Form 8-K - Current report
13 Januar 2025 - 2:57PM
Edgar (US Regulatory)
false
0001082554
0001082554
2025-01-13
2025-01-13
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15 (d) of
the
Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):
January 13, 2025
United Therapeutics Corporation
(Exact Name of Registrant as Specified in
its Charter)
| Delaware |
|
000-26301 |
|
52-1984749 |
| (State or Other |
|
(Commission |
|
(I.R.S. Employer |
| Jurisdiction of |
|
File Number) |
|
Identification Number) |
| Incorporation) |
|
|
|
|
| 1000 Spring Street |
|
|
| Silver Spring, MD |
|
20910 |
| (Address of Principal Executive Offices) |
|
(Zip Code) |
Registrant’s telephone number, including
area code: (301) 608-9292
Check the appropriate box below if the Form 8-K filing
is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ¨ | Written communications pursuant to Rule 425 under the Securities
Act (17 CFR 230.425) |
| ¨ | Soliciting material pursuant to Rule 14a-12 under the Exchange
Act (17 CFR 240.14a-12) |
| ¨ | Pre-commencement communications pursuant to Rule 14d-2(b) under
the Exchange Act (17 CFR 240.14d-2(b)) |
| ¨ | Pre-commencement communications pursuant to Rule 13e-4(c) under
the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant
to Section 12(b) of the Act:
| Title of each class |
|
Trading symbol(s) |
|
Name of each exchange on which
registered |
| Common Stock, par value $0.01 per share |
|
UTHR |
|
Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging
growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of
the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging
growth company ¨
If an emerging
growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with
any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
| Item 7.01. |
Regulation FD Disclosure. |
On January 13, 2025, United Therapeutics Corporation intends to
provide an overview and update on the company’s business during a presentation and Q&A session at the 43rd annual J.P. Morgan
Healthcare Conference in San Francisco. The presentation will include the slides furnished as Exhibit 99.1 hereto and incorporated
herein by reference. These slides will also be posted to the United Therapeutics website at https://ir.unither.com/events-and-presentations.
As
previously announced, the presentation will take place on January 13, 2025, from 2:15 p.m. to 2:55 p.m., Pacific Standard Time,
and can be accessed via a live webcast on the United Therapeutics website at https://ir.unither.com/events-and-presentations.
An archived, recorded version of the session will be available approximately 24 hours after the session ends and can be accessed at the
same location for 30 days.
The information in this Item 7.01 and the related Item 9.01, including
Exhibit 99.1 attached hereto, is being furnished and shall not be deemed “filed” for purposes of Section 18 of the
Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section,
nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act as amended,
regardless of any general incorporation language in such filing.
SIGNATURE
Pursuant to the requirements of the Securities
Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| |
UNITED THERAPEUTICS CORPORATION |
| |
|
|
| Dated: January 13, 2025 |
By: |
/s/ Paul A. Mahon |
| |
Name: |
Paul A. Mahon |
| |
Title: |
General Counsel |
Exhibit 99.1

| United Therapeutics:
Enabling Inspiration
J.P. MORGAN HEALTHCARE CONFERENCE
JANUARY 13, 2025 |

| 2 INTRODUCTION
Safe Harbor Statement
MIROKIDNEY®, MIROLIVERELAP®, ORENITRAM®, REMODULIN®, TYVASO®, and TYVASO DPI® are registered trademarks of United Therapeutics Corporation and/or its subsidiaries.
UHEART™, UKIDNEY™, ULOBE™, ULUNG™, and UTHYMOKIDNEY™ are trademarks of United Therapeutics Corporation and/or its subsidiaries.
2
All statements in this presentation are made as of January 13, 2025. We undertake no obligation to
publicly update or revise these statements, whether as a result of new information, future events, or
otherwise.
Statements included in this presentation that are not historical in nature are “forward-looking statements”
within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements
include, among others, statements related to our revenue growth expectations, the timing and success of
our pipeline programs, our planned manufacturing and field force expansions, our organ manufacturing
efforts, and similar statements concerning anticipated future events and expectations.
We caution you that these statements are not guarantees of future performance and are subject to
numerous evolving risks and uncertainties that we may not be able to accurately predict or assess, including
the risk factors that we describe in our Securities and Exchange Commission filings, including our most
recent Annual Report on Form 10-K or Quarterly Report on Form 10-Q. Any of these factors could cause
actual results to differ materially from the expectations we express or imply in this presentation.
This presentation and any related discussions or statements are intended to educate investors about our
company. Sometimes that process includes reporting on the progress and results of clinical trials or other
developments with respect to our products. This presentation and any related discussions or statements are
not intended to promote our products, to suggest that our products are safe and effective for any use other
than what is consistent with their FDA-approved labeling, or to provide all available information regarding
the products, their risks, or related clinical trial results. Anyone seeking information regarding the use of one
of our products should consult the full prescribing information for the product available on our website at
www.unither.com. |

| 3 WHO WE ARE
Enabling Inspiration
Founded to save a daughter’s life
Structured as a Public Benefit Corporation
3
Rare Lung
Diseases
Pediatric
Oncology
Organs &
Organ Alternatives
OUR FOCUS |
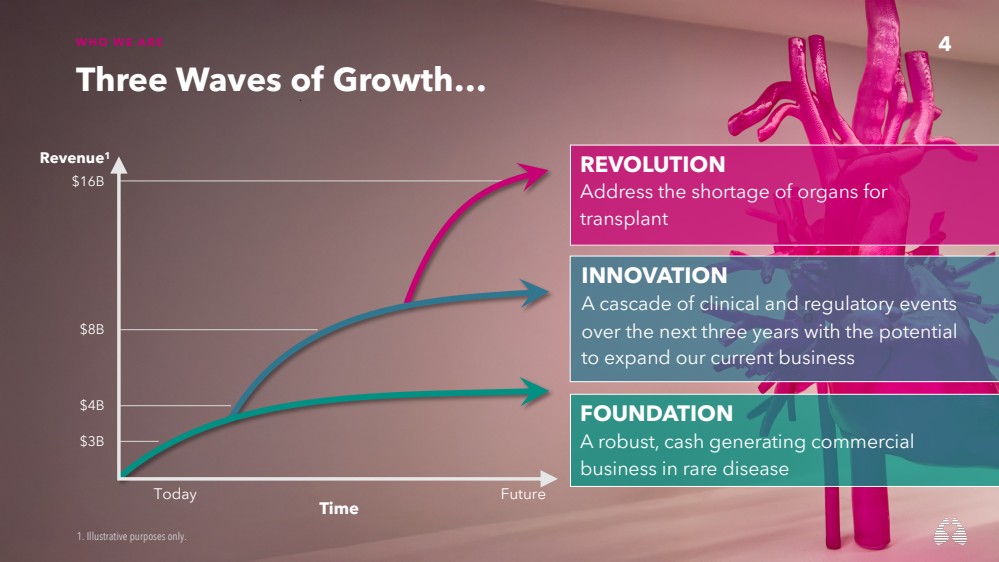
| 4 WHO WE ARE
Three Waves of Growth…
FOUNDATION
A robust, cash generating commercial
business in rare disease
INNOVATION
A cascade of clinical and regulatory events
over the next three years with the potential
to expand our current business
1. Illustrative purposes only.
$3B
$4B
$8B
$16B
Time Today Future
Revenue1
REVOLUTION
Address the shortage of organs for
transplant
4 |
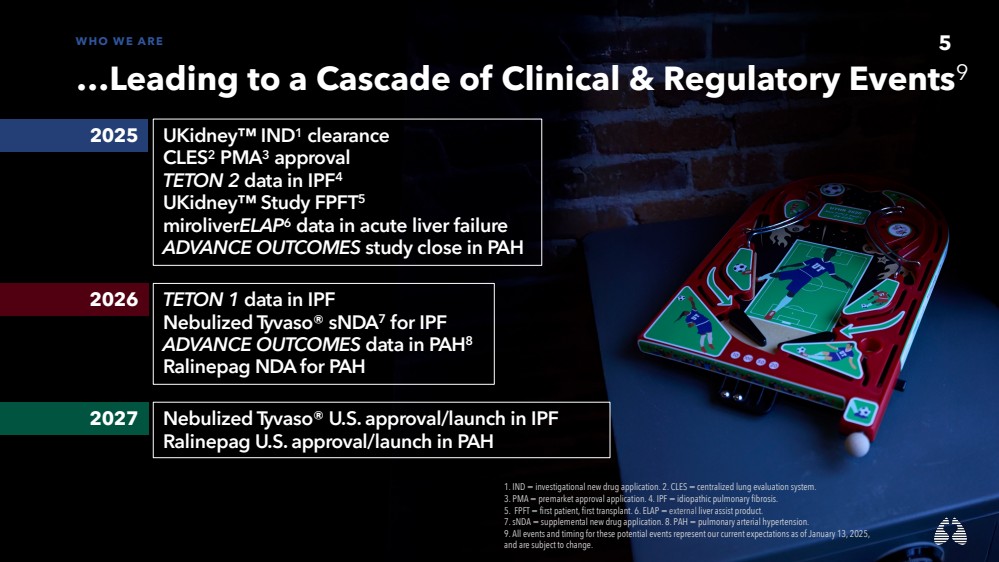
| 5 WHO WE ARE
…Leading to a Cascade of Clinical & Regulatory Events9
2025 UKidney™ IND1 clearance
CLES2 PMA3 approval
TETON 2 data in IPF4
UKidney™ Study FPFT5
miroliverELAP6 data in acute liver failure
ADVANCE OUTCOMES study close in PAH
2026 TETON 1 data in IPF
Nebulized Tyvaso® sNDA7 for IPF
ADVANCE OUTCOMES data in PAH8
Ralinepag NDA for PAH
2027 Nebulized Tyvaso® U.S. approval/launch in IPF
Ralinepag U.S. approval/launch in PAH
5
1. IND = investigational new drug application. 2. CLES = centralized lung evaluation system.
3. PMA = premarket approval application. 4. IPF = idiopathic pulmonary fibrosis.
5. FPFT = first patient, first transplant. 6. ELAP = external liver assist product.
7. sNDA = supplemental new drug application. 8. PAH = pulmonary arterial hypertension.
9. All events and timing for these potential events represent our current expectations as of January 13, 2025,
and are subject to change. |
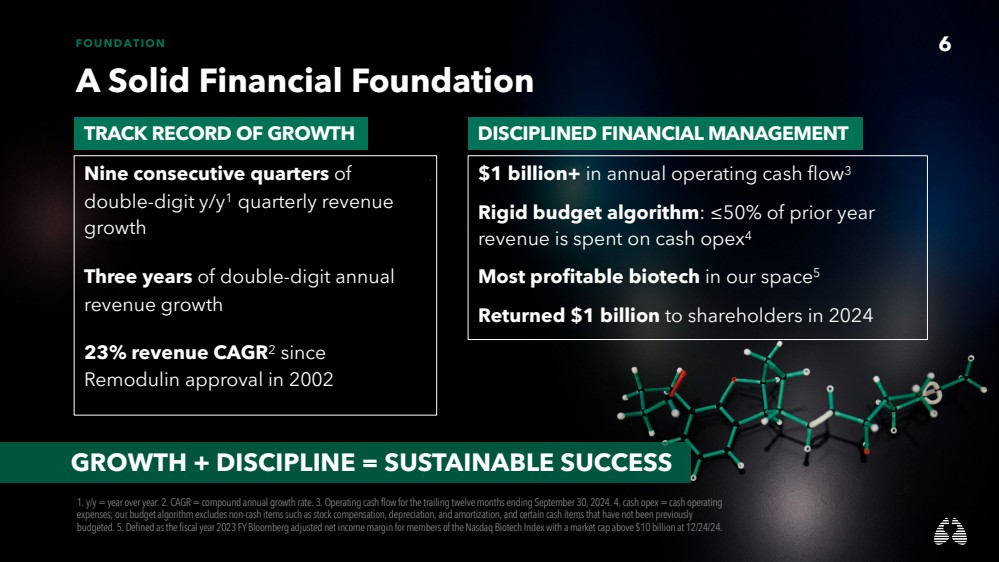
| 6 FOUNDATION
A Solid Financial Foundation
Nine consecutive quarters of
double-digit y/y1 quarterly revenue
growth
Three years of double-digit annual
revenue growth
23% revenue CAGR2 since
Remodulin approval in 2002
1. y/y = year over year. 2. CAGR = compound annual growth rate. 3. Operating cash flow for the trailing twelve months ending September 30, 2024. 4. cash opex= cash operating
expenses; our budget algorithm excludes non-cash items such as stock compensation, depreciation, and amortization, and certain cash items that have not been previously
budgeted. 5. Defined as the fiscal year 2023 FY Bloomberg adjusted net income margin for members of the Nasdaq Biotech Index with a market cap above $10 billion at 12/24/24.
$1 billion+ in annual operating cash flow3
Rigid budget algorithm: ≤50% of prior year
revenue is spent on cash opex4
Most profitable biotech in our space5
Returned $1 billion to shareholders in 2024
6
TRACK RECORD OF GROWTH DISCIPLINED FINANCIAL MANAGEMENT
GROWTH + DISCIPLINE = SUSTAINABLE SUCCESS |

| 7 FOUNDATION
Market Leadership with a Robust Platform Approach
INHALED
The most prescribed prostacyclin in the U.S.
ORAL
11 sequential quarters of y/y quarterly revenue growth
PARENTERAL
The most prescribed parenteral prostacyclin in the U.S.
7 |
| 8 INNOVATION
Leading with Innovation
8
A RELENTLESS PURSUIT OF INNOVATION OUR LONG-TERM VISION
Tyvaso® IPF
Ralinepag PAH
Durable, strong
cash flow from our
current portfolio
+
Innovative
research to create
a sustainable
business |

| 9 INNOVATION
TETON Program in IPF
9
TREPROSTINIL PIONEER
Sir John Robert Vane. Photograph. Wellcome Collection. Source: Wellcome Collection.
SIR JOHN R. VANE FRS
NOBEL LAUREATE |
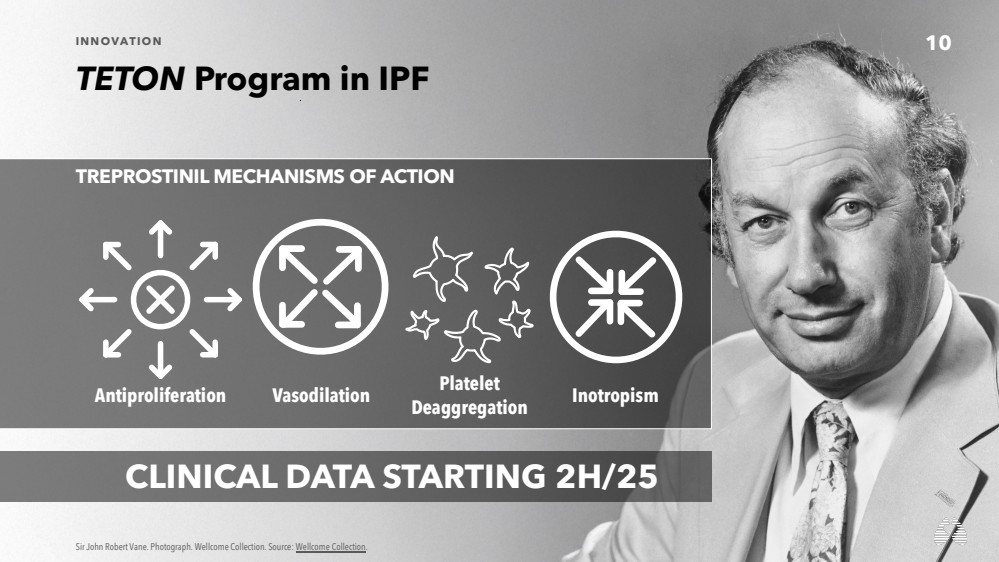
| 10 INNOVATION
TETON Program in IPF
CLINICAL DATA STARTING 2H/25
10
Sir John Robert Vane. Photograph. Wellcome Collection. Source: Wellcome Collection.
TREPROSTINIL MECHANISMS OF ACTION
Platelet
Deaggregation Antiproliferation Vasodilation Inotropism |
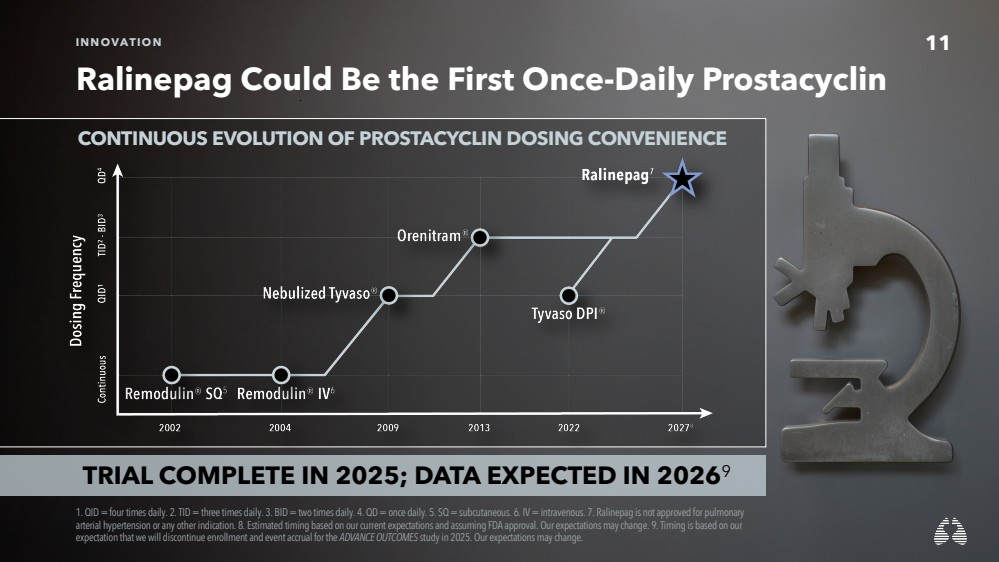
| 11 INNOVATION
Ralinepag Could Be the First Once-Daily Prostacyclin
1. QID = four times daily. 2. TID = three times daily. 3. BID = two times daily. 4. QD = once daily. 5. SQ = subcutaneous. 6. IV= intravenous. 7. Ralinepag is not approved for pulmonary
arterial hypertension or any other indication. 8. Estimated timing based on our current expectations and assuming FDA approval. Our expectations may change. 9. Timing is based on our
expectation that we will discontinue enrollment and event accrual for the ADVANCE OUTCOMES study in 2025. Our expectations may change.
TRIAL COMPLETE IN 2025; DATA EXPECTED IN 20269
11
CONTINUOUS EVOLUTION OF PROSTACYCLIN DOSING CONVENIENCE |
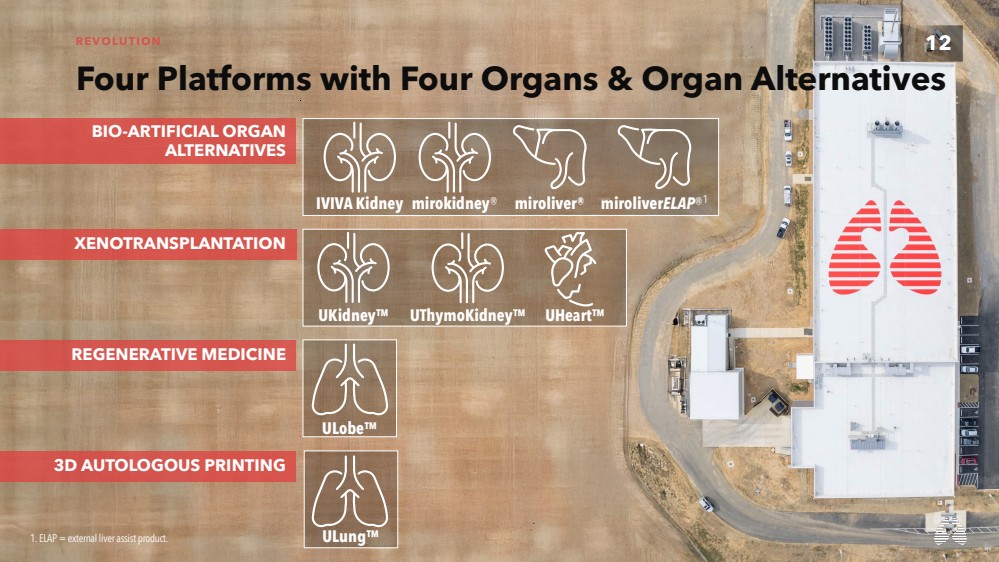
| 12 REVOLUTION
Four Platforms with Four Organs & Organ Alternatives
12
UKidney™ UThymoKidney™ UHeart™
XENOTRANSPLANTATION
BIO-ARTIFICIAL ORGAN
ALTERNATIVES
REGENERATIVE MEDICINE
3D AUTOLOGOUS PRINTING
IVIVA Kidney mirokidney® miroliverELAP® miroliver 1 ®
ULobe™
1. ELAP = external liver assist product. ULung™ |
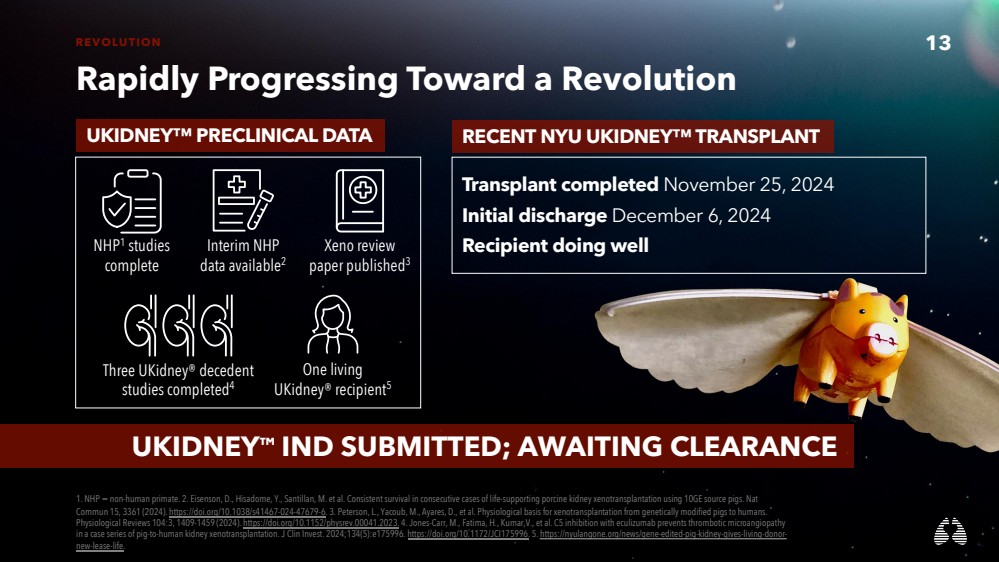
| 13 REVOLUTION
Rapidly Progressing Toward a Revolution
UKIDNEY™ IND SUBMITTED; AWAITING CLEARANCE
UKIDNEY™ PRECLINICAL DATA RECENT NYU UKIDNEY™ TRANSPLANT
Transplant completed November 25, 2024
Initial discharge December 6, 2024
Recipient doing well
13
NHP1 studies
complete
Xeno review
paper published3
Interim NHP
data available2
Three UKidney® decedent
studies completed4
One living
UKidney® recipient5
1. NHP = non-human primate. 2. Eisenson, D., Hisadome, Y., Santillan, M. et al. Consistent survival in consecutive cases of life-supporting porcine kidney xenotransplantation using 10GE source pigs. Nat
Commun 15, 3361 (2024). https://doi.org/10.1038/s41467-024-47679-6. 3. Peterson, L., Yacoub, M., Ayares, D., et al. Physiological basis for xenotransplantation from genetically modified pigs to humans.
Physiological Reviews 104:3, 1409-1459 (2024). https://doi.org/10.1152/physrev.00041.2023. 4. Jones-Carr, M., Fatima, H., Kumar,V., et al. C5 inhibition with eculizumab prevents thrombotic microangiopathy
in a case series of pig-to-human kidney xenotransplantation. J Clin Invest. 2024;134(5):e175996. https://doi.org/10.1172/JCI175996. 5. https://nyulangone.org/news/gene-edited-pig-kidney-gives-living-donor-new-lease-life. |

| 1414
Pictured, left to right: Mr. Willie Bennett, Mrs. Towana Looney, Martine Rothblatt, Bina Rothblatt. |

| 15 REVOLUTION
Rapidly Progressing Toward a Revolution
15 |
| 1616 |
| 1717 |
| 1818 |
| 19 REVOLUTION
Uniquely Positioned to Help Patients and
Create Lasting Shareholder Value
19
PBC PHILOSOPHY When our patients succeed, we all
succeed
CASH FLOW + INNOVATION A unique combination of strong cash
flow and a revolutionary pipeline
36 MONTHS OF CATALYSTS A three-year cascade of potential
clinical data and regulatory catalysts
starts in 2025
NO OTHER BIOTECH HAS THIS COMPELLING PROFILE |
v3.24.4
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
United Therapeutics (NASDAQ:UTHR)
Historical Stock Chart
Von Dez 2024 bis Jan 2025

United Therapeutics (NASDAQ:UTHR)
Historical Stock Chart
Von Jan 2024 bis Jan 2025
