Avacta Group strengthens executive team with appointment of Michelle Morrow, PhD as Chief Scientific Officer
15 Oktober 2024 - 8:00AM
UK Regulatory
Avacta Group strengthens executive team with appointment
of Michelle Morrow, PhD as Chief Scientific Officer
Avacta Group plc
("Avacta" or "the Group" or "the Company")
Avacta Group strengthens executive team
with appointment of Michelle Morrow, PhD as Chief
Scientific Officer
London, Oct. 15, 2024 – Avacta Group plc
(AIM: AVCT), a life sciences company developing
innovative, targeted oncology drugs, today announced the
appointment of seasoned scientific leader Michelle Morrow, PhD as
Chief Scientific Officer with effect from November 4, 2024. Dr.
Morrow brings nearly 20 years’ experience in oncology therapeutics
and antibody research at both biotech and pharmaceutical companies.
She will fill a key role in the management team at Avacta as the
Company evolves into a therapeutics-focused biotech. She most
recently served at invoX Pharma as Senior Vice President (SVP),
Head of invoX Therapeutics Innovation, leading a discovery and
preclinical research team that was established following the
Company’s acquisition of F-star Therapeutics, where she held the
position of SVP, Head of Research.
Christina
Coughlin, MD, PhD, Chief Executive Officer of Avacta,
commented: “We are thrilled to welcome Michelle to the
team, she brings decades of experience in translating early science
into innovative and effective medicines for patients. Michelle’s
scientific leadership is exemplified by an outstanding track record
in advancing novel oncology candidates from target validation
through preclinical research to Phase 2 clinical proof-of-concept.
Michelle will be a significant asset to the Avacta team in driving
our programs forward and we look forward to benefiting from her
wealth of expertise.”
Michelle
Morrow, PhD, Chief Scientific Officer designate of Avacta,
commented: “I am delighted to join Avacta at such an
exciting time in its development. The progress made in the AVA6000
clinical trial, and the potential of the pre|CISION™ platform are
very exciting. I look forward to leveraging my experience in
oncology drug development to help further drive the Company’s
pipeline and explore the full potential of our therapies to benefit
patients’ lives.”
During her time at F-star, Dr. Morrow made
significant contributions to progressing bispecific candidates from
discovery and into clinical trials and led F-star’s biology and
translational research functions. Prior to that, she established an
immuno-oncology preclinical modelling group supporting projects
across the Medimmune and AstraZeneca portfolio and held Discovery
Project Leader roles for Imfinzi®, an approved
anti-PD-L1 antibody, and volrustomig, an anti- PD-1/CTLA-4
bispecific antibody.
Dr. Morrow earned her
PhD in Immunology from the University of Cambridge, UK and
conducted post-doctoral research into childhood leukaemia at the
Institute of Child Health, London.
-Ends-
For further information from
Avacta Group plc, please contact:
Avacta Group
plc
Michael Vinegrad, Group Communications Director
|
https://avacta.com/ |
Peel Hunt (Nomad and
Broker)
James Steel / Chris Golden / Patrick Birkholm
|
www.peelhunt.com
|
ICR
Consilium
Mary-Jane Elliott / Jessica Hodgson / Sukaina Virji |
avacta@consilium-comms.com
|
About Avacta Group plc
- https://avacta.com/
Avacta Group is a UK-based life
sciences company focused on improving healthcare outcomes through
targeted cancer treatments and diagnostics.
Avacta Therapeutics: a clinical stage oncology
biotech division that is harnessing the proprietary pre|CISION
platform technology to develop novel, highly targeted cancer
drugs.
The pre|CISION™ platform is a highly specific
substrate for fibroblast activation protein (FAP) which is
upregulated in most solid tumors compared with healthy tissues. The
pre|CISION™ platform harnesses this tumor specific protease to
cleave pre|CISION™ peptide drug conjugates and pre|CISION™
antibody/Affimer® drug conjugates in the tumor
microenvironment, thus releasing active payload in the tumor and
reducing systemic exposure and toxicity, allowing dosing to be
optimized to deliver the best outcomes for patients.
The lead pre|CISION™ program AVA6000, a peptide
drug conjugate form of doxorubicin, is in Phase 1 studies. It has
shown an improvement in safety and tolerability in clinical trials
to date compared with standard doxorubicin and preliminary signs of
clinical activity in multiple patients.
Avacta Diagnostics focuses on supporting
healthcare professionals and broadening access to diagnostics.
To register for news alerts by email go
to https://avacta.com/investors/investor-news-email-alerts/


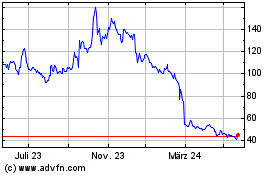
Avacta (LSE:AVCT)
Historical Stock Chart
Von Okt 2024 bis Nov 2024
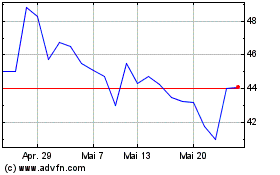
Avacta (LSE:AVCT)
Historical Stock Chart
Von Nov 2023 bis Nov 2024