Kazia Therapeutics Shares Rise Premarket After Key FDA Designation
06 Juli 2022 - 1:52PM
Dow Jones News
By Colin Kellaher
U.S.-listed shares of Kazia Therapeutics Ltd. jumped more than
15% in premarket trading Wednesday after the Australian
biotechnology company said the U.S. Food and Drug Administration
awarded rare-pediatric-disease designation to its lead program in a
rare and highly-aggressive childhood brain cancer.
Kazia said the designation covers paxalisib for the treatment of
atypical rhabdoid/teratoid tumors, fast-growing tumors that begin
in the brain and spinal cord and usually occur in children ages 3
and under.
The FDA's rare-pediatric-disease designation covers diseases
with serious or life-threatening manifestations that mainly affect
fewer than 200,000 people in the U.S. under the age of 18.
The agency awards priority-review vouchers to drug makers upon
approval of drugs that are granted the designation, and those
vouchers can be used to obtain priority review for another drug or
sold to other companies.
Kazia's U.S. shares, which closed Tuesday at $4.17, were
recently up 16% to $4.84 in premarket trading.
Write to Colin Kellaher at colin.kellaher@wsj.com
(END) Dow Jones Newswires
July 06, 2022 07:37 ET (11:37 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
Kazia Therapeutics (ASX:KZA)
Historical Stock Chart
Von Nov 2024 bis Dez 2024
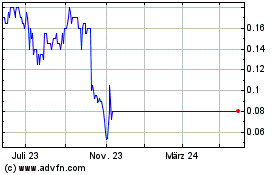
Kazia Therapeutics (ASX:KZA)
Historical Stock Chart
Von Dez 2023 bis Dez 2024
Echtzeit-Nachrichten über Kazia Therapeutics Limited (Australische Börse): 0 Nachrichtenartikel
Weitere Kazia Therapeutics Limited News-Artikel