Teva Shares Drop 11% After Alvotech Gets FDA Complete Response Letter
14 April 2023 - 8:28PM
Dow Jones News
By Chris Wack
Teva Pharmaceutical Industries Ltd. shares were down 11% to
$8.24 on Friday after the company said that the U.S. Food and Drug
Administration issued a letter that rejected the Biologics License
Application for AVT02, a high-concentration biosimilar candidate
for Humira adalimumab, from its partner Alvotech.
The Israel-based, drug company said the FDA issued a complete
response letter, which stated that the application couldn't be
approved at this time based on deficiencies associated with
Alvotech's manufacturing facility that must be satisfactorily
resolved.
Teva said that additional review of the details following the
recent FDA's re-inspection and letter are being assessed to
determine next steps. The company also said it remains optimistic
about additional compounds in the pipeline and further progress
with AVT02.
Alvotech shares were down as well, declining 19% to $11.06 in
midday trading.
Write to Chris Wack at chris.wack@wsj.com
(END) Dow Jones Newswires
April 14, 2023 14:13 ET (18:13 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
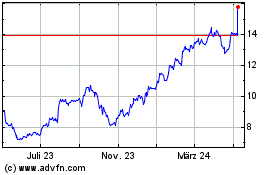
Teva Pharmaceutical Indu... (NYSE:TEVA)
Historical Stock Chart
Von Mär 2024 bis Apr 2024
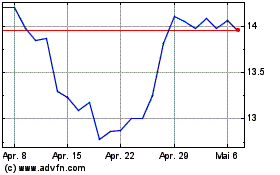
Teva Pharmaceutical Indu... (NYSE:TEVA)
Historical Stock Chart
Von Apr 2023 bis Apr 2024