Achieve Life Sciences Announces Addition to the U.S. Russell 3000® and Russell Microcap® Indexes
01 Juli 2024 - 2:00PM

Achieve Life Sciences, Inc. (Nasdaq: ACHV), a late-stage
pharmaceutical company committed to the global development and
commercialization of cytisinicline for smoking cessation and
nicotine dependence, announced that the company has been added to
the U.S. Russell 3000® and Russell Microcap® Indexes, which
will be effective today, Monday, July 1, 2024, at the opening of
the U.S. equity markets.
“We are proud to be included in the U.S. Russell 3000® and
Russell Microcap® Indexes and see this as an opportunity to enhance
Achieve’s visibility among investors,” said John Bencich, Achieve’s
Chief Executive Officer. “This recognition reflects Achieve’s solid
business fundamentals and market potential for cytisinicline as a
treatment for nicotine dependence. We are dedicated to advancing
our strategic priorities to deliver value for our stakeholders and
remain committed to addressing the worldwide crisis of smoking and
nicotine vaping addiction. We are currently anticipating a new drug
application submission for the treatment of smoking cessation in
the United States in the first half of 2025.”
The Company also recently announced that it
entered into a non-binding term sheet to refinance and extend the
maturity date of its outstanding term loans with Silicon Valley
Bank and its affiliates (“SVB”). The term sheet provides for a
tranched facility amount of up to $20 million, maturing June 1,
2028, with interest-only payments through at least December 31,
2025. The parties anticipate closing on the refinanced loan on or
before the current loan maturity date of August 1, 2024; however,
the term sheet is non-binding, and there is no assurance that the
Company will be able to enter into a definitive agreement with SVB
on the terms provided in the term sheet, or any at all.
About FTSE RussellRussell U.S. indexes are
reconstituted annually, capturing the 4,000 largest U.S. stocks as
of April 30, 2024, ranking them by total market capitalization.
Membership in the U.S. all-cap Russell 3000® Index, which remains
in place for one year, means automatic inclusion in the large-cap
Russell 1000® Index or small-cap Russell 2000® Index, as well as
the appropriate growth and value style indexes. FTSE Russell
determines membership for its Russell indexes primarily by
objective, market-capitalization rankings, and style attributes,
and are widely used by investment managers and institutional
investors for index funds and as benchmarks for active investment
strategies.
For more information on the Russell 3000®,
Russell Microcap Indexes, and the Russell indexes reconstitution,
go to the “Russell Reconstitution” section on the FTSE Russell
website.
About Achieve and Cytisinicline Achieve’s focus
is to address the global smoking health and nicotine addiction
epidemic through the development and commercialization of
cytisinicline. There are an estimated 28 million adults in the
United States alone who smoke combustible cigarettes.1 Tobacco use
is currently the leading cause of preventable death that is
responsible for more than eight million deaths worldwide and nearly
half a million deaths in the United States annually.2,3 More than
87% of lung cancer deaths, 61% of all pulmonary disease deaths, and
32% of all deaths from coronary heart disease are attributable to
smoking and exposure to secondhand smoke.3
In addition, there are over 11 million adults in the United
States who use e-cigarettes, also known as vaping.1 In 2023,
approximately 2.1 million middle and high school students in the
United States reported using e-cigarettes.4 Currently, there are no
FDA-approved treatments indicated specifically as an aid to
nicotine e-cigarette cessation.
Cytisinicline is a plant-based alkaloid with a high binding
affinity to the nicotinic acetylcholine receptor. It is believed to
aid in treating nicotine addiction for smoking and e-cigarette
cessation by interacting with nicotine receptors in the brain,
reducing the severity of withdrawal symptoms, and reducing the
reward and satisfaction associated with nicotine products.
Cytisinicline is an investigational product candidate being
developed for the treatment of nicotine addiction and has not been
approved by the Food and Drug Administration for any indication in
the United States. For more information on cytisinicline and
Achieve visit www.achievelifesciences.com.
Forward Looking StatementsThis press release
contains forward-looking statements within the meaning of the “safe
harbor” provisions of the Private Securities Litigation Reform Act
of 1995, including, but not limited to, statements regarding the
effects of being added to the U.S. Russell 3000® and Russell®
Microcap Indexes, the timing and nature of cytisinicline clinical
development and regulatory submissions, the potential market size
for cytisinicline, and the potential benefits of cytisinicline. All
statements other than statements of historical fact are statements
that could be deemed forward-looking statements. Achieve may not
actually achieve its plans or product development goals in a timely
manner, if at all, or otherwise carry out its intentions or meet
its expectations or projections disclosed in these forward-looking
statements. These statements are based on management’s current
expectations and beliefs and are subject to a number of risks,
uncertainties and assumptions that could cause actual results to
differ materially from those described in the forward-looking
statements, including, among others, the risk that cytisinicline
may not demonstrate the hypothesized or expected benefits; the risk
that Achieve may not be able to obtain additional financing to fund
the development of cytisinicline; the risk that cytisinicline will
not receive regulatory approval or be successfully commercialized;
the risk that new developments in the smoking cessation landscape
require changes in business strategy or clinical development plans;
the risk that Achieve’s intellectual property may not be adequately
protected; general business and economic conditions; risks related
to the impact on our business of macroeconomic and geopolitical
conditions, including inflation, rising interest rates, increased
volatility in the debt and equity markets, actual or perceived
instability in the global banking system, global health crises and
pandemics and geopolitical conflict and the other factors described
in the risk factors set forth in Achieve’s filings with the
Securities and Exchange Commission from time to time, including
Achieve’s Annual Reports on Form 10-K and Quarterly Reports on Form
10-Q. Achieve undertakes no obligation to update the
forward-looking statements contained herein or to reflect events or
circumstances occurring after the date hereof, other than as may be
required by applicable.
Investor Relations ContactNicole
Jonesachv@cg.capital(404) 736-3838
Media ContactGlenn
SilverGlenn.Silver@Finnpartners.com(646) 871-8485
References1Cornelius ME, Loretan CG, Jamal A,
et al. Tobacco Product Use Among Adults – United States, 2021. MMWR
Morb Mortal Wkly Rep 2023;72:475–483.2World Health Organization.
WHO Report on the Global Tobacco Epidemic, 2019. Geneva: World
Health Organization, 2017.3U.S. Department of Health and Human
Services. The Health Consequences of Smoking – 50 Years of
Progress. A Report of the Surgeon General, 2014.4Birdsey J,
Cornelius M, Jamal A, et al. Tobacco Product Use Among U.S. Middle
and High School Students — National Youth Tobacco Survey, 2023.
MMWR Morb Mortal Wkly Rep 2023;72:1173–1182.
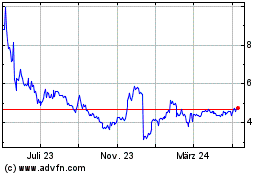
Achieve Life Sciences (NASDAQ:ACHV)
Historical Stock Chart
Von Jun 2024 bis Jul 2024
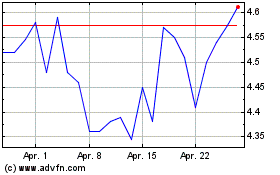
Achieve Life Sciences (NASDAQ:ACHV)
Historical Stock Chart
Von Jul 2023 bis Jul 2024