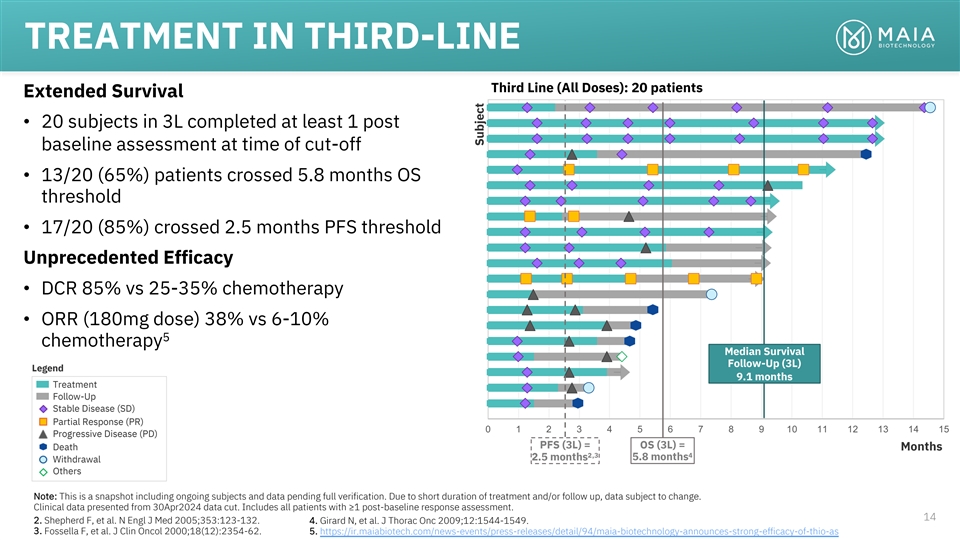
TREATMENT IN THIRD-LINE Third Line (All Doses): 20 patients Extended
Survival • 20 subjects in 3L completed at least 1 post baseline assessment at time of cut-off • 13/20 (65%) patients crossed 5.8 months OS threshold • 17/20 (85%) crossed 2.5 months PFS threshold Unprecedented Efficacy • DCR
85% vs 25-35% chemotherapy • ORR (180mg dose) 38% vs 6-10% 5 chemotherapy Median Survival Follow-Up (3L) Legend 9.1 months Treatment Follow-Up Stable Disease (SD) Partial Response (PR) 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Progressive Disease
(PD) PFS (3L) = OS (3L) = Death Months 2,3 4 5.8 months 2.5 months Withdrawal Others Note: This is a snapshot including ongoing subjects and data pending full verification. Due to short duration of treatment and/or follow up, data subject to change.
Clinical data presented from 30Apr2024 data cut. Includes all patients with ≥1 post-baseline response assessment. 14 2. Shepherd F, et al. N Engl J Med 2005;353:123-132. 4. Girard N, et al. J Thorac Onc 2009;12:1544-1549. 3. Fossella F, et al.
J Clin Oncol 2000;18(12):2354-62. 5. https://ir.maiabiotech.com/news-events/press-releases/detail/94/maia-biotechnology-announces-strong-efficacy-of-thio-as Subject
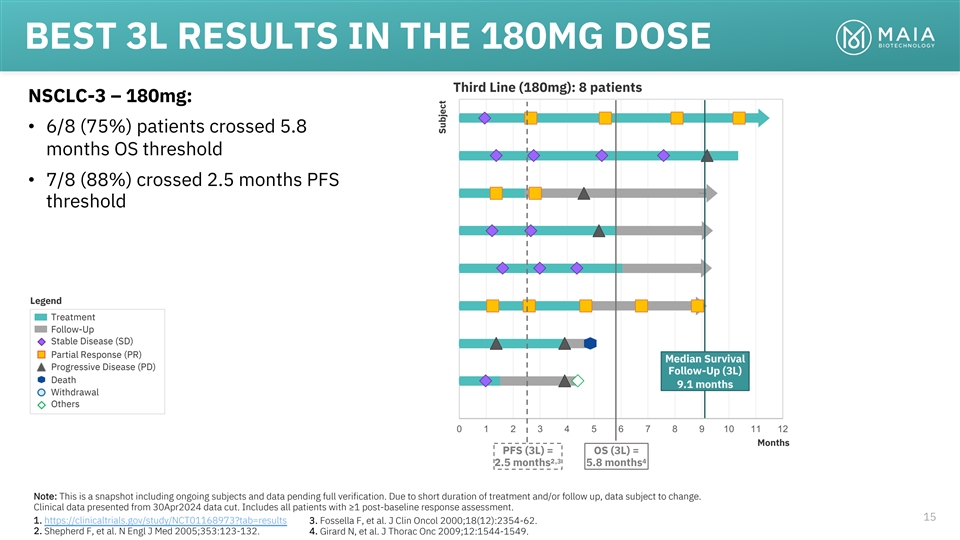
BEST 3L RESULTS IN THE 180MG DOSE Third Line (180mg): 8 patients
NSCLC-3 – 180mg: • 6/8 (75%) patients crossed 5.8 months OS threshold • 7/8 (88%) crossed 2.5 months PFS threshold Legend Treatment Follow-Up Stable Disease (SD) Partial Response (PR) Median Survival Progressive Disease (PD)
Follow-Up (3L) Death 9.1 months Withdrawal Others 0 1 2 3 4 5 6 7 8 9 10 11 12 Months PFS (3L) = OS (3L) = 2,3 4 2.5 months 5.8 months Note: This is a snapshot including ongoing subjects and data pending full verification. Due to short duration of
treatment and/or follow up, data subject to change. Clinical data presented from 30Apr2024 data cut. Includes all patients with ≥1 post-baseline response assessment. 15 1. https://clinicaltrials.gov/study/NCT01168973?tab=results 3. Fossella F,
et al. J Clin Oncol 2000;18(12):2354-62. 2. Shepherd F, et al. N Engl J Med 2005;353:123-132. 4. Girard N, et al. J Thorac Onc 2009;12:1544-1549. Subject
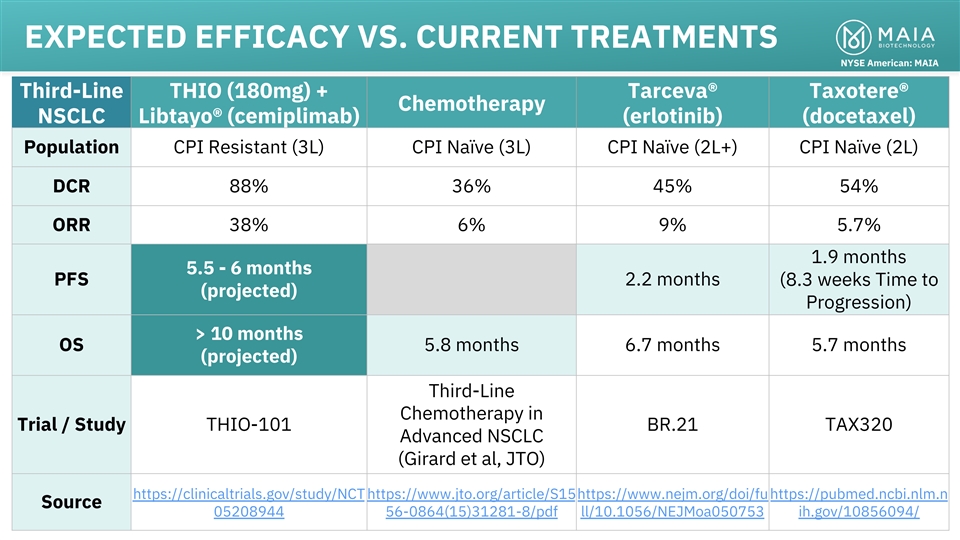
EXPECTED EFFICACY VS. CURRENT TREATMENTS NYSE American: MAIA Third-Line
THIO (180mg) + Tarceva® Taxotere® Chemotherapy NSCLC Libtayo® (cemiplimab) (erlotinib) (docetaxel) Population CPI Resistant (3L) CPI Naïve (3L) CPI Naïve (2L+) CPI Naïve (2L) DCR 88% 36% 45% 54% ORR 38% 6% 9% 5.7% 1.9
months 5.5 - 6 months PFS 2.2 months (8.3 weeks Time to (projected) Progression) > 10 months OS 5.8 months 6.7 months 5.7 months (projected) Third-Line Chemotherapy in Trial / Study THIO-101 BR.21 TAX320 Advanced NSCLC (Girard et al, JTO)
https://clinicaltrials.gov/study/NCT https://www.jto.org/article/S15https://www.nejm.org/doi/fu https://pubmed.ncbi.nlm.n Source 05208944 56-0864(15)31281-8/pdf ll/10.1056/NEJMoa050753 ih.gov/10856094/ 16

NYSE American: MAIA PLANNED UPCOMING TRIALS 17 17
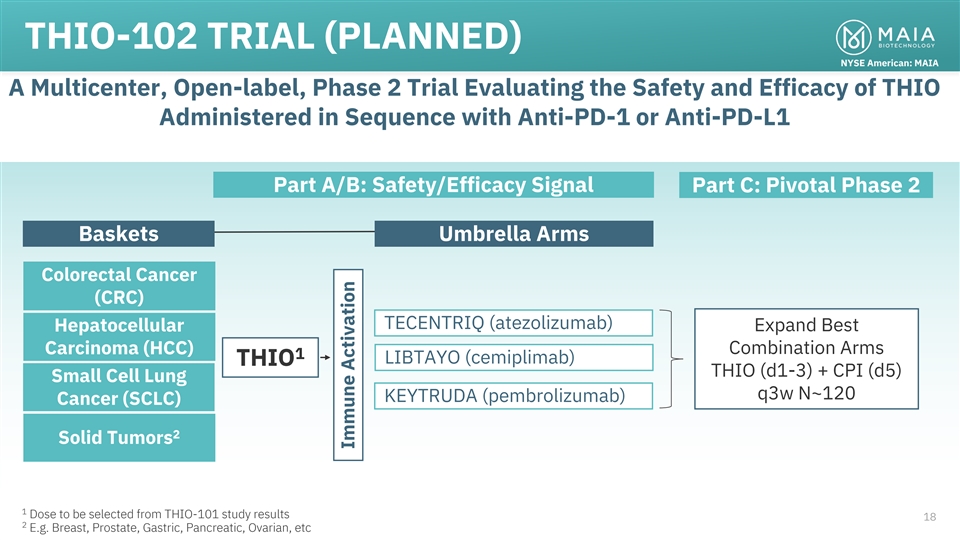
THIO-102 TRIAL (PLANNED) NYSE American: MAIA A Multicenter, Open-label,
Phase 2 Trial Evaluating the Safety and Efficacy of THIO Administered in Sequence with Anti-PD-1 or Anti-PD-L1 Part A/B: Safety/Efficacy Signal Part C: Pivotal Phase 2 Baskets Umbrella Arms Colorectal Cancer (CRC) TECENTRIQ (atezolizumab)
Hepatocellular Expand Best Carcinoma (HCC) Combination Arms 1 LIBTAYO (cemiplimab) THIO THIO (d1-3) + CPI (d5) Small Cell Lung q3w N~120 KEYTRUDA (pembrolizumab) Cancer (SCLC) 2 Solid Tumors 1 Dose to be selected from THIO-101 study results 18 2
E.g. Breast, Prostate, Gastric, Pancreatic, Ovarian, etc Immune Activation
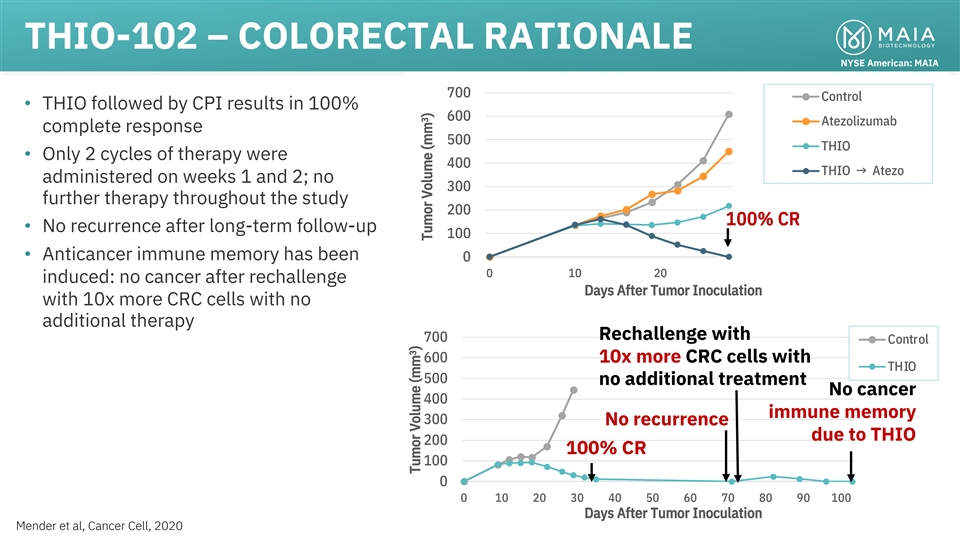
THIO-102 – COLORECTAL RATIONALE NYSE American: MAIA 700 Control
• THIO followed by CPI results in 100% 600 Atezolizumab complete response 500 THIO • Only 2 cycles of therapy were 400 THIO → Atezo administered on weeks 1 and 2; no 300 further therapy throughout the study 200 100% CR • No
recurrence after long-term follow-up 100 • Anticancer immune memory has been 0 0 10 20 induced: no cancer after rechallenge Days After Tumor Inoculation with 10x more CRC cells with no additional therapy Rechallenge with 700 Control 600 10x
more CRC cells with THIO 500 no additional treatment No cancer 400 immune memory 300 No recurrence due to THIO 200 100% CR 100 0 0 10 20 30 40 50 60 70 80 90 100 Days After Tumor Inoculation 19 Mender et al, Cancer Cell, 2020 3 Tumor Volume (mm ) 3
Tumor Volume (mm )
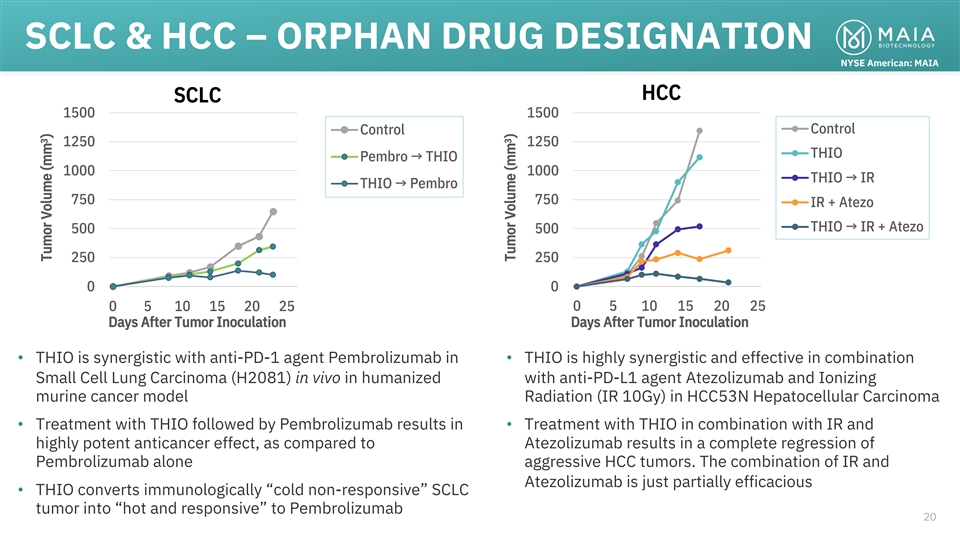
SCLC & HCC – ORPHAN DRUG DESIGNATION NYSE American: MAIA HCC
SCLC 1500 1500 Control Control 1250 1250 THIO Pembro → THIO 1000 1000 THIO → IR THIO → Pembro 750 750 IR + Atezo THIO → IR + Atezo 500 500 250 250 0 0 0 5 10 15 20 25 0 5 10 15 20 25 Days After Tumor Inoculation Days After
Tumor Inoculation • THIO is synergistic with anti-PD-1 agent Pembrolizumab in • THIO is highly synergistic and effective in combination Small Cell Lung Carcinoma (H2081) in vivo in humanized with anti-PD-L1 agent Atezolizumab and
Ionizing murine cancer model Radiation (IR 10Gy) in HCC53N Hepatocellular Carcinoma • Treatment with THIO followed by Pembrolizumab results in • Treatment with THIO in combination with IR and highly potent anticancer effect, as compared
to Atezolizumab results in a complete regression of Pembrolizumab alone aggressive HCC tumors. The combination of IR and Atezolizumab is just partially efficacious • THIO converts immunologically “cold non-responsive” SCLC tumor
into “hot and responsive” to Pembrolizumab 20 3 Tumor Volume (mm ) 3 Tumor Volume (mm )
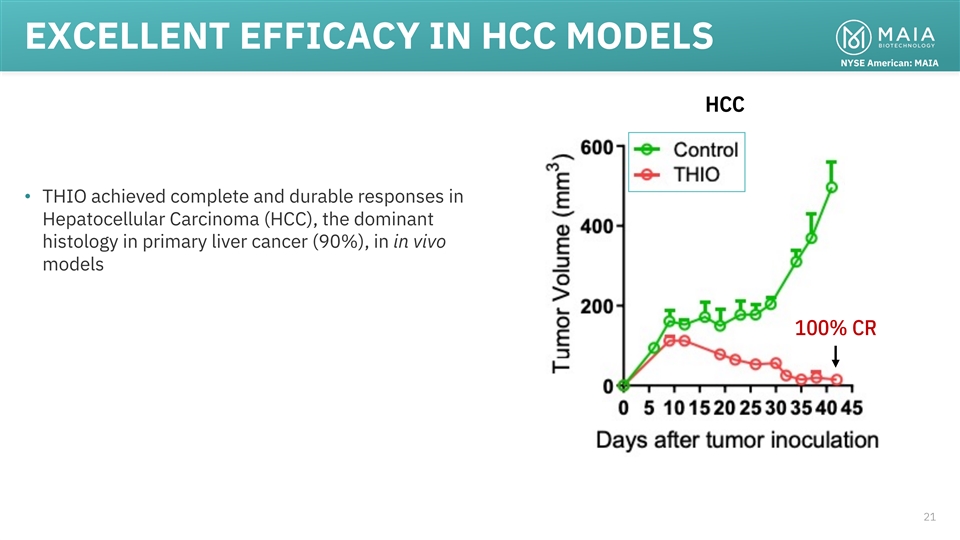
EXCELLENT EFFICACY IN HCC MODELS NYSE American: MAIA HCC • THIO
achieved complete and durable responses in Hepatocellular Carcinoma (HCC), the dominant histology in primary liver cancer (90%), in in vivo models 100% CR 21
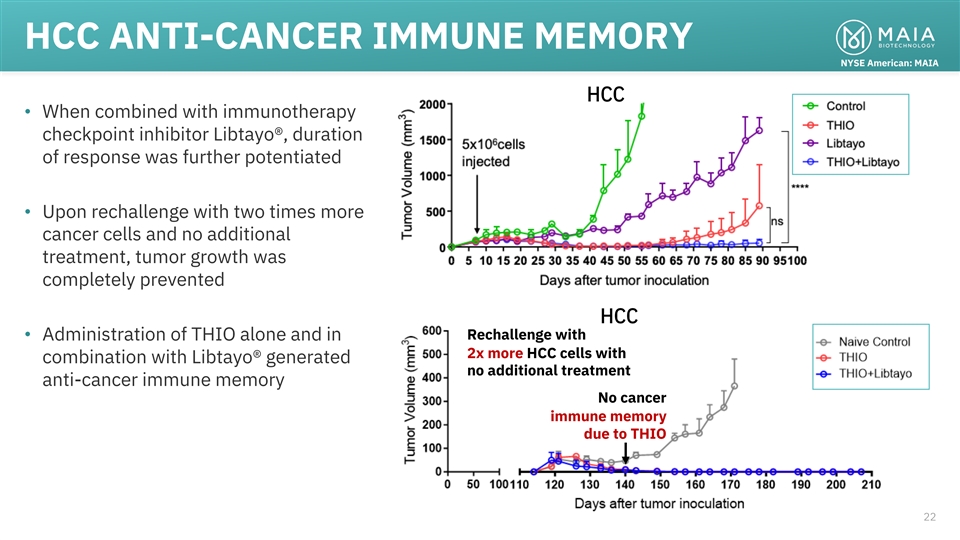
HCC ANTI-CANCER IMMUNE MEMORY NYSE American: MAIA HCC • When
combined with immunotherapy checkpoint inhibitor Libtayo®, duration of response was further potentiated • Upon rechallenge with two times more cancer cells and no additional treatment, tumor growth was completely prevented HCC Rechallenge
with • Administration of THIO alone and in 2x more HCC cells with combination with Libtayo® generated no additional treatment anti-cancer immune memory No cancer immune memory due to THIO 22
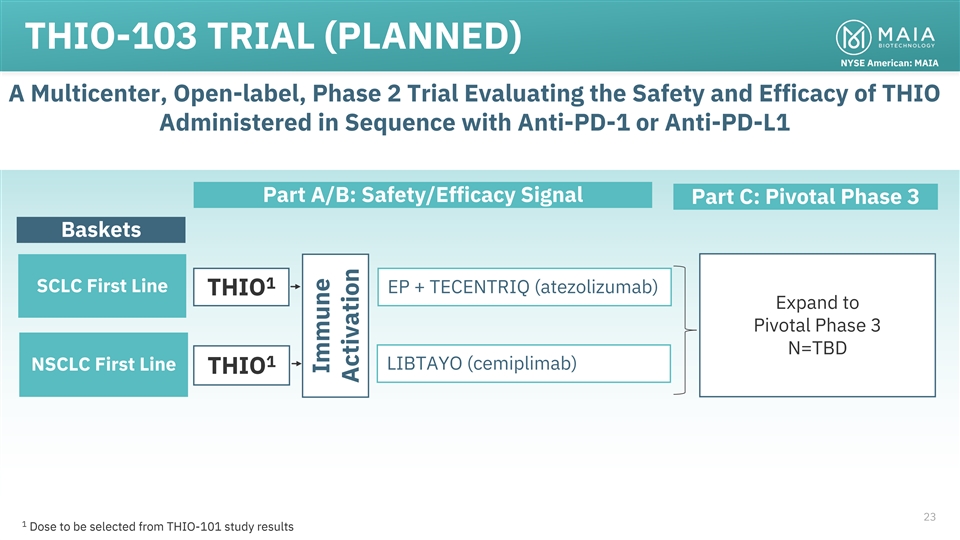
THIO-103 TRIAL (PLANNED) NYSE American: MAIA A Multicenter, Open-label,
Phase 2 Trial Evaluating the Safety and Efficacy of THIO Administered in Sequence with Anti-PD-1 or Anti-PD-L1 Part A/B: Safety/Efficacy Signal Part C: Pivotal Phase 3 Baskets 1 SCLC First Line EP + TECENTRIQ (atezolizumab) THIO Expand to Pivotal
Phase 3 N=TBD 1 LIBTAYO (cemiplimab) NSCLC First Line THIO 23 1 Dose to be selected from THIO-101 study results Immune Activation

NYSE American: MAIA INVESTMENT OPPORTUNITY 24 24

EXCLUSIVITY AND INTELLECTUAL PROPERTY NYSE American: MAIA Goal: New
Chemical Entity (NCE) Marketing Exclusivity • THIO has never been previously approved by the FDA for commercialization • Robust exclusivity • US: 7 years; EU, Japan, other markets: 10 years Robust and Growing Patent Portfolio for
THIO • 5 issued patents • 29 pending patent applications Current patents/provisional applications broadly cover the following key areas: • Telomere targeting compounds (2034+) • THIO’s immunogenic treatment strategy:
sequential combination with CPIs (2041) 25

EXPERIENCED MANAGEMENT TEAM NYSE American: MAIA Vlad Vitoc, MD, MBA
Sergei Gryaznov, PhD Jeffrey Himmelreich, MBA Founder and CEO Chief Scientific Officer Head of Finance • 24+ years in Oncology Pharma/ • 25+ years as Scientist • 20+ years of financial Biotech: Commercial, Medical • Expert
Drug Discovery and expertise • 12 compounds launched Development, Oncology • CFO for privately held and across 20+ tumor types with 120+ publications publicly traded companies • Leadership roles at • Head of the J&J in
the healthcare and Bayer (Nexavar), Oligonucleotide Center of manufacturing industries Astellas (Tarceva, Xtandi), Excellence Worldwide • Active CPA licensed in the Cephalon (Treanda), • Expert of telomeres and state of Pennsylvania and
Novartis (Zometa), telomerase in cancer, co- is a Chartered Global Incyte (Jakafi) inventor of THIO Management Accountant 26
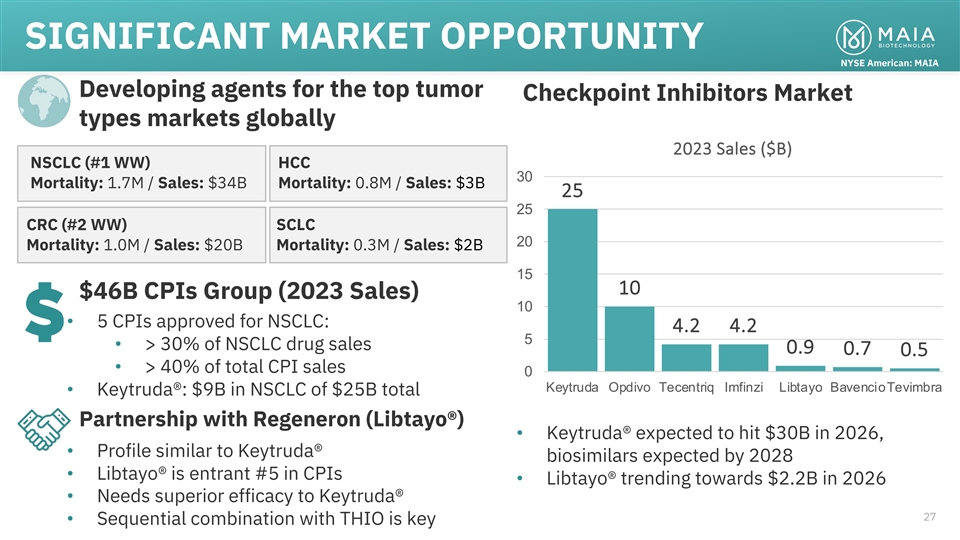
SIGNIFICANT MARKET OPPORTUNITY NYSE American: MAIA Developing agents
for the top tumor Checkpoint Inhibitors Market types markets globally 2023 Sales ($B) NSCLC (#1 WW) HCC 30 Mortality: 1.7M / Sales: $34B Mortality: 0.8M / Sales: $3B 25 25 CRC (#2 WW) SCLC 20 Mortality: 1.0M / Sales: $20B Mortality: 0.3M / Sales:
$2B 15 10 $46B CPIs Group (2023 Sales) 10 • 5 CPIs approved for NSCLC: 4.2 4.2 5 • > 30% of NSCLC drug sales 0.9 0.7 0.5 • > 40% of total CPI sales 0 Keytruda Opdivo Tecentriq Imfinzi Libtayo BavencioTevimbra •
Keytruda®: $9B in NSCLC of $25B total Partnership with Regeneron (Libtayo®) • Keytruda® expected to hit $30B in 2026, • Profile similar to Keytruda® biosimilars expected by 2028 • Libtayo® is entrant #5 in
CPIs • Libtayo® trending towards $2.2B in 2026 • Needs superior efficacy to Keytruda® 27 • Sequential combination with THIO is key
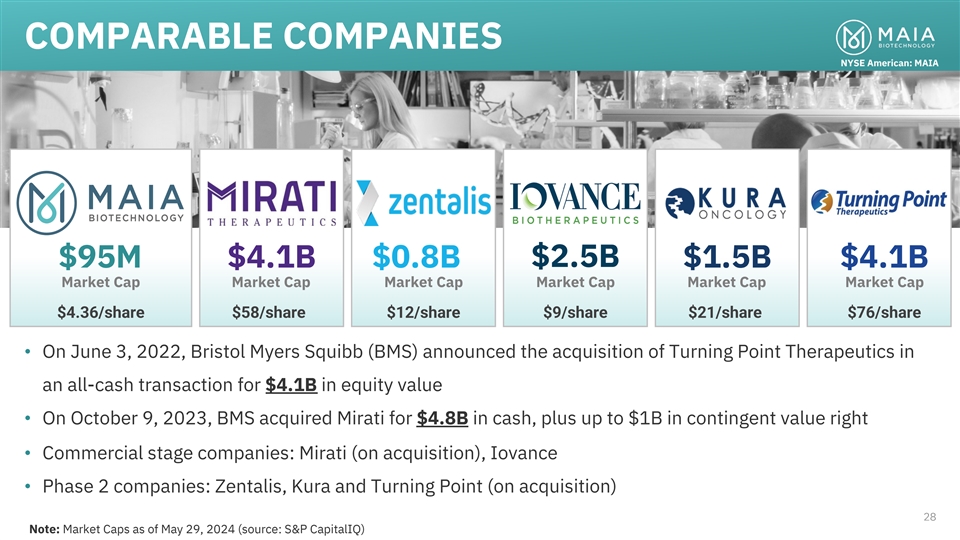
COMPARABLE COMPANIES NYSE American: MAIA $2.5B $95M $4.1B $0.8B $1.5B
$4.1B Market Cap Market Cap Market Cap Market Cap Market Cap Market Cap $4.36/share $58/share $12/share $9/share $21/share $76/share • On June 3, 2022, Bristol Myers Squibb (BMS) announced the acquisition of Turning Point Therapeutics in an
all-cash transaction for $4.1B in equity value • On October 9, 2023, BMS acquired Mirati for $4.8B in cash, plus up to $1B in contingent value right • Commercial stage companies: Mirati (on acquisition), Iovance • Phase 2
companies: Zentalis, Kura and Turning Point (on acquisition) 28 28 Note: Market Caps as of May 29, 2024 (source: S&P CapitalIQ)
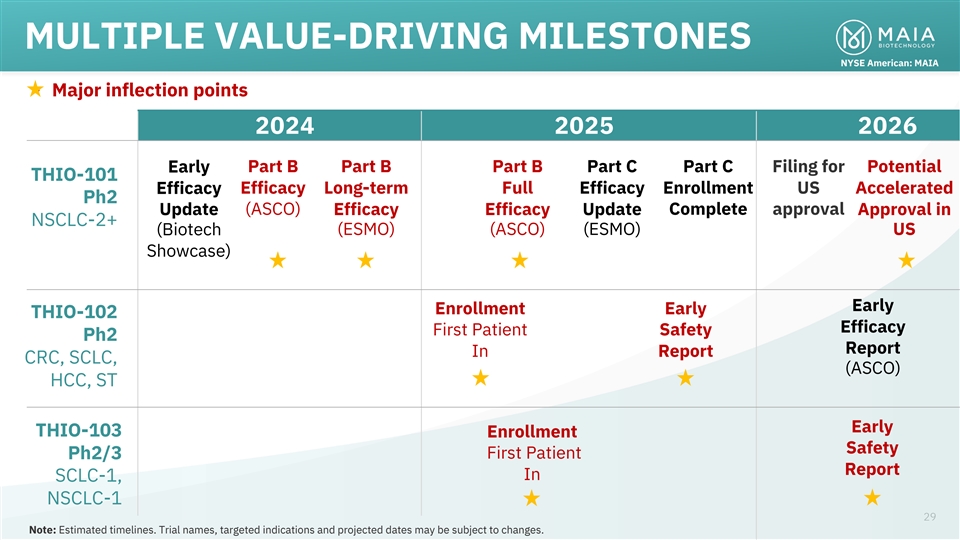
MULTIPLE VALUE-DRIVING MILESTONES NYSE American: MAIA • Major
inflection points 2024 2025 2026 Part B Part B Part B Part C Part C Filing for Potential Early THIO-101 Efficacy Long-term Full Efficacy Enrollment US Accelerated Efficacy Ph2 (ASCO) Complete approval Update Efficacy Efficacy Update Approval in
NSCLC-2+ (ESMO) (ASCO) (ESMO) US (Biotech Showcase) Early Enrollment Early THIO-102 Efficacy First Patient Safety Ph2 Report In Report CRC, SCLC, (ASCO) HCC, ST Early THIO-103 Enrollment Safety First Patient Ph2/3 Report In SCLC-1, NSCLC-1 29 Note:
Estimated timelines. Trial names, targeted indications and projected dates may be subject to changes.

NYSE American: MAIA THANK YOU Investor Relations Contact +1 (872)
270-3518 ir@maiabiotech.com MAIA Biotechnology, Inc. 444 West Lake Street, Suite 1700 Chicago, IL 60606 30 30

NYSE American: MAIA APPENDIX 31 31
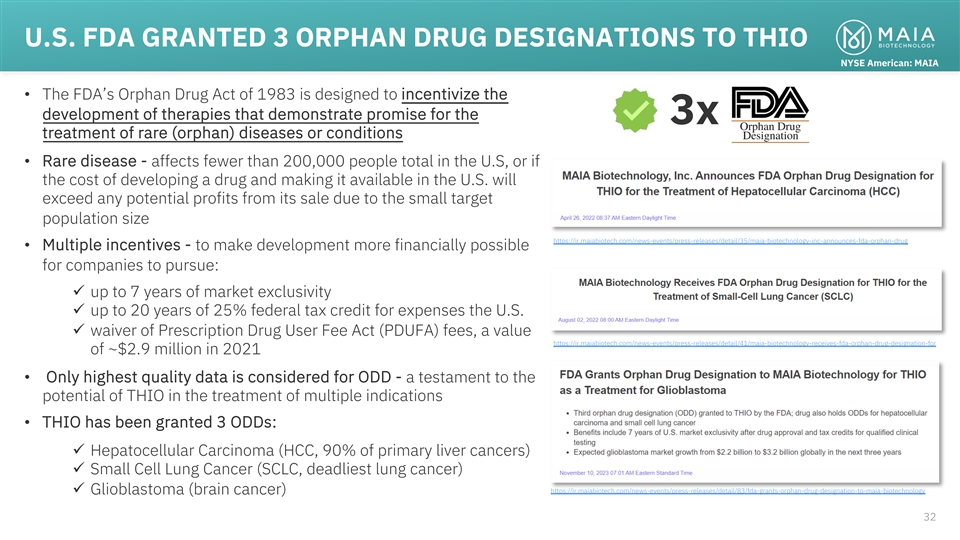
U.S. FDA GRANTED 3 ORPHAN DRUG DESIGNATIONS TO THIO NYSE American: MAIA
• The FDA’s Orphan Drug Act of 1983 is designed to incentivize the development of therapies that demonstrate promise for the 3x treatment of rare (orphan) diseases or conditions • Rare disease - affects fewer than 200,000 people
total in the U.S, or if the cost of developing a drug and making it available in the U.S. will exceed any potential profits from its sale due to the small target population size
https://ir.maiabiotech.com/news-events/press-releases/detail/35/maia-biotechnology-inc-announces-fda-orphan-drug • Multiple incentives - to make development more financially possible for companies to pursue: ü up to 7 years of market
exclusivity ü up to 20 years of 25% federal tax credit for expenses the U.S. ü waiver of Prescription Drug User Fee Act (PDUFA) fees, a value
https://ir.maiabiotech.com/news-events/press-releases/detail/41/maia-biotechnology-receives-fda-orphan-drug-designation-for of ~$2.9 million in 2021 • Only highest quality data is considered for ODD - a testament to the potential of THIO in
the treatment of multiple indications • THIO has been granted 3 ODDs: ü Hepatocellular Carcinoma (HCC, 90% of primary liver cancers) ü Small Cell Lung Cancer (SCLC, deadliest lung cancer)
https://ir.maiabiotech.com/news-events/press-releases/detail/83/fda-grants-orphan-drug-designation-to-maia-biotechnology ü Glioblastoma (brain cancer) 32

Exhibit 99.2 Vlad Vitoc, MD, MBA Chief Executive Officer 444 West Lake
Street, Suite 1700 - Chicago, IL 60606 (312) 416-8592 vvitoc@maiabiotech.com (215) 971-5488 maiabiotech.com Telomere Targeting Immunotherapies for Cancer MAIA is an immuno-oncology company focused on the development and commercialization of
first-in-class drugs intended to meaningfully improve and extend the lives of people with hard-to-treat cancers. We are exploring new science for cancer therapy utilizing a novel dual mechanism of action: telomere targeting and immunogenicity. Our
lead program is THIO, a first-in-class anticancer agent in clinical development for the treatment of Non-Small Cell Lung Cancer (NSCLC) in patients. Company Highlights Clinical Programs THIO-101 THIO-102 (planning) Ph 2 trial of THIO + Libtayo®
(cemiplimab) Ph 2 trial of THIO + checkpoint inhibitors • Go-to-market trial in second-line+ NSCLC • Go-to-market trial in late line of therapy in multiple • Objectives: select most efficacious dose and expand tumor types:
Colorectal Cancer (CRC), into pivotal trial; file for accelerated approval in Hepatocellular Carcinoma (HCC, 90% of primary 2025 type of liver cancers), Small Cell Lung Cancer • Enrollment completed earlier than expected in Feb (SCLC) and
Solid Tumors of any type (ST) 2024; trial nearing completion • Objectives: select most efficacious combination by • Topline data expected mid-2024; long-term data on tumor type and expand into pivotal trials (12+ second half of 2024
possible market entry indications) • Preliminary Overall Response Rate for best dose • File for accelerated approvals in 2026 and beyond 180mg: ü 38% ORR in third-line vs. 6-10% with SoC THIO-103 (planning) ü 75% of patients
crossed 5.8 months OS Ph 2/3 trial of THIO + checkpoint inhibitors threshold in third-line • First-line NSCLC and SCLC • Preliminary Disease Control Rate (DCR), best • Expand to Breast, Prostate, Pancreatic, Ovarian, predictor for
overall survival benefit (meta-analysis Gastric Cancer, etc. of 74 trials worldwide): • Objectives: confirmatory for accelerated approvals ü 85% DCR in third-line vs. 25-35% with Soc from THIO-101 and THIO-102 THIO is a Unique Direct
Telomere Targeting Agent • Potential to be used in combination with other anticancer and immune therapies • Novel dual mechanism of action: telomere targeting + immunogenic • 3 FDA Orphan Drug Designations: HCC, SCLC, and
Glioblastoma • Excellent efficacy: achieved complete and durable responses in HCC in vivo models (peer-reviewed published study) • Featured in multiple renowned scientific publications including Cancer Cell and Nature Partnership with
Regeneron Cap Table • Clinical supply agreement: Regeneron provides NYSE American: MAIA Libtayo® for THIO-101 1 2 • Equivalent to $32M non-dilutive participation Share Price $4.36 Float 14M (largest financing move to date) 1 Market
Cap $95M Insider • Potential to expand existing relationship and target 2 Holdings 27% FD Shares new companies 2 Cash $8M 2 35M Outstanding 1 2. As of Apr 25, 2024 1. As of May 30, 2024

MAIA Biotechnology’s goal is to bring revolutionary cancer
treatments to the market, with the only direct telomere targeting agent in clinical development. MAIA is developing agents for the top tumor types markets globally. Significant Market Opportunity • Cancer is the most dominant of the
age-related disease categories and has life altering impacts in the lives of patients and their close ones • The number of people aged 80 years or older is expected to triple between 2020 and 2050 to reach 426 million • Approximately 40%
of people alive today are projected to be diagnosed with a cancer type in their lifetime, and 20% will die of it • NSCLC is the leading tumor type: Mortality 1.7M / Sales $32B (2022) • CRC is second: Mortality 1M / Sales $20B (2022)
Strong and Growing IP Portfolio • Potential for receiving NCE marketing exclusivity • 5 patents issued, 29 patent applications pending Next Generation Potential Telomere Targeting Therapeutics in R&D • 84 new molecules
engineered; same mechanism of action as THIO • Following THIO to commercial stage within 4-5 years Robust Pipeline PHASE 1 PHASE 2 PHASE 3 COLLABORATION & RIGHTS THIO Telomere targeting agent Worldwide rights Patient Enrollment (THIO
→ Libtayo®) NSCLC-2+ THIO-101 owned by MAIA Complete Worldwide rights Ph 2 THIO-102 CRC, HCC, SCLC, ST (THIO → CPI) owned by MAIA Planning Worldwide rights Ph 2/3 THIO-103 NSCLC-1, SCLC-1 (THIO → CPI) owned by MAIA Planning nd
2 Generation Telomere targeting agents IND MAIA-2021-020 Multiple Ind. Enabling Developed in-house IND MAIA-2022-012 Multiple Ind. Enabling fully-owned by MAIA MAIA-2021-029 Multiple Indications Vlad Vitoc, MD, MBA Founder, Chairman, and Chief
Executive Officer • 24+ years in Pharma/Biotech: Commercial, Medical, • 12 compounds launched across 20+ tumor types • Leadership roles at Bayer (Nexavar), Astellas (Tarceva, Xtandi), Cephalon (Treanda), Novartis (Zometa), and
Incyte (Jakafi) Scan QR Code to DISCLAIMER: This information is published solely for informational purposes and is not to be construed as a solicitation or an offer to buy any security access our or related financial instrument or to participate in
any trading strategy. The summary may include “forward-looking statements” with the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Exchange Act of 1934 and are intended to be covered by the safe harbor
provisions for investor 2 forward looking statements. This information is supplied from sources we believe to be reliable but we cannot guarantee accuracy. This document is presentation furnished to you solely for your information.
Exhibit 99.3
MAIA Biotechnology Reveals New Clinical Data Showing THIO’s Strong Efficacy in Non-Small Cell Lung Cancer
THIO’s favorable disease control and overall response rates exceed reported
standard-of-care data in third line treatment
CHICAGO, IL –
June 04, 2024 - MAIA Biotechnology, Inc., (NYSE American: MAIA) (“MAIA”, the “Company”), a clinical-stage biopharmaceutical company developing targeted immunotherapies for cancer, today announced new efficacy data from its Phase
2 THIO-101 clinical trial evaluating THIO sequenced with the immune checkpoint inhibitor (CPI) cemiplimab (Libtayo®) in patients with advanced non-small cell lung cancer (NSCLC) who failed 2 or more standard-of-care therapy regimens. Updated results show a favorable overall
response rate (ORR) of 38% and a disease control rate (DCR) of 85% from THIO + CPI in third-line treatment. The new data was presented in a poster session at the American Society of Clinical Oncology (ASCO) 2024 Annual Meeting on June 3, 2024.
The primary objectives of THIO-101 Phase 2 trial are to examine the safety and tolerability of THIO as an
anticancer drug and as an immune system primer, and to examine the clinical efficacy of THIO in the form of ORR. At the time of the most recent data cut-off (April 30, 2024), all evaluable patients had
completed ≥1 post-baseline assessment.
Results from third-line treatment:
| |
• |
|
Disease control rate (DCR) was 85% for THIO vs. standard of care DCR of 25–35% for chemotherapy1 |
| |
• |
|
65% of patients crossed the 5.8-month overall survival (OS) threshold2 |
| |
• |
|
85% of patients crossed the 2.5-month progression-free survival (PFS)
threshold3-4 |
| |
• |
|
Median survival follow-up time is currently 9.1 months (n=20)
|
Results from third-line treatment with THIO 180mg (optimal dose selection)
| |
• |
|
Median PFS of 5.5 months (24.1 weeks) |
| |
• |
|
78% OS rate at 6 months |
| |
• |
|
38% ORR vs. standard of care 6–10% for chemotherapy5
|
| |
• |
|
75% of patients crossed the 5.8-month OS threshold5 |
| |
• |
|
88% of patients crossed the 2.5-month PFS threshold6-7 |
| |
• |
|
Median survival follow-up time is currently 9.1 months (n=8)
|
| 1 |
Matsumoto H, et al. Transl Lung Cancer Res 2021;10:2278–89. |
| 2 |
Girard N, et al. J Thorac Onc 2009;12:1544-1549. |
| 3 |
Shepherd F, et al. N Engl J Med 2005;353:123-132.
|
| 4 |
Fossella F, et al. J Clin Oncol 2000;18(12):2354-62.
|
“All exceptional measures of efficacy in our trial to date have exceeded our own expectations and
outperformed standard of care treatments,” said Vlad Vitoc, M.D., MAIA’s Chairman and Chief Executive Officer. “The data presented at ASCO advances THIO’s excellent clinical profile as a strong, safe, and highly effective
alternative for patients who progressed following chemotherapy and other available treatments. We eagerly anticipate full efficacy data from THIO-101 in the second half of this year.”
To date, treatment with THIO + cemiplimab has been generally well tolerated in a heavily pre-treated patient
population. Full enrollment in THIO-101 was completed on February 19, 2024, earlier than expected as per trial design. The Company expects that THIO-101 will be the
first completed clinical study of a telomere targeting agent in the field of cancer drug discovery and treatment.
The poster and updated Company
presentations can be accessed on the company’s website.
About THIO
THIO (6-thio-dG or 6-thio-2’-deoxyguanosine) is a first-in-class investigational telomere-targeting agent currently in clinical development to
evaluate its activity in Non-Small Cell Lung Cancer (NSCLC). Telomeres, along with the enzyme telomerase, play a fundamental role in the survival of cancer cells and their resistance to current therapies. The
modified nucleotide 6-thio-2’-deoxyguanosine (THIO) induces telomerase-dependent telomeric DNA modification, DNA damage responses, and selective cancer cell death.
THIO-damaged telomeric fragments accumulate in cytosolic micronuclei and activates both innate (cGAS/STING) and adaptive (T-cell) immune responses. The sequential treatment with THIO followed by PD-(L)1 inhibitors resulted in profound and persistent tumor regression in advanced, in vivo cancer models by induction of cancer type–specific immune memory. THIO is presently developed as a second or later
line of treatment for NSCLC for patients that have progressed beyond the standard-of-care regimen of existing checkpoint inhibitors.
About THIO-101, a Phase 2 Clinical Trial
THIO-101 is a multicenter, open-label, dose finding Phase 2 clinical trial. It is the first trial designed to evaluate
THIO’s anti-tumor activity when followed by PD-(L)1 inhibition. The trial is testing the hypothesis that low doses of THIO administered prior to cemiplimab (Libtayo®) will enhance and prolong immune response in patients with advanced NSCLC who previously did not respond or developed resistance and progressed after first-line treatment regimen containing
another checkpoint inhibitor. The trial design has two primary objectives: (1) to evaluate the safety and tolerability of THIO administered as an anticancer compound and a priming immune activator (2) to assess the clinical efficacy of
THIO using Overall Response Rate (ORR) as the primary clinical endpoint. Treatment with cemiplimab (Libtayo®) followed by THIO has been generally well-tolerated to date in a heavily pre-treated population. For more information on this Phase II trial, please visit ClinicalTrials.gov using the identifier NCT05208944.
About MAIA Biotechnology, Inc.
MAIA is a targeted therapy, immuno-oncology company focused on the development and commercialization of potential first-in-class drugs with novel mechanisms of action that are intended to meaningfully improve and extend the lives of people with cancer. Our lead program is THIO, a potential
first-in-class cancer telomere targeting agent in clinical development for the treatment of NSCLC patients with telomerase-positive cancer cells. For more information,
please visit www.maiabiotech.com.
Forward Looking Statements
MAIA cautions that all statements, other than statements of historical facts contained in this press release, are forward-looking statements. Forward-looking
statements are subject to known and unknown risks, uncertainties, and other factors that may cause our or our industry’s actual results, levels or activity, performance or achievements to be materially different from those anticipated by such
statements. The use of words such as “may,” “might,” “will,” “should,” “could,” “expect,” “plan,” “anticipate,” “believe,” “estimate,”
“project,” “intend,” “future,” “potential,” or “continue,” and other similar expressions are intended to identify forward looking statements. However, the absence of these words does not mean that
statements are not forward-looking. For example, all statements we make regarding (i) the initiation, timing, cost, progress and results of our preclinical and clinical studies and our research and development programs, (ii) our ability to
advance product candidates into, and successfully complete, clinical studies, (iii) the timing or likelihood of regulatory filings and approvals, (iv) our ability to develop, manufacture and commercialize our product candidates and to
improve the manufacturing process, (v) the rate and degree of market acceptance of our product candidates, (vi) the size and growth potential of the markets for our product candidates and our ability to serve those markets, and
(vii) our expectations regarding our ability to obtain and maintain intellectual property protection for our product candidates, are forward looking. All forward-looking statements are based on current estimates, assumptions and expectations by
our management that, although we believe to be reasonable, are inherently uncertain. Any forward-looking statement expressing an expectation or belief as to future events is expressed in good faith and believed to be reasonable at the time such
forward-looking statement is made. However, these statements are not guarantees of future events and are subject to risks and uncertainties and other factors beyond our control that may cause actual results to differ materially from those expressed
in any forward-looking statement. Any forward-looking statement speaks only as of the date on which it was made. We undertake no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future
events or otherwise, except as required by law. In this release, unless the context requires otherwise, “MAIA,” “Company,” “we,” “our,” and “us” refers to MAIA Biotechnology, Inc. and its
subsidiaries.
Investor Relations Contact
+1
(872) 270-3518
ir@maiabiotech.com
v3.24.1.1.u2
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Maia Biotechnology (AMEX:MAIA)
Historical Stock Chart
Von Nov 2024 bis Dez 2024

Maia Biotechnology (AMEX:MAIA)
Historical Stock Chart
Von Dez 2023 bis Dez 2024
