As filed with the United States Securities and Exchange Commission on December
11, 2024
Registration
No. 333-282993
UNITED
STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
F-1
(Amendment No. 3)
REGISTRATION STATEMENT
UNDER THE SECURITIES ACT OF 1933
MAINZ
BIOMED N.V.
(Exact name of registrant as specified in its charter)
| The
Netherlands |
|
8731 |
|
Not
Applicable |
(State or other jurisdiction
of
incorporation or organization) |
|
(Primary Standard Industrial
Classification Code Number) |
|
(I.R.S. Employer
Identification Number) |
Mainz
Biomed N.V.
Robert Koch Strasse 50
55129 Mainz
Germany
Telephone: 0049 6131 5542860
(Address
of principal executive offices, including zip code, and telephone number, including area code)
Vcorp
Services, LLC
25 Robert Pitt Drive, Suite 204
Monsey, NY 10952
Telephone:
(Name,
address, including zip code, and telephone number, including area code, of agent of service)
Copies
to:
William Rosenstadt,
Esq.
Mengyi “Jason” Ye, Esq.
Tim Dockery, Esq.
Ortoli Rosenstadt LLP
366 Madison Avenue
New York, New York 10022
Telephone: (212) 588-0022 |
|
M.
Ali Panjwani, Esq.
Pryor
Cashman LLP
7
Times Square, New York
New
York 10036-6569
Telephone: (212) 421-4100 |
Approximate
date of commencement of proposed sale to the public: As soon as practicable after this Registration Statement becomes effective.
If
any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415
under the Securities Act of 1933, check the following box. ☒
If
this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act of 1933,
check the following box and list the Securities Act registration statement number of the earlier effective registration statement for
the same offering. ☐
If
this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act of 1933, check
the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same
offering. ☐
If
this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act of 1933, check
the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same
offering. ☐
Indicate
by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933.
Emerging
growth company ☒
If
an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant
has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant
to Section 7(a)(2)(B) of the Securities Act. ☐
The
Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the
registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective
in accordance with Section 8(a) of the Securities Act of 1933 or until this Registration Statement shall become effective
on such date as the United States Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
The
information in this preliminary prospectus is not complete and may be changed. The securities may not be sold until the registration
statement filed with the United States Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to
sell, nor does it seek an offer to buy, the securities in any jurisdiction where such offer or sale is not permitted.
| PRELIMINARY PROSPECTUS |
|
SUBJECT TO COMPLETION |
|
DATED DECEMBER
11, 2024 |
Up to 1,147,776 Ordinary Units
Each Ordinary Unit Consisting of
One Ordinary Share,
One Class A Warrant to purchase One Ordinary
Share and
One Class B Warrant to purchase One Ordinary
Share
Up To 1,147,776 Pre-Funded Units
Each Pre-Funded Unit Consisting of
One Pre-Funded Warrant to purchase One Ordinary
Share,
One Class A Warrant to purchase One Ordinary
Share and
One Class B Warrant to purchase One Ordinary
Share
Up To 3,443,328 Ordinary Shares underlying
the Class A Warrants,
the Class B Warrants, and the Pre-Funded
Warrants

Mainz
Biomed N.V.
We are offering on a “best efforts”
basis up to 1,147,776 ordinary units of Mainz Biomed N.V. with each ordinary unit consisting of one ordinary share, one Class A ordinary
share purchase warrant (“Class A Warrant”) and one Class B ordinary share purchase warrant (“Class B Warrant”).
Our ordinary shares are traded on the Nasdaq Capital Market under the symbol “MYNZ.” The last reported sale price of our
ordinary shares on December 6, 2024 as reported on Nasdaq, was $6.97 per share rounded to the nearest whole cent, which is the per ordinary
unit offering price we have assumed for purposes of this preliminary prospectus.
We are also offering to each purchaser of
ordinary units that would otherwise result in the purchaser’s beneficial ownership exceeding 4.99% of our outstanding ordinary
shares immediately following the consummation of this offering the opportunity to purchase pre-funded units in lieu of ordinary units
with each pre-funded unit consisting of one pre-funded warrant to purchase an ordinary share, one Class A Warrant and one Class B Warrant.
The purchase price of each pre-funded unit will be equal to the price per ordinary unit minus $0.0001. The number of ordinary units we
are offering will be decreased on a one-for-one basis. We do not intend to apply for any listing of the ordinary units, the pre-funded
units, the pre-funded warrants, the Class A Warrants or the Class B Warrants on any securities exchange or nationally recognized trading
system, and we do not expect a market to develop for any of such securities. We are also registering the ordinary shares issuable from
time to time upon the exercise of the pre-funded warrants, the Class A Warrants and the Class B Warrants offered hereby. We refer to
the ordinary units, the pre-funded units, the pre-funded warrants, the Class A Warrants and the Class B Warrants offered hereby as the
offered securities.
Each pre-funded warrant will be exercisable
for one ordinary share, and the remaining exercise price of each pre-funded warrant will equal $0.0001 per ordinary share. The pre-funded
warrants will be immediately exercisable and may be exercised at any time until all of the pre-funded warrants are exercised in full.
Each Class A Warrant will be exercisable for
one ordinary share. The exercise price of each Class A Warrant will equal the per ordinary unit offering price hereunder, which we have
assumed to be $6.97 based on the closing price of our ordinary shares rounded to the nearest whole cent on the Nasdaq Capital Market
on December 6, 2024. The Class A Warrants will be immediately exercisable and may be exercised until the fifth anniversary of the date
of issuance.
Each Class B Warrant will be exercisable for
one ordinary share. The exercise price of each Class B Warrant will equal the per ordinary unit offering price hereunder, which we have
assumed to be $6.97 based on the closing price of our ordinary shares rounded to the nearest whole cent on the Nasdaq Capital Market
on December 6, 2024. The Class B Warrants will be immediately exercisable and may be exercised until the earlier of (i) first anniversary
of the date of issuance or (ii) 30 days following the public disclosure of results from the eAArly Detect 2 study.
A holder of pre-funded warrants, Class A Warrants
and Class B Warrants will not have the right to exercise any portion of such warrants if the holder, together with its affiliates, would
beneficially own in excess of 4.99% (or, at the election of the holder, 9.99%) of the number of ordinary shares outstanding immediately
after giving effect to such exercise.
On December 3, 2024 we effectuated a 1:40
reverse stock split of our ordinary shares. All share and per share amounts in this prospectus (except where otherwise indicated) account
for this reverse stock split. Any share and per share amounts incorporated by reference into this prospectus from documents that we have
previously fielded with the U.S. Securities and Exchange Commission (the “SEC”) do not account for such reverse stock split.
The
public offering price for the offered securities will be determined at the time of pricing and may be at a discount to the current market
price at the time. Therefore, the assumed public offering price used throughout this prospectus may not be indicative of the final offering
price. The final public offering price will be determined through negotiation between us, the placement agent and the investors based
upon a number of factors, including our history and our prospects, the industry in which we operate, our past and present operating results,
the previous experience of our executive officers and the general condition of the securities markets at the time of this offering.
This is a best-efforts offering and the placement
agent does not have an obligation to purchase any securities. Because there is no minimum offering amount required as a condition to
closing this offering, we may sell fewer than all of the securities offered hereby, which may significantly reduce the amount of proceeds
received by us, and investors in this offering will not receive a refund in the event that we do not sell a number of securities sufficient
to pursue the business goals outlined in this prospectus. Because there is no minimum offering amount, investors could be in a position
where they have invested in our company, but we are unable to fulfill our objectives due to a lack of interest in this offering. Also,
any proceeds from the sale of securities offered by us will be available for our immediate use despite uncertainty about whether we would
be able to use such funds to effectively implement our business plan.
This offering will terminate on December 24,
2024, unless we decide to terminate the offering (which we may do at any time in our discretion) prior to that date. We will have one
closing for all the securities purchased in this offering. The public offering price per ordinary unit and per pre-funded unit will be
fixed for the duration of this offering. The offering will settle delivery versus payment (“DVP”)/receipt versus payment
(“RVP”). Accordingly, we and the placement agent have not made any arrangements to place investor funds in an escrow account
or trust account since the placement agent will not receive investor funds in connection with the sale of the securities offered hereunder.
We are both an “emerging growth company”
and a “foreign private issuer” under applicable U.S. Securities and Exchange Commission rules and will be eligible for reduced
public company disclosure requirements. See section titled “Prospectus Summary – Implications of Being an Emerging Growth
Company” and “Prospectus Summary – Implications of being a Foreign Private Issuer” for additional information.
Investing
in our securities involves a high degree of risk. You should carefully consider the matters described under the caption “Risk Factors”
beginning on page 13.
Neither
the U.S. Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined
if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
| |
Per
Ordinary Unit | | |
Per
Pre-Funded
Unit | | |
Total | |
| Public
offering price | |
$ | | | |
$ | | | |
$ | | |
| Placement
Agent Fees(1) | |
$ | | | |
$ | | | |
$ | | |
| Proceeds
to us, before expenses(2) | |
$ | | | |
$ | | | |
$ | | |
| (1) | The
placement agent, Maxim Group LLC, will receive compensation in addition to the placement
agent fees. See “Plan of Distribution” for a description of compensation payable
to the placement agent. |
| (2) | The
total estimated expenses related to this offering are set forth in the section entitled “Expenses
Relating to this Offering.” |
If we complete this offering, net proceeds will
be delivered to us on the closing date.
The
placement agent expects to deliver the offered securities on or about ,
2024.
Maxim
Group LLC
The
date of this prospectus is , 2024
TABLE
OF CONTENTS
This
prospectus is part of a registration statement on Form F-1 that we filed with the SEC. You should read this prospectus and the information
and documents incorporated herein by reference carefully. Such documents contain important information you should consider when making
your investment decision. See “Where You Can Find Additional Information” and “Incorporation of Documents by Reference”
in this prospectus.
You
should rely only on the information contained in this prospectus (inclusive of the documents incorporated by reference herein), any amendment
or supplement to this prospectus or any free writing prospectus prepared by or on our behalf. Neither we nor the placement agent have
authorized any other person to provide you with different or additional information. Neither we nor the placement agent take responsibility
for, nor can we provide assurance as to the reliability of, any other information that others may provide. The placement agent is not
making an offer to sell these securities in any jurisdiction where the offer or sale is not permitted. The information contained in this
prospectus or incorporated by reference in this prospectus is accurate only as of the date of this prospectus or such other date stated
in this prospectus, and our business, financial condition, results of operations and/or prospects may have changed since those dates.
Except
as otherwise set forth in this prospectus, neither we nor the placement agent have taken any action to permit a public offering of these
securities outside the United States or to permit the possession or distribution of this prospectus outside the United States.
Persons outside the United States who come into possession of this prospectus must inform themselves about and observe any restrictions
relating to the offering of these securities and the distribution of this prospectus outside the United States.
Unless
the context otherwise requires, in this prospectus, the term(s) “we”, “us”, “our”, “Company”,
“our company”, “our business” and “Mainz Biomed” refer to Mainz Biomed N.V.
All share and per share amounts in this prospectus
(except where otherwise indicated) account for a 1:40 reverse stock split of our ordinary shares that occurred on December 3, 2024. Any
share and per share amounts incorporated by reference into this prospectus from documents that we have previously fielded with the SEC
do not account for such reverse stock split.
PROSPECTUS
SUMMARY
The
following summary highlights, and should be read in conjunction with, the more detailed information contained elsewhere in this prospectus
or incorporated by reference into this prospectus. You should read carefully the entire document or documents incorporated by reference
in this prospectus, including our historical financial statements and related notes incorporated by reference herein, to understand our
business, the offered securities and the other considerations that are important to your investment decision. You should pay special
attention to the “Risk Factors” section beginning on page 13 as well as the risk factors described under the heading “Risk
Factors” in our Annual Report on Form 20-F for the year ended December 31, 2023, filed on April 9, 2024.
We
are a public company under Dutch law. We were incorporated on March 8, 2021 as a private limited liability company (besloten
vennootschap met beperkte aansprakelijkheid) under Dutch law. We were formed to acquire PharmGenomics GmbH (“PharmGenomics”),
a German company with limited liability, and we acquired PharmGenomics on September 20, 2021. On November 9, 2021, we converted
into a Dutch public company with limited liability (naamloze vennootschap). The address for our principal place of business is
Robert Koch Strasse 50, 55129 Mainz, Germany, and the telephone number is +49 6131 5542860.
We
have registered our ordinary shares under the Exchange Act, and we intend to make our current and periodic reports and other information
(including interactive data files) filed with or furnished to the SEC, pursuant to Section 13(a) or 15(d) of the Exchange Act,
available free of charge through our website as soon as reasonably practicable after those reports and other information are electronically
filed with, or furnished to, the SEC. The SEC maintains a website at http://www.sec.gov that contains reports and other information
regarding issuers that file electronically with the SEC, and all of our reports and other information filed or submitted publicly with
the SEC may also be found there.
Information
on our website or any other website is not incorporated by reference into this annual report and does not constitute a part of this annual
report. We have included our website address as an inactive textual reference only.
General
We
develop and market in-vitro diagnostic (“IVD”) tests for early cancer detection, initially focusing on colorectal cancer
(“CRC”) and advanced precancerous lesions. Our flagship product, ColoAlert, is currently available in European markets. Additionally,
we are developing a next-generation mRNA-based colorectal cancer screening test, with plans for future launch in both the United States
and Europe. While we operated a clinical diagnostic laboratory through 2023 and early 2024, our primary business model focuses on distributing
IVD kits to third-party laboratories across Europe and the United States.
Our
research and development efforts are aimed at expanding and diversifying our product portfolio. In 2023 and early 2024, we managed a
government-funded R&D project for early pancreatic cancer detection, which provided non-refundable grant income to cover a portion
of related project costs. Although this funding has concluded, we have continued our R&D activities in 2024, albeit at a reduced
level.
We are headquartered in Mainz, Germany where
we have approximately 25 employees.
Products
and Product Candidates
Our
mission is to enhance disease diagnosis by applying cutting-edge genetic diagnostic technologies, enabling earlier and more accurate
detection for timely and improved treatment. Alongside our current ColoAlert CRC screening test, we are developing an advanced mRNA-based
CRC screening test, as well as PancAlert, a product candidate for pancreatic cancer detection. Our approach integrates proprietary, validated
biomarkers with reliable diagnostic tools, further strengthened by machine learning and artificial intelligence to optimize accuracy
and applicability. We strive to make the diagnosis of various diseases more effective by using the latest genetic diagnostic technologies.
Enabling earlier detection of these diseases allows for earlier and better therapy for affected individuals. In addition to offering
the CRC screening test, ColoAlert, we are currently developing our product candidate PancAlert for the detection of pancreatic cancer.
We aim to use proprietary, known and existing biomarkers in applicable and reliable diagnostic tools.
ColoAlert
and Our Next Generation Colorectal Cancer Screening Test
We currently offer ColoAlert, a CE-IVD certified
diagnostic test for CRC to laboratories across Europe. The CE-IVD marking indicates that our diagnostic test complies with the European
In-Vitro Diagnostic Devices Directive (IVDD 98/79/EC) Its simple, at-home collection process makes it easier for individuals to
participate in CRC screening, promoting early detection and increasing the likelihood of effective treatment.
According the World Health Organization, as
of July 2023 CRC is the third most commonly diagnosed cancer in the world. 1 Due to its ability to move into large, adjacent
areas leading to other cancers, including pancreatic cancer and other gastro-intestinal cancers, the World Health Organization suggests
as of February 2022, CRC is the second leading cause of cancer death globally.2 Further, Global Market Insights anticipates
that the annual market for CRC diagnostics will surpass $30 billion by 20323. Colorectal cancer screening presents a significant
opportunity in the molecular diagnostics market.
In the intestines, epithelial cells are continuously
shed into the stool. This includes not only healthy cells but also cells from polyps and colon cancer. Using advanced genetic diagnostic
techniques like PCR analysis—which amplifies DNA from a small sample into millions of copies—these shed cells can be isolated
and examined for genetic mutations, enhancing the early detection of CRC.
ColoAlert is a multitarget test that analyzes
stool samples for both genetic abnormalities and hidden (occult) blood by combining a fecal immunochemical test (“FIT”) for
detection of human hemoglobin with the PCR results of specific tumor DNA markers. The genetic analysis includes quantifying human DNA
and detecting specific somatic point mutations in the KRAS (codon 12/13) and BRAF (codon 600) genes. An independent clinical study led
by Professor Matthias Dollinger and conducted with 566 patients at the University Hospitals of Leipzig and Halle-Wittenberg, Germany,
demonstrated ColoAlert’s high sensitivity (85%) and specificity (92%), with a patient satisfaction rate of 98%. The selected genetic
markers enhance the diagnostic precision of the occult blood test, increasing the clinical value of the test. Since this study, we have
upgraded the occult blood test component of ColoAlert to a fully automated version, further improving the test’s overall sensitivity.
Early screening for CRC has the potential
to dramatically impact its treatment and prevention and, ultimately, save lives. For example, the Journal of the National Cancer Institute
has reported in 2022 that diagnostic tests with a sensitivity of 10% for advanced adenomas (“AA”), a type of pre-cancerous
polyp often attributed to CRC, could reduce mortality rates for adults over 45 by 47% and that diagnostic tests with
a sensitivity of 76% for AA could reduce mortality rates for adults over 45 by 67%.5 Most CRC screening programs currently
recommend beginning screening at age 50. However, there is a growing trend to lower this starting age. For example, the U.S. Food and
Drug Administration (the “FDA”) recently recommended starting CRC screening at age 45. This means that as members of Generation
X age into their 40s and 50s they become part of the age group recommended to begin testing for CRC. In 2023, the American Cancer Society
highlighted the rapid progression of CRC among younger individuals, noting that CRC diagnoses in people under 55 increased from 11% in
1995 to 20% in 2019. Given the rising prevalence of CRC in younger populations, we anticipate that screening guidelines will continue
to lower the starting age, particularly for methods like ColoAlert that can detect cancer in its early stages. Additional factors supporting
CRC screening include a family history of CRC, risk factors such as obesity, irritable bowel syndrome (IBS), inflammatory bowel disease
(IBD), high consumption of red meat, alcohol, and nicotine, as well as pre-existing conditions like breast cancer or type 2 diabetes.
Until
February 2023, we licensed the ColoAlert test from the Norwegian research and development firm ColoAlert AS. In February 2023, we acquired
the test and its related intellectual property from ColoAlert AS under an agreement that includes: (i) a $2 million cash payment, to
be made over the next four years, (ii) the issuance of 300,000 ordinary restricted shares, and (iii) a revenue share capped at $1 per
test sold over a 10-year period.
In
the European Union, the ColoAlert PCR kit (“ColoAlert Lab Kit Core II”) is CE-IVD certified under the current In-Vitro Diagnostics
Directive 98/79/EC (IVD-D). As of May 26, 2022, IVD products in the EU are regulated by the In-Vitro Diagnostics Regulation, EU 2017/746
(IVD-R), which supersedes the IVD-D. The ColoAlert sample collection kit has already been successfully registered under the IVD-R, and
we are currently assessing the requirements to certify our ColoAlert PCR kit under these new regulations. ColoAlert is validated for
use on the Roche LightCycler 480 II, and Mainz BioMed plans to validate it for additional real-time PCR instruments used in laboratories
worldwide, which may accelerate market adoption. The ColoAlert PCR kits are manufactured at our facility in Mainz, Germany.
| 1 | https://www.who.int/news-room/fact-sheets/detail/colorectal-cancer |
| 2 | https://www.who.int/news-room/fact-sheets/detail/cancer |
| 3 | https://www.gminsights.com/industry-analysis/colorectal-cancer-diagnostics-market |
| 54 | https://academic.oup.com/view-large/365872704 |
In
January 2022, we entered into a Technology Rights Agreement concerning a portfolio of novel mRNA biomarkers developed at the Université
de Sherbrooke (“UdeS Biomarkers”). mRNA testing can detect molecular changes in cells even before visible abnormalities or
symptoms manifest. mRNA biomarkers often reflect the dynamic changes in gene expression that occur during the progression of adenomas
to advanced stages. As adenomas evolve, certain genes may be upregulated or downregulated, and RNA biomarkers can capture these changes,
providing insights into the stage of adenoma development. mRNA biomarkers are highly specific to particular stages or types of adenomas.
By targeting RNA molecules associated with the advanced stage of adenomas, we believe that these biomarkers can distinguish between advanced
adenomas and less advanced forms or benign conditions.
Through
our agreement with the Université de Sherbrooke, we obtained an exclusive, unilateral option to acquire a license for the UdeS
Biomarkers. We exercised this option on February 15, 2023, by entering into an Assignment Agreement to acquire the intellectual property
rights for these biomarkers. In exchange, we agreed to (i) pay €25,000 in cash and (ii) provide a 2% profit share of net sales from
any products incorporating the UdeS biomarkers.
Historical and Proposed
Clinical Trials
The
UdeS Biomarkers consist of five gene expression markers shown to be highly effective in detecting colorectal cancer (CRC) lesions, including
advanced precancerous lesions, AAs, a type of precancerous polyp often associated with CRC. In a UdeS-sponsored study evaluating these
biomarkers, results demonstrated sensitivities of 75% for detecting AA and 95% for CRC, with a specificity of 96%.

Clinical validation and
trials – Statistics and Proposed Timeline
In
relation to the UdeS Biomarkers, we initiated two feasibility studies to assess our next-generation mRNA CRC screening test, combining
UdeS Biomarkers with FIT tests. The first study, ColoFuture, is an international, multicenter clinical study across Europe designed to
validate the effectiveness of the UdeS biomarkers, specifically their capability to identify advanced precancerous lesions or AA while
enhancing sensitivity and specificity for CRC detection. ColoFuture includes 662 participants, covering individuals with average CRC
risk and those at increased risk or known to have CRC or AA. Enrollment in ColoFuture concluded in late 2023.
Additionally,
we conducted a U.S.-based multicenter study called “eAArly DETECT,” which evaluates feasibility and stability in 450 participants,
including those with average CRC risk and individuals at elevated risk or known to have CRC or AA. eAArly DETECT was completed by the
end of 2023. These studies aim to identify the optimal combination of biomarkers, potentially including mRNA and housekeeping biomarkers
alongside a FIT test, for our next-generation product. This product will undergo further testing in the eAArly DETECT v2 study and in
the evaluation in our pivotal FDA PMA study, labeled “reconAAsense.” Both ColoFuture and eAArly DETECT studies utilized an
advanced machine learning and AI-driven algorithm, developed in partnership with Liquid Biosciences, based in California.
In October 2023, we announced the results
of the ColoFuture study, which demonstrated a sensitivity of 94% for colorectal cancer (CRC) and 97% specificity, along with an 80% sensitivity
for Aas and 95% specificity. In December 2023, we released topline results from our eAArly DETECT clinical study in the U.S., which reported
a sensitivity of 97% for CRC, 97% specificity, and an 82% sensitivity for advanced adenomas and 97% specificity. These topline results
reaffirm the positive findings from ColoFuture, our European counterpart, which were reported in October 2023.
The
eAArly DETECT study enrolled 254 evaluable subjects across 21 sites in the United States, featuring a design similar to that of ColoFuture.
Patients aged 45 and older were invited to participate when referred for a colonoscopy, whether for CRC screening (average risk), follow-up
on a positive non-invasive test, imaging, or symptoms. Additionally, individuals already diagnosed with CRC were included prior to receiving
treatment. Participants who agreed to provide a stool sample before the colonoscopy (or treatment for identified CRC patients) were eligible.
Subjects were classified following a central pathology review: CRC, advanced adenoma, non-advanced adenoma, no findings, or non-colorectal
cancer. Each subject’s outcome was then compared to the results of the next-generation mRNA-based CRC screening test.
In
June 2024, the Company presented pivotal data from its largest cohort to date during a poster session at the American Society of Clinical
Oncology (ASCO) 2024 Annual Meeting in Chicago, Illinois. This data combined results from the ColoFuture and eAArly DETECT studies, along
with additional patients collected since the initial study results were reported, underscoring the significance of our innovative screening
approach.
The
combined analysis included 690 clinical subjects from 30 specialized gastroenterology centers across Europe and the United States, incorporating
previously unexamined and unreported samples. This highlighted the exceptional efficacy of Mainz Biomed’s multimodal screening
test, which integrates the Fecal Immunochemical Test (FIT) with proprietary mRNA biomarkers, supported by an advanced AI and machine
learning algorithm. This comprehensive approach allows for precise differentiation between colorectal cancer (CRC), AAs, non-advanced
adenomas, and samples with no pathological findings.
| | |
ColoFuture | | |
eAArly
DETECT | | |
Pooled
study | |
| CRC
Sensitivity | |
| 94 | % | |
| 97 | % | |
| 92 | % |
| CRC
Specificity | |
| 97 | % | |
| 97 | % | |
| 90 | % |
| AA
Sensitivity | |
| 80 | % | |
| 82 | % | |
| 82 | % |
| AA
Specificity | |
| 95 | % | |
| 97 | % | |
| 90 | % |
| Location | |
| EU | | |
| US | | |
| EU
& US | |
| # of Participants | |
| 220 | | |
| 254 | | |
| 690 | |
The
following tables set out the CRC and AA sensitivity and specificity results of our next-generation mRNA CRC screening test as compared
to some competing products.

Recent Developments
In November 2024, we entered into a Collaboration
Agreement with Life Technologies Corporation, a subsidiary of Thermo Fisher Scientific Inc. (“Thermo Fisher”). Thermo Fisher
is a world leader in serving science, with annual revenue over $40 billion in its latest fiscal year. The collaboration agreement provides
us the opportunity to develop our mRNA based next generation assays on the Thermo Fisher Extraction (King Fisher) and PCR (QuantStudio)
platforms. Life Science Technologies will contribute development resources and provide the extraction and PCR instrumentation and consumables
during development at significant discounts to market. There is no minimum cost expenditure in connection with the development of the
assays. All of the development work will occur in our facility in Mainz, Germany.
| |
 |
 |
|
| |
Thermo KingFisher APEX |
QuantStudio™ 7 Pro Dx
(Thermo Fisher Scientific) |
|
| 5 | Chung
D, et al. N Engl J Med 2024;390:973-983 |
| 6 | Imperiale
T, et al. N Engl J Med 2024;390:984-93 |
| 7 | Barnell
E, JAMA, 2023;330(18):1760-1768 |
| 8 | Barnell E, et al. JAMA, 2023;330(18):1760-1768 |
| 9 | Imperiale T, et al. N Engl J Med 2024;390:984-93 |
| 10 | Barnell E, JAMA, 2023;330(18):1760-1768 |
| 11 | Chung D, et al. N Engl J Med 2024;390:973-983 |
Expansion of ColoAlert in Europe
We have advanced
ColoAlert’s commercial presence in Germany, leveraging our headquarters proximity to Frankfurt. In the near term, Germany will
remain our focal market, as we aim to enlarge our footprint by building new laboratory partnerships and increasing product visibility.
Through our medical
lab partners in Germany, ColoAlert is offered to medical professionals overseeing CRC screening in Germany. This group is not limited
to the 1,800 gastroenterologists who primarily conduct colonoscopies but extends to 55,000 general practitioners and over 7,000 specialized
practices in gynecology and urology, who customarily initiate CRC screening, predominantly through the FIT stool test. For individuals
with statutory health insurance, ColoAlert is an out-of-pocket expense. However, it may be reimbursed on a case-by-case basis for those
with private insurance. Out of Germany’s 84 million populace, approximately 10 million have private health coverage. To facilitate
sales, we are developing targeted marketing resources and planning to engage physicians and healthcare providers at medical conferences.
Our strategy focuses
on partnering with laboratories that have a stronghold in CRC screening. These partnerships enable us to reach broader physician networks
affiliated with these labs. Approximately 12 lab chains in Germany process about 64% of the FIT tests, amounting to roughly 3 million
tests annually. By partnering with significant lab chains and independent laboratories, we aim to amplify ColoAlert’s market penetration.
We are not currently
seeking statutory reimbursement for ColoAlert, as we believe our next-generation test, currently under development, is more attuned to
the stringent criteria set by German regulatory and reimbursement agencies. With enhanced sensitivity and specificity for detecting early-stage
colorectal cancer and advanced adenomas, this forthcoming product is poised for inclusion in statutory reimbursement schemes, thereby
establishing a solid market presence in anticipation of its release.
Mainz Biomed is progressively
extending its operations to additional European markets that are accustomed to personal health expenditures, as ColoAlert is not included
in statutory health insurance programs in these regions. Currently, our reach includes established connections in the United Kingdom,
Spain & Portugal, Italy, Austria.
Through our commercial
team, we aim to expand our reach within other European markets.. Our growth strategy is to target clinical labs directly, bolstering
our sales efforts with specialized training for sales reps, educational seminars for physicians, and joint marketing initiatives to heighten
ColoAlert’s profile.
As we pursue these expansions, we remain committed to adapting our commercial strategies
to align with the healthcare payment practices prevalent in each locale. We recognize the importance of catering to markets with a predisposition
towards out-of-pocket payments for health services, which presents a favorable environment for ColoAlert’s integration and acceptance
in the near term, while pursuing statutory reimbursement for our next generation product.
PancAlert
We
are in the early stages of developing PancAlert, a stool-based screening test aimed at detecting pancreatic cancer. According to the
Global Cancer Observatory, over 460,000 cases of pancreatic cancer were diagnosed worldwide in 2018. Due to its asymptomatic early stages,
this disease is often detected too late, making pancreatic cancer one of the most lethal malignancies, with over 430,000 annual deaths
reported by the Global Cancer Observatory. In the United States, the SEER program estimated 62,210 new cases and 49,830 deaths from pancreatic
cancer in the same year. Between 2012 and 2018, SEER reported that the 5-year survival rate was approximately 44% for localized cases,
15% for regional cases, and only 3% for distant-stage disease. Studies have indicated that asymptomatic patients diagnosed incidentally
during other medical examinations have significantly better prognoses than those presenting with characteristic symptoms, such as rapid
weight loss or back pain.
The
average age of onset for pancreatic cancer is 71 years for men and 75 years for women, with age being a significant risk factor similar
to other cancers. Most patients are over 50, with the majority of diagnoses occurring between the ages of 60 and 80. Despite being the
seventh most common cancer, pancreatic cancer ranks as the third leading cause of cancer-related death in the European Union, underscoring
the dire prognosis for patients. Although survival rates for pancreatic cancer have improved in recent decades, there remains an urgent
need for enhanced early diagnostic methods.
A
definitive diagnosis of pancreatic cancer is currently made through a series of investigations, including imaging scans, blood tests,
and biopsies, which are typically conducted only in symptomatic patients. However, recent research indicates that the disease can persist
for an extended period without presenting symptoms, highlighting a crucial opportunity for early detection. Since pancreatic cancer initiates
at the molecular level, genetic diagnostic methods show promise for early identification. Biomarkers associated with pancreatic cancer
can be found in the stool, primarily via pancreatic juice, facilitating user-friendly sample collection.
Our
development strategy involves selecting and validating a specific panel of biomarkers, establishing an appropriate method for sample
preparation, and validating the detection and measurement technology using purchased or clinically defined samples (biopsies, pancreatic
juice, stool, and others). The next steps include transitioning to routine diagnostics using stool samples, optimizing the process, and
conducting clinical evaluations to assess its potential as a screening tool for the early detection of pancreatic cancer.
Our
goal is to establish PancAlert as the world’s first pancreatic cancer screening test utilizing Real-Time PCR-based multiplex detection
of molecular-genetic biomarkers in stool samples. We have recently partnered with Liquid Biosciences, an artificial intelligence and
machine learning company, to further evaluate the most promising candidates for disease-specific biomarkers. The platform technology
we are using will also allow for the easy integration of additional biomarkers as needed.
We have entered into an agreement with Microba
Life Sciences to conduct a pilot research project utilizing Microba’s proprietary metagenomic sequencing technology and bioinformatic
tools to potentially discover novel microbiome biomarkers for pancreatic cancer detection. We believe that this relationship could provide
multiple diagnostic opportunities including discovery of diagnostic and prognostic biomarkers. Such faecal microbiota-based screening
could also be applicable to other gastrointestinal cancers. There is no minimum cost expenditure in connection with
the agreement.
Strategy
Realignment
Between
July 2024 and October 2024, we restructured our operations to concentrate on: (1) our ColoAlert business in Europe, (2) the development
of its next-generation product, and (3) planning and conducting the eAArly Detect 2 clinical study in the U.S. in 2025, aimed at validating
the strong clinical performance of our next-generation mRNA-based CRC screening test in an average-risk population.
In
line with this focus, we implemented cost reduction measures, including a 65% reduction in personnel, decreased external consulting expenses,
and the sale or closure of its European Oncology Lab (EOL) business in St. Ingbert, Germany. We believe that these cost reductions will
position the business for success in 2025 and beyond.
Legal Proceedings
In connection with a right of first refusal granted
to an investment bank in connection with our initial public offering in 2021, Mainz filed a lawsuit against such investment bank in 2024
in the New York State Supreme Court in New York County, asking the court to determine our and the investment banks rights and obligations
under the relevant contracts by and between us and the investment bank. Shortly after our filing of such lawsuit, the investment
bank initiated arbitration proceedings against us with the Financial Industry Regulatory Authority (“FINRA”). Thereafter,
we requested the arbitration panel stay its proceeding in light of the existing lawsuit. The arbitration panel agreed with us and
on September 13, 2024, issued an order formally staying the arbitration proceeding.
Except as set out above, we are not involved in,
or aware of, any legal or administrative proceedings contemplated or threatened by any governmental authority or any other party. As
of the date of this prospectus supplement, no director, officer or affiliate is a party adverse to us in any legal proceeding or has an
adverse interest to us in any legal proceeding.
Convertible Note Development
We have reached an agreement with the holder
of our remaining convertible debenture, which has approximately $1.4 million in principal outstanding as of the date hereof, for the
repayment of such convertible debenture. Pursuant to this agreement, we would pay approximately $0.4 million on such convertible note
plus any accrued and unpaid interest thereon at or shortly after the successful conclusion of this offering and thereafter we would pay
$100,000 of the unpaid interest (plus a redemption premium of 8% and any accrued but unpaid interest) each month for ten months starting
on January 31, 2025. The holder of the convertible debenture has agreed that it will be unable to convert any portion of the convertible
debenture that remains outstanding until July 1, 2025, but it may thereafter utilize the conversion provisions of the convertible debenture.
As a result, we do not know the exact amount of the cash payments that we will have to make under the convertible debenture, but it will
range from a minimum of approximately $1.0 million (plus accrued and unpaid interest) to $1.5 million (plus accrued but unpaid interest).
The funds for such repayment will come from cash on hand, the net proceeds of this offering and, if applicable, any future financings.
IMPLICATIONS
OF BEING AN EMERGING GROWTH COMPANY
We
qualify as and elect to be an “emerging growth company” as defined in the Jumpstart our Business Startups Act of 2012,
or the JOBS Act. An emerging growth company may take advantage of specified reduced reporting and other burdens that are otherwise applicable
generally to public companies. These provisions include, but not limited to:
| ● | reduced
disclosure about the emerging growth company’s executive compensation arrangements
in our periodic reports, proxy statements and registration statements; and |
| ● | an
exemption from the auditor attestation requirement in the assessment of our internal control
over financial reporting pursuant to the Sarbanes-Oxley Act of 2002. |
We
may take advantage of these provisions for up to five years or such earlier time that we are no longer an emerging growth company.
We would cease to be an emerging growth company if we have more than $1.07 billion in annual gross revenue, have more than $700 million
in market value of our shares of ordinary shares held by non-affiliates or issue more than $1.0 billion of non-convertible debt
over a three-year period.
Implications
of Being a Foreign Private Issuer
We
are a foreign private issuer within the meaning of the rules under the Securities Exchange Act of 1934, as amended (the
“Exchange Act”). As such, we are exempt from certain provisions applicable to United States domestic public companies.
For example:
| ● | we
are not required to provide as many Exchange Act reports or provide periodic and current
reports as frequently, as a domestic public company; |
| ● | for
interim reporting, we are permitted to comply solely with our home country requirements,
which are less rigorous than the rules that apply to domestic public companies; |
| ● | we
are not required to provide the same level of disclosure on certain issues, such as executive
compensation; |
| ● | we
are exempt from provisions of Regulation FD aimed at preventing issuers from making
selective disclosures of material information; |
| ● | we
are not required to comply with the sections of the Exchange Act regulating the solicitation
of proxies, consents or authorizations in respect of a security registered under the Exchange Act;
and |
| ● | we
are not required to comply with Section 16 of the Exchange Act requiring insiders
to file public reports of their share ownership and trading activities and establishing insider
liability for profits realized from any “short-swing” trading transaction. |
Summary
Risk Factors
An investment in the offered securities involves
a high degree of risk. If any of the factors below or in the section entitled “Risk Factors” occurs, our business, financial
condition, liquidity, results of operations and prospects could be materially and adversely affected.
Risks
Related to Our Business Generally
| ● | We
are an early revenue stage company and have incurred operating losses since inception, and
we do not know when we will attain profitability. An investment in our securities is highly
risky and could result in a complete loss of your investment if we are unsuccessful in our
business plans. |
| ● | Terms
of subsequent financings may adversely impact your investment. |
| ● | Our
inability to manage growth could harm our business. |
| ● | We
substantially depend upon our management. |
| ● | Our
financial statements for the fiscal year ended December 31, 2023 include an explanatory paragraph
from our auditor indicating that there is substantial doubt about our ability to continue
as a going concern. |
| ● | You
may face difficulties protecting your interests, and your ability to protect your rights
through the U.S. federal courts may be limited because we are incorporated under the
laws of the Netherlands, a substantial portion of our assets are in the European Union and
substantial portion of our directors and executive officers reside outside the United States. |
Risks
Related to Our Technology and Business Strategy
| ● | We
may fail to generate sufficient revenue from our relationships with our clients or laboratory
partners to achieve and maintain profitability. |
| ● | Our
success depends heavily on our ColoAlert screening tests. |
| ● | Sales
of our diagnostic tests could be adversely impacted by the reluctance of physicians to adopt
the use of our tests and by the availability of competing diagnostic tests. |
| ● | We
may not succeed in establishing, maintaining and strengthening ColoAlert and other brands
associated with our products, which would materially and adversely affect acceptance of our
diagnostic tests, and our business, revenues and prospects. |
| ● | We
might decide not to incorporate the UdeS Biomarkers after we conclude additional studies
on such biomarkers. |
| ● | We
may face technology transfer challenges and expenses in adding new tests to our portfolio
and in expanding our reach into new geographical areas. |
| ● | We
may depend on possible future collaborations to develop and commercialize many of our diagnostic
test candidates and to provide the manufacturing, regulatory compliance, sales, marketing
and distribution capabilities required for the success of our business. |
| ● | If
we are unable to obtain and enforce patents and to protect our trade secrets, others could
use our technology to compete with us, which could create undue competition and pricing pressures.
There is no certainty that any future patent applications will result in the issuance of
patents or that issued patents, if we receive any, will be deemed enforceable. |
| ● | Results
of FDA required studies may not create desired clinical performance resulting in follow-on
studies delaying the launch of the product in the US. |
Risks
Related to Regulations
| ● | Our
global operations expose us to numerous and sometimes conflicting legal and regulatory requirements,
and violations of these requirements could harm our business. |
| ● | Our
business is subject to various complex laws and regulations. We could be subject to significant
fines and penalties if we or our partners fail to comply with these laws and regulations. |
| ● | We
will have to maintain facilities, or maintain relationships with third party laboratories,
for the manufacture and use of diagnostic tests. Our ability to provide services and pursue
our research and development and commercialization efforts may be jeopardized if these facilities
were to be harmed or rendered inoperable. |
| ● | We
anticipate being required to obtain regulatory approval of our diagnostic test products to
enter new markets. |
Risks Related to the Offered Securities
and this Offering
| ● | This
is a best-efforts offering, no minimum amount of securities is required to be sold and we
may not raise the amount of capital we believe is required for our business plans. |
| ● | The
market price of our ordinary shares may be volatile and may fluctuate in a way that is disproportionate
to our operating performance. |
| ● | You
will experience immediate and substantial dilution as a result of this offering. |
| ● | You
may experience dilution of your ownership interests if we issue additional ordinary shares
or preferred shares. |
| ● | We
do not intend to pay dividends, and there will thus be fewer ways in which you are able to
make a gain on your investment. |
| ● | We
do not intend to pay dividends, and there will thus be fewer ways in which you are able to
make a gain on your investment. |
| ● | FINRA
sales practice requirements may limit your ability to buy and sell our ordinary shares, which
could depress the price of our shares. |
| ● | Volatility
in our ordinary shares price may subject us to securities litigation. |
| ● | We
have been notified by Nasdaq that we are not in compliance with certain standards which Nasdaq
requires listed companies meet for their respective securities to continue to be listed and
traded on its exchange. If we are unable to regain compliance with such continued listing
requirements, Nasdaq may choose to delist our securities from its exchange or may subject
us to additional restrictions, which may adversely affect the liquidity and trading price
of our securities. |
| ● | In
an effort to regain compliance with the Minimum Bid Price Requirement, we enacted a reverse
stock split, and the public market may react negatively to this reverse stock split. |
| ● | We
have broad discretion in the use of the net proceeds from this offering and may not use them
effectively. |
| ● | There
is no public market for the pre-funded warrants, the Class A Warrants and the Class B Warrants
being offered in this offering. |
| ● | Holders
of the pre-funded warrants, the Class A Warrants and the Class B Warrants purchased in this
offering will have no rights as ordinary shareholders until such holders exercise such respective
warrants and acquire our ordinary shares, except as otherwise provided in the respective
warrants. |
Offering
Summary
Ordinary Units Offered:
|
|
1,147,776 ordinary units at an assumed offering price of $6.97 per
ordinary unit, the closing price of our ordinary shares on the Nasdaq Capital Market on December 6, 2024, rounded to the nearest whole
cent. Each ordinary unit consists of one ordinary share, one Class A Warrant and one Class B Warrant |
| |
|
|
Pre-Funded Units Offered:
|
|
Up
to 1,147,776 pre-funded units at an assumed offering price of $6.97 per pre-funded unit, the closing
price of our ordinary shares on the Nasdaq Capital Market on December 6, 2024, rounded to the
nearest whole cent minus $0.0001. We are offering to each purchaser of ordinary units that would
otherwise result in the purchaser’s beneficial ownership exceeding 4.99% of our outstanding
ordinary shares immediately following the consummation of this offering the opportunity to purchase
pre-funded units in lieu of ordinary units. Each pre-funded unit consists of one pre-funded warrant
to purchase an ordinary share, one Class A Warrant and one Class B Warrant.
For each pre-funded unit we sell (without
regard to any limitation on exercise set forth therein), the number of ordinary units we are offering will be decreased on a one-for-one
basis.
|
| Pre-Funded Warrants: |
|
Each pre-funded warrant will be exercisable for one ordinary share.
The remaining exercise price of each pre-funded warrant will equal $0.0001 per ordinary share. The pre-funded warrants will be immediately
exercisable (subject to the beneficial ownership cap) and may be exercised at any time until all of the pre-funded warrants are exercised
in full. |
| |
|
|
| Class A Warrants: |
|
Each Class A Warrant will be exercisable for one ordinary share. The exercise price of each Class
A Warrant will equal the per ordinary unit offering price hereunder, which we have assumed to be $6.97 based on the closing price
of our ordinary shares rounded to the nearest whole cent on the Nasdaq Capital Market on December 6, 2024. The Class A Warrants will
be immediately exercisable (subject to the beneficial ownership cap) and may be exercised until the fifth anniversary from the date
of issuance. |
| |
|
|
| Class B Warrants: |
|
Each Class B Warrant will be exercisable for one ordinary share. The exercise price of each Class
B Warrant will equal to the per ordinary unit offering price hereunder, which we have assumed to be $6.97 based on the closing price
of our ordinary shares rounded to the nearest whole cent on the Nasdaq Capital Market on December 6, 2024. The Class B Warrants will
be immediately exercisable (subject to the beneficial ownership cap) and may be exercised until the earlier of (i) the first anniversary
of the date of issuance or (ii) 30 days following the public disclosure of results from the eAArly Detect 2 study. |
| |
|
|
| Beneficial Ownership Limitation: |
|
A holder of pre-funded warrants, Class
A Warrants and Class B Warrants will not have the right to exercise any portion of those warrants if the holder, together with its
affiliates, would beneficially own in excess of 4.99% (or, at the election of the holder, 9.99%) of the number of ordinary shares
outstanding immediately after giving effect to such exercise.
|
| Shares Outstanding After the Offering*: |
|
3,149,276 |
| |
|
|
| Use of Proceeds: |
|
We estimate that we will receive net proceeds of approximately $7,125,000 from this offering, after deducting the placement agent
fees and estimated offering expenses of approximately $875,000 payable by us. However, because this is a best-efforts offering
and there is no minimum offering amount required as a condition to the closing of this offering, the actual offering amount, the
placement agent’s fees and net proceeds to us are not presently determinable and may be substantially less than the maximum
amounts set forth on the cover page of this prospectus.
We intend to use the net proceeds from
this offering for the eAArly Detect 2 study, the development of our next generation screening product, the commercial expansion of
our ColoAlert product, repayment of convertible debt and for general corporate purposes. |
| Market for the Offered Securities: |
|
Our
ordinary shares are currently listed on the Nasdaq Capital Market under the symbol “MYNZ”.
The closing price of our ordinary shares rounded to the nearest whole cent on the Nasdaq
Capital Market on December 6, 2024 was $6.97.
There is no market for any of our pre-funded
warrants, Class A Warrants or Class B Warrants, and we do not anticipate that one will develop for any of these securities. We do
not intend to apply for our pre-funded warrants, Class A Warrants or Class B Warrants to be traded on any public market or quotation
system.
|
| Risk Factors: |
|
See “Risk Factors” for a discussion of the factors you should consider before deciding to invest in our securities. |
| |
|
|
| Reasonable Best Efforts: |
|
We have agreed to offer and sell the securities offered hereby to the purchasers through the placement agent. The placement agent is not required to buy or sell any specific number or dollar amount of the securities offered hereby, but it will use its reasonable best efforts to solicit offers to purchase the securities offered by this prospectus. See “Plan of Distribution” on page 42 of this prospectus. |
| |
* |
Shares
outstanding after the offering (i) is based on 2,001,500 ordinary shares that are outstanding as of the date of this prospectus,
(ii) assumes an offering price of $6.97 per share, the closing price of our ordinary shares rounded to the nearest whole cent on
the Nasdaq Capital Market on December 6, 2024, (iii) assumes that any pre-funded warrants sold hereunder are immediately exercised
and (iv) excludes: |
| |
● |
up to 104,167 ordinary shares underlying warrants that are outstanding
as of the date hereof; |
| |
|
|
| |
● |
up to 2,295,552 ordinary shares
underlying the Class A Warrants and Class B Warrants that are being offered hereby; |
| ● | up
to 69,679 ordinary shares underlying options that we have granted as of the date hereof;
and |
| ● | up
to 348,122 ordinary shares underlying principal as of the date hereof under outstanding convertible
notes (assuming their conversion at the floor price contained in such notes). |
Summary
Financial Data
The
following tables summarize our financial data. We derived the summary financial statement data for the years ended December 31,
2023 and 2022 set forth below from our audited financial statements included in our Annual Report on Form 20-F filed on April 9, 2024
(each of which are incorporated by reference into this prospectus), and for the six months ended June 30, 2024 and 2023 from our unaudited
financial statements for the six months ended June 30, 2024 included in our report on Form 6-K filed on October 18, 2024 (which
are incorporated by reference into this prospectus). Our financial statements have been prepared in accordance with International Financial
Reporting Standards as issued by the International Accounting Standards Board. Our historical results are not necessarily indicative
of the results that may be expected in the future. You should read the information presented below together with “Management’s
Discussion and Analysis of Financial Condition and Results of Operations,” our financial statements, the notes to those statements
and the other financial information incorporated by reference into this prospectus.
Summary
of Operations in U.S. Dollars
| | |
Six Months
Ended
June 30, | | |
Years
Ended
December 31, | |
| | |
2024 | | |
2023 | | |
2023 | | |
2022 | |
| Revenues | |
$ | 520,773 | | |
$ | 499,049 | | |
$ | 895,479 | | |
$ | 529,877 | |
| Cost of Revenues | |
| 201,735 | | |
| 211,310 | | |
| 385,820 | | |
| 347,726 | |
| GROSS PROFIT | |
| 319,038 | | |
| 287,739 | | |
| 509,659 | | |
| 182,151 | |
| | |
| | | |
| | | |
| | | |
| | |
| OPERATING EXPENSES | |
| | | |
| | | |
| | | |
| | |
| Research and Development | |
| 3,242,622 | | |
| 5,481,229 | | |
| 9,590,393 | | |
| 5,019,366 | |
| Sales and marketing | |
| 2,361,105 | | |
| 3,992,975 | | |
| 6,158,477 | | |
| 6,396,906 | |
| General and administrative | |
| 4,522,639 | | |
| 5,227,181 | | |
| 11,405,471 | | |
| 15,209,919 | |
| Total operating expenses | |
| 10,126,366 | | |
| 14,701,385 | | |
| 27,154,341 | | |
| 26,626,191 | |
| Operating loss | |
| (9,807,328 | ) | |
| (14,413,646 | ) | |
| (26,644,682 | ) | |
| (26,444,040 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| OTHER INCOME/(EXPENSE) | |
| (1,216,434 | ) | |
| (398,997 | ) | |
| 348,955 | | |
| 56,094 | |
| | |
| | | |
| | | |
| | | |
| | |
| NET LOSS | |
$ | (11,023,762 | ) | |
$ | (14,812,643 | ) | |
| (26,295,727 | ) | |
| (26,387,336 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| TOTAL COMPREHENSIVE
LOSS | |
$ | (11,086,128 | ) | |
$ | (14,963,239 | ) | |
$ | (26,800,221 | ) | |
$ | (26,337,633 | ) |
Balance
Sheet in U.S. Dollars
| | |
As
of
June 30,
2024 (unaudited)
| | |
As
of December 31, 2023
(audited)
| |
| Cash | |
$ | 977,764 | | |
$ | 7,070,925 | |
| Total
Current Assets | |
| 2,389,703 | | |
| 8,979,896 | |
| Total
Assets | |
| 8,545,030 | | |
| 15,409,028 | |
| Total
Current Liabilities | |
| 10,013,944 | | |
| 9,236,936 | |
| Total
Liabilities | |
| 12,592,730 | | |
| 12,159,802 | |
Working
Deficit | |
$ | (7,624,241 | ) | |
$ | (257,040 | ) |
| Total
Stockholders’ Equity (Deficit) | |
| (4,138,700 | ) | |
| 3,249,226 | |
RISK
FACTORS
An investment in the offered securities
carries a significant degree of risk. You should carefully consider the following risks, as well as the other information contained in
this prospectus (or incorporated by reference herein), including risk factors in the documents incorporated herein by reference and our
historical financial statements and related notes incorporated by reference herein, before you decide to purchase the offered securities.
Any one of these risks and uncertainties has the potential to cause material adverse effects on our business, prospects, financial condition
and operating results which could cause actual results to differ materially from any forward-looking statements expressed by us and a
significant decrease in the value of the offered securities. Refer to “Special Note Regarding Forward-Looking Statements”.
We
may not be successful in preventing the material adverse effects that any of the following risks and uncertainties may cause. These potential
risks and uncertainties may not be a complete list of the risks and uncertainties facing us. There may be additional risks and uncertainties
that we are presently unaware of, or presently consider immaterial, that may become material in the future and have a material adverse
effect on us. You could lose all or a significant portion of your investment due to any of these risks and uncertainties.
Risks Related to the Offered Securities
and this Offering
This
is a best-efforts offering, no minimum amount of securities is required to be sold and we may not raise the amount of capital we believe
is required for our business plans.
The
placement agent has agreed to use its reasonable best efforts to solicit offers to purchase the securities being offered in this offering.
The placement agent has no obligation to buy any of the securities from us or to arrange for the purchase or sale of any specific number
or dollar amount of the securities. There is no required minimum number of securities or amount of proceeds that must be sold as a condition
to completion of this offering. Because there is no minimum offering amount required as a condition to the closing of this offering,
the actual offering amount, placement agent fees and proceeds to us are not presently determinable and may be substantially less than
the maximum amounts set forth above. We may sell fewer than all of the securities offered hereby, which may significantly reduce the
amount of proceeds received by us, and investors in this offering will not receive a refund in the event that we do not sell an amount
of securities sufficient to fund for our operations as described in the “Use of Proceeds” section herein. Thus, we may not
raise the amount of capital we believe is required for our operations in the short-term and even if we raise the maximum offering amount
in this public offering, we will need to raise additional funds in the future, which may not be available or available on terms acceptable
to us.
The
market price of our ordinary shares may be volatile and may fluctuate in a way that is disproportionate to our operating performance.
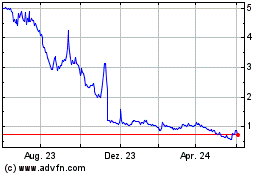
The public market for our ordinary shares
has a limited history. Our ordinary shares began trading on the Nasdaq Capital Market on November 5, 2021, and since that date they
have had a high closing price of $1,110.40 per share and a low closing price of $6.79 per share. The daily trading volume and our per
ordinary share market price may decrease significantly after the date of this annual report. The value of our ordinary shares could decline
due to the impact of any of the following factors upon the market price of our ordinary shares:
| ● | sales
or potential sales of substantial amounts of our ordinary shares; |
| ● | announcements
about us or about our competitors; |
| ● | litigation
and other developments relating to our intellectual property or other proprietary rights
or those of our competitors; |
| ● | conditions
in the diagnostic test industry; |
| ● | governmental
regulation and legislation; |
| |
● |
variations in our anticipated
or actual operating results; |
| |
● |
change in securities analysts’
estimates of our performance, or our failure to meet analysts’ expectations; |
| |
● |
change in general economic
trends; and |
| |
● |
investor perception of
our industry or our prospects. |
Many
of these factors are beyond our control. These fluctuations often have been unrelated or disproportionate to the operating performance
of these companies. As a consequence, there may be periods of several days or more when trading activity in our shares is minimal
or non-existent, as compared to a seasoned issuer which has a large and steady volume of trading activity that will generally support
continuous sales without an adverse effect on share price. A broad or active public trading market for our ordinary shares may not develop
or be sustained.
You
will experience immediate and substantial dilution as a result of this offering.
You will incur immediate and substantial dilution
as a result of this offering. After giving effect to the sale by us of 1,147,776 ordinary shares (assuming no pre-funded units are sold
in this offering) at an assumed public offering price of $6.97 per ordinary unit and after deducting the placement agent fees and estimated
offering expenses payable by us, investors in this offering can expect an immediate dilution of $2.74 per ordinary share on a pro forma
as adjusted basis (see Dilution).
In addition, you may experience further dilution)
upon the exercise of outstanding warrants, the Class A Warrants and the Class B Warrants being offered hereunder and outstanding options
and the conversion of outstanding convertible debt.
You
may experience dilution of your ownership interests if we issue additional ordinary shares or preferred shares.
In
the future, we may issue our authorized but previously unissued equity securities, resulting in the dilution of the ownership interests
of our present shareholders. Any such issuances could dilute your voting and economic interest. For example, as of December 6, 2024,
we had approximately $1,392,487 in principal and interest outstanding under convertible promissory notes. If all of such amounts were
to be converted at the floor price contained in such notes, we would issue approximately 348,122 ordinary shares, an amount that is equal
to 15% of the outstanding shares on the date hereof and 10% of the outstanding shares after this offering, assuming a per ordinary unit
price of $6.97 and that any pre-funded warrants issued hereunder are immediately exercised.
We
may issue additional ordinary shares or other securities that are convertible into or exercisable for ordinary shares in order to raise
additional capital, or in connection with hiring or retaining employees, directors, or consultants, or in connection with future acquisitions
of licenses to technology or diagnostic tests in connection with future business acquisitions, or for other business purposes. The future
issuance of any such additional ordinary shares or other securities, including those underlying the warrants and options we have issued
and granted, would dilute the voting power of our current shareholders, could dilute the net tangible book value per share at the time
of such future issuance and may create downward pressure on the trading price of our ordinary shares.
We
may also issue preferred shares having rights, preferences, and privileges senior to the rights of our ordinary shares with respect to
dividends, rights to share in distributions of our assets if we liquidate our company or voting rights. Any preferred shares may also
be convertible into ordinary shares on terms that would be dilutive to holders of ordinary shares.
We
do not intend to pay dividends, and there will thus be fewer ways in which you are able to make a gain on your investment.
We
have never paid any cash or stock dividends, and we do not intend to pay any dividends for the foreseeable future. To the extent that
we require additional funding currently not provided for in our financing plan, our funding sources may prohibit the payment of any dividends.
Because we do not intend to declare dividends, any gain on your investment will need to result from an appreciation in the price of our
ordinary shares. There will therefore be fewer ways in which you will be able to make a gain on your investment. Our articles of association
prescribe that any profits in any financial year will be distributed first to holders of preferred shares, if outstanding.
We
do not intend to pay dividends, and there will thus be fewer ways in which you are able to make a gain on your investment.
We
have never paid any cash or stock dividends, and we do not intend to pay any dividends for the foreseeable future. To the extent that
we require additional funding currently not provided for in our financing plan, our funding sources may prohibit the payment of any dividends.
Because we do not intend to declare dividends, any gain on your investment will need to result from an appreciation in the price of our
ordinary shares. There will therefore be fewer ways in which you will be able to make a gain on your investment. Our articles of association
prescribe that any profits in any financial year will be distributed first to holders of preferred shares, if outstanding.
FINRA
sales practice requirements may limit your ability to buy and sell our ordinary shares, which could depress the price of our shares.
FINRA
rules require broker-dealers to have reasonable grounds for believing that an investment is suitable for a customer before recommending
that investment to the customer. Prior to recommending speculative low-priced securities to their non-institutional customers, broker-dealers
must make reasonable efforts to obtain information about the customer’s financial status, tax status and investment objectives,
among other things. Under interpretations of these rules, FINRA believes that there is a high probability such speculative low-priced
securities will not be suitable for at least some customers. Thus, FINRA requirements may make it more difficult for broker-dealers to
recommend that their customers buy our ordinary shares, which may limit your ability to buy and sell our shares, have an adverse effect
on the market for our shares and, thereby, depress their market prices.
Volatility
in our ordinary shares price may subject us to securities litigation.
The
market for our ordinary shares may have, when compared to seasoned issuers, significant price volatility, and we expect that our share
price may continue to be more volatile than that of a seasoned issuer for the indefinite future. In the past, plaintiffs have often initiated
securities class action litigation against a company following periods of volatility in the market price of its securities. We may, in
the future, be the target of similar litigation. Securities litigation could result in substantial costs and liabilities and could divert
management’s attention and resources.
We
have been notified by Nasdaq that we are not in compliance with certain standards which Nasdaq requires listed companies meet for their
respective securities to continue to be listed and traded on its exchange. If we are unable to regain compliance with such continued
listing requirements, Nasdaq may choose to delist our securities from its exchange or may subject us to additional restrictions, which
may adversely affect the liquidity and trading price of our securities.
Our
securities are currently listed on Nasdaq. In May 2024, we received written notice (the “Notice”) from the Listing Qualifications
Department of Nasdaq notifying us that, based on the closing bid price of our ordinary shares for the last 30 consecutive trading days,
we no longer complied with the minimum bid price requirement for continued listing on the Nasdaq Capital Market. Nasdaq Listing Rule
5550(a)(2) requires listed securities to maintain a minimum bid price of $1.00 per share (the “Minimum Bid Price Requirement”),
and Nasdaq Listing Rule 5810(c)(3)(A) provides that a failure to meet the Minimum Bid Price Requirement exists if the deficiency continues
for a period of 30 consecutive trading days. Pursuant to the Nasdaq Listing Rules, we were provided an initial compliance period of 180
calendar days to regain compliance with the Minimum Bid Price Requirement. To regain compliance, the closing bid price of the ordinary
shares has to be at least $1.00 per share for a minimum of 10 consecutive trading days prior to November 25, 2024, and we must otherwise
satisfy The Nasdaq Capital Market’s requirements for listing.
On November 27, 2024,
we received a staff determination letter (the “Determination Letter”) from Nasdaq notifying us that we had not regained compliance
with the Minimum Bid Price Requirement by November 25, 2024, and are not eligible for a second 180-day period due to our failure to comply
with the minimum stockholders’ equity initial listing requirement for The Nasdaq Capital Market. Additionally, the Determination
Letter also noted that we no longer meet the minimum $2,500,000 minimum stockholders’ equity requirement for continued listing
on the Nasdaq Capital Market set forth in Listing Rule 5550(b)(1) based on our unaudited financial statements for the six-month period
ended June 30, 2024. Pursuant to Listing Rule 5810(d)(2), this deficiency is an additional basis for delisting.
The Determination
Letter sets out that, unless we request an appeal of this determination, by December 4, 2024, our securities will be scheduled for delisting
from The Nasdaq Capital Market and will be suspended at the opening of business on December 6, 2024. Prior to December 4, 2024, we intend
to request an appeal on the Determination Letter, which request will automatically stay any suspension or delisting action pending the
hearing and the expiration of any additional extension period granted by the Nasdaq Hearing Panel (the “Panel”) following
the hearing. In that regard, pursuant to the Nasdaq Listing Rules, the Panel has the authority to grant an extension not to exceed 180
days from the date of the Determination Letter.
We have taken steps
to regain compliance with the Minimum Bid Requirement, including holding an annual general meeting on November 13, 2024 at which our
shareholders approved a measure to enact a reverse stock split with a 1:2 to 1:100 ratio, such ratio to be determined by our board of
directors and enacting such reversed stock split with a ratio of 1:40 on December 3, 2024. Additionally, we believe that following the
completion of this offering (assuming that we sell all of the securities offered hereby), we believe that we will meet the minimum $2,500,000
minimum stockholders’ equity requirement for continued listing on the Nasdaq Capital Market. Despite these efforts, Nasdaq might
not grant us a discretionary extension of up to an additional 180 days. Even if it does, we might not be able to regain compliance within
that additional extension, and Nasdaq may move to delist our ordinary shares.
If
Nasdaq delists our ordinary shares from trading on its exchange and we are not able to list our ordinary shares on another national securities
exchange, our ordinary shares may be quoted on an over-the-counter market. However, if this were to occur, we could face significant
material adverse consequences, including:
| ● | a
limited availability of market quotations for our securities; |
| ● | reduced
liquidity for our securities; |
| ● | a
determination that our ordinary shares are a “penny stock”, which will require
brokers trading in such ordinary shares to adhere to more stringent rules and possibly result
in a reduced level of trading activity in the secondary trading market for our ordinary shares; |
| ● | a
limited amount of news and analyst coverage; and |
| ● | a
decreased ability to issue additional securities or obtain additional financing in the future. |
As
a result, an investor would likely find it more difficult to trade, or to obtain accurate price quotations for, our securities if our
securities are de-listed from Nasdaq. Delisting would likely also reduce the visibility, liquidity and value of our securities,
including as a result of reduced institutional investor interest in our company, and may increase the volatility of our securities.
In an effort to regain compliance with
the Minimum Bid Price Requirement, we enacted a reverse stock split, and the public market may react negatively to this reverse stock
split.
We enacted a 1-for-40 reverse stock split
of our ordinary shares on in an effort to regain compliance with Nasdaq’s Minimum Bid Price Requirement. To regain compliance,
the closing bid price of the ordinary shares has to be at least $1.00 per share for a minimum of 10 consecutive trading days and, at
Nasdaq’s discretion, up to 20 consecutive trading days. The reverse stock split might not be sufficient to regain compliance with
the Minimum Bid Price Requirement.
Regardless of whether the reverse stock split
achieves its goal of obtaining compliance with the Minimum Bid Price Requirement, the market price per ordinary share could drop as a
result of such reverse stock split. Historically, the market price of small-cap companies that enact reverse stock splits often decrease
significantly following such split due to fears of dilution irrespective of the performance, prospects, management or results of operation
of the company that enacted such reverse stock split. Our market price may drop significantly as a result of this reverse stock split.
We
have broad discretion in the use of the net proceeds from this offering and may not use them effectively.
Our
management will have broad discretion in the application of the net proceeds from this offering, including for any of the purposes described
in the section entitled “Use of Proceeds,” and you will not have the opportunity as part of your investment decision to assess
whether the net proceeds are being used appropriately. Because of the number and variability of factors that will determine our use of
the net proceeds from this offering, their ultimate use may vary substantially from their currently intended use. The failure by our
management to apply these funds effectively could harm our business.
There is no public market for the pre-funded
warrants, Class A Warrants or Class B Warrants being offered in this offering.
There is no established public trading market
for the pre-funded warrants, Class A Warrants or Class B Warrants being offered in this offering, and we do not expect a market to develop
for any of these securities. In addition, we do not intend to apply to list any of these warrants on any securities exchange or nationally
recognized trading system, including Nasdaq. Without an active market, the liquidity of these warrants will be limited.
Holders of the pre-funded warrants,
Class A Warrants and Class B Warrants purchased in this offering will have no rights as ordinary shareholders until such holders exercise
such warrants and acquire our ordinary shares, except as otherwise provided in such warrants.
Until holders of the pre-funded warrants,
Class A Warrants or Class B Warrants acquire shares of our ordinary shares upon exercise of such warrants, they will have no rights with
respect to our ordinary shares underlying such warrants. Upon exercise of such warrants, the holders will be entitled to exercise the
rights of an ordinary shareholder only as to matters for which the record date occurs after the exercise.
SPECIAL
NOTE REGARDING FORWARD-LOOKING STATEMENTS
This
prospectus and the documents incorporated by reference herein contain statements that constitute “forward-looking statements”.
Any statements that are not statements of historical facts may be deemed to be forward-looking statements. These statements appear in
a number of different places in this prospectus and the documents incorporated by reference herein and, in some cases, can be identified
by words such as “anticipates”, “estimates”, “projects”, “expects”, “contemplates”,
“intends”, “believes”, “plans”, “may”, “will”, or their negatives or other
comparable words, although not all forward-looking statements contain these identifying words. Forward-looking statements in this prospectus
and the documents incorporated by reference herein may include, but are not limited to, statements and/or information related to: strategy,
future operations, projected production capacity, projected sales or rentals, projected costs, expectations regarding demand and acceptance
of our products, availability of material components, trends in the market in which we operate, plans and objectives of management.
We
believe that we have based our forward-looking statements on reasonable assumptions, estimates, analysis and opinions made in light of
our experience and our perception of trends, current conditions and expected developments, as well as other factors that we believe to
be relevant and reasonable in the circumstances at the date that such statements are made, but which may prove to be incorrect. Although
management believes that the assumption and expectations reflected in such forward-looking statements are reasonable, we may have made
misjudgments in preparing such forward-looking statements. Assumptions have been made regarding, among other things: our expected production
capacity; labor costs and material costs, no material variations in the current regulatory environment and our ability to obtain financing
as and when required and on reasonable terms. Readers are cautioned that the foregoing list is not exhaustive of all factors and assumptions
which may have been used.
The
forward-looking statements, including the statements contained in the sections entitled Risk Factors, Description of Business and Management’s
Discussion and Analysis of Financial Conditions and Results of Operations and elsewhere in this prospectus and the documents incorporated
by reference herein, are subject to known and unknown risks, uncertainties and other factors that may cause actual results to be materially
different from those expressed or implied by such forward-looking statements.
Although
management has attempted to identify important factors that could cause actual results to differ materially from those contained in forward-looking
statements, there may be other factors that cause results not to be as anticipated, estimated or intended. Forward-looking statements
might not prove to be accurate, as actual results and future events could differ materially from those anticipated in such forward-looking
statements or we may have mad misjudgments in the course of preparing the forward-looking statements. Accordingly, readers should not
place undue reliance on forward-looking statements. We wish to advise you that these cautionary remarks expressly qualify, in their entirety,
all forward-looking statements attributable to our company or persons acting on our company’s behalf. We do not undertake to update
any forward-looking statements to reflect actual results, changes in assumptions or changes in other factors affecting such statements,
except as, and to the extent required by, applicable securities laws. You should carefully review the cautionary statements and risk
factors contained in this prospectus, the documents incorporated by reference herein and other documents that we may file from time to
time with the securities regulators.
USE
OF PROCEEDS
Based
upon an assumed offering price of $6.97 per ordinary unit (the last reported sale price rounded to the nearest whole cent of our ordinary
shares as reported on the Nasdaq Capital Market on December 6, 2024), we estimate that we will receive gross proceeds from this offering
of approximately $8,000,000, and net proceeds of approximately $7,125,000, after deducting placement agent fees and estimated offering
expenses of approximately $875,000 payable by us. However, because this is a best-efforts offering and there is no minimum offering amount
required as a condition to the closing of this offering, the actual offering amount, the placement agent’s fees and net proceeds
to us are not presently determinable and may be substantially less than the maximum amounts set forth on the cover page of this prospectus.
We intend to use the net proceeds from this
offering for the eAArly Detect 2 study, the development of our next generation screening product, the commercial expansion of our ColoAlert
product, repayment of convertible debt and for general corporate purposes. A payment of approximately $420,000 from the proceeds of this
offering will be made on the convertible debenture shortly after the closing. We also anticipate that subsequent monthly payments of
$100,000 towards the convertible debenture will also be made starting January 31, 2025, although there may be alternate sources of available
funds outside of this offering.
A $1.00 increase or decrease in the assumed public offering price
of $6.97 per ordinary unit would increase or decrease the net proceeds from this offering by approximately $1,067,432 assuming that the
number of ordinary units offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the estimated
placement agent fees and offering expenses payable by us. Similarly, each increase or decrease of 100,000 ordinary units offered would
increase or decrease our net proceeds by approximately $648,210, assuming the assumed public offering price remains the same, and after
deducting estimated placement agent fees and estimated offering expenses payable by us.
Pending
our use of the net proceeds from this offering, we may invest the net proceeds in a variety of capital preservation investments, including
short-term, investment grade, interest bearing instruments and U.S. government securities. As of the date of this prospectus, we
do not intend to use the proceeds from this offering to make principal payments, scheduled or early, on any of our outstanding debt,
however we may reassess this intention not to make additional payments on our outstanding debt immediately after the completion of this
offering.
DIVIDEND
POLICY
We
have not paid any dividends on our ordinary shares since incorporation. Our management anticipates that we will retain all future earnings
and other cash resources for the future operation and development of our business. We do not intend to declare or pay any cash dividends
in the foreseeable future. Payment of any future dividends will be at the Board’s discretion, subject to applicable law, after
taking into account many factors including our operating results, financial condition and current and anticipated cash needs.
Under
Dutch law, we may only pay dividends to the extent our shareholders’ equity (eigen vermogen) exceeds the sum of the paid-up
and called-up share capital plus the reserves required to be maintained by Dutch law or by our articles of association. As of June 30,
2024, our shareholders’ equity did not exceed this amount, and we do not anticipate it to do so in the near future.
Our
articles of association prescribe that profits in any financial year will be distributed first to holders of our preferred shares, if
any are outstanding. Any remaining profits may be reserved by our Board of Directors.
CAPITALIZATION
AND INDEBTEDNESS
The
following table sets forth the capitalization of Mainz Biomed N.V. on:
| |
● |
a pro forma
basis, giving effect since July 1, 2024 to (i) the issuance of a convertible note for net
proceeds of $1,340,000, and (ii) the conversion of $7,389,303 in principal and interest on
convertible notes and the receipt of net proceeds of $6,076,427, through the issuance of
1,281,836 ordinary shares; and |
| |
● |
a pro
forma, as adjusted basis, additionally giving effect to the sale by us of 1,147,776 ordinary units, at an assumed public offering
price of $6.97 per ordinary unit, after deducting placement agent fees and estimated offering expenses. |
The
pro forma and pro forma as adjusted information below is illustrative only and our capitalization following the completion of this offering
will be adjusted based on the actual public offering price and other terms of this offering determined at pricing. You should read this
table together with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our
audited financial statements and the related notes appearing elsewhere in this prospectus and the documents incorporated by reference
herein and our unaudited consolidated pro forma information appearing elsewhere in this prospectus and the documents incorporated by
reference herein.
| | |
As of June 30, 2024 | |
| | |
Actual | | |
Pro Forma | | |
Pro Forma,
as adjusted | |
| Cash | |
$ | 977,764 | | |
$ | 8,394,191 | | |
$ | 15,519,191 | |
| | |
| | | |
| | | |
| | |
| Debt: | |
| | | |
| | | |
| | |
| Convertible debt - related party | |
$ | 32,140 | | |
$ | 32,140 | | |
$ | 32,140 | |
| Convertible promissory note at fair value | |
| 5,842,003 | | |
| 52,591 | | |
| 52,591 | |
| Silent partnership | |
| 762,892 | | |
| 762,892 | | |
| 762,892 | |
| Silent partnership - related party | |
| 267,206 | | |
| 267,206 | | |
| 267,206 | |
| Total Debt | |
$ | 6,904,241 | | |
$ | 1,114,829 | | |
$ | 1,114,829 | |
| | |
| | | |
| | | |
| | |
| Stockholders’ Equity: | |
| | | |
| | | |
| | |
| Share capital | |
| 276,378 | | |
| 290,478 | | |
| 303,104 | |
| Share premium | |
| 54,136,785 | | |
| 67,588,415 | | |
| 74,700,789 | |
| Reserve | |
| 22,314,598 | | |
| 22,314,598 | | |
| 22,314,598 | |
| Accumulated deficit | |
| (80,351,783 | ) | |
| (80,611,674 | ) | |
| (80,611,674 | ) |
| Accumulated other comprehensive income
(loss) | |
| (514,678 | ) | |
| (514,678 | ) | |
| (514,678 | ) |
| Total Stockholders’ Equity | |
| (4,138,700 | ) | |
| 9,067,139 | | |
| 16,192,139 | |
| Total Capitalization | |
$ | 2,765,541 | | |
$ | 10,181,968 | | |
$ | 17,306,968 | |
The pro forma as adjusted information above
assumes the sale of all of the ordinary units offered hereby. Because this is a best-efforts offering and there is no minimum offering
amount required as a condition to the closing of this offering, the actual offering amount, the placement agent’s fees and net
proceeds to us are not presently determinable and may be substantially less than the maximum amounts set forth on the cover page of this
prospectus. As a result, the pro forma as adjusted information provided herein may be substantially different.
DILUTION
If you invest in the offered securities, your
interest in our ordinary shares will be diluted to the extent of the difference between the offering price per ordinary unit or pre-funded
unit and the as adjusted net tangible book value per ordinary share after the offering. Dilution results from the fact that the per ordinary
unit offering price is substantially in excess of the book value per ordinary share attributable to the existing shareholders for our
presently outstanding ordinary shares. Our net tangible book value attributable to shareholders at June 30, 2024 was $(7,344,754),
or approximately $(11.48) per ordinary share. Net tangible book value per ordinary share as of June 30, 2024 represents the amount
of total assets less intangible assets and total liabilities, divided by the number of ordinary shares outstanding.
After giving effect since July 1, 2024 to
(i) the issuance of a convertible note for net proceeds of $1,340,000, and (ii) the conversion of $7,389,303 in principal and interest
on convertible notes and the receipt of net proceeds of $6,076,427, through the issuance of 1,281,836 ordinary shares (the “Post-June 30,
2024 Issuances”), our pro forma net tangible book value as of June 30, 2024 would have been approximately $5,861,085, or approximately
$3.05 per ordinary share, based on 1,921,500 ordinary shares outstanding on a pro forma basis.
Our pro forma as adjusted net tangible book value of our ordinary shares
as of June 30, 2024 gives further effect to the assumed sale of 1,147,776 ordinary units at the assumed public offering price of
$6.97 per ordinary unit, after deducting the placement agent fees and estimated offering expenses. Our pro forma as adjusted net tangible
book value as of June 30, 2024, which gives effect to the net proceeds from the offering and the issuance of additional shares in
the offering but does not take into consideration any other changes in our net tangible book value after June 30, 2024, will be approximately
$12,986,085, or $4.23 per ordinary share. This would result in dilution to investors in this offering of approximately $2.74, or approximately
39% from the assumed offering price of $6.97 per ordinary unit. Pro forma as adjusted net tangible book value per ordinary share would
increase to the benefit of present shareholders by $15.71 per share attributable to the purchase of the ordinary shares by investors in
this offering.
The following table sets forth the estimated net
tangible book value per ordinary share after the offering and the dilution to persons purchasing ordinary shares.
| Assumed
offering price per ordinary unit |
|
$ |
|
|
|
$ |
6.97 |
|
| Pro
forma net tangible book value per ordinary share before the offering |
|
$ |
3.05 |
|
|
|
|
|
| Increase
per ordinary share attributable to this offering |
|
$ |
1.18 |
|
|
|
|
|
| Pro
forma as adjusted net tangible book value after the offering |
|
$ |
|
|
|
$ |
4.23 |
|
| Pro
forma as adjusted dilution per ordinary share to new investors in this offering |
|
$ |
|
|
|
$ |
2.74 |
|
If
any ordinary shares are issued upon exercise of outstanding warrants or options, you may experience further dilution.
The following table summarizes, as of December 6, 2024, the differences
between the number of ordinary shares purchased from us, the total cash consideration paid, and the average price per share paid by our
existing shareholders and by our new investors purchasing ordinary units in our public offering at the assumed public offering price of
$6.97 per ordinary unit, as disclosed on the cover page of this prospectus, before deducting estimated placement agent fees and estimated
offering expenses payable by us:
| | |
Shares Purchased | | |
Total Consideration | | |
Average | |
| | |
Number | | |
Percent | | |
Amount | | |
Percent | | |
Price Per
Share | |
| Existing shareholders | |
| 2,001,500 | | |
| 64 | % | |
| 67,878,893 | | |
| 89 | % | |
$ | 33.91 | |
| New investors | |
| 1,147,776 | | |
| 36 | % | |
| 8,000,000 | | |
| 11 | % | |
$ | 6.97 | |
| Total | |
| 3,149,276 | | |
| 100 | % | |
$ | 76,623,607 | | |
| 100 | % | |
| | |
The pro forma as adjusted information above
assumes the sale of all of the ordinary units offered hereby and the immediate exercise of any pre-funded warrants sold hereby. Because
this is a best-efforts offering and there is no minimum offering amount required as a condition to the closing of this offering, the
actual amount of securities sold hereby and net proceeds to us are not presently determinable and may be substantially less than the
amounts used to calculate the dilution information herein. As a result, the pro forma as adjusted information provided herein may be
substantially different.
For this section entitled “Dilution”,
we have allocated the entire per ordinary unit offering price to the ordinary share contained therein and have not allocated any value
to the Class A Warrant or the Class B Warrant.
MARKET
FOR OUR SECURITIES
Our
ordinary shares began trading on the Nasdaq Capital Market on November 4, 2021 under the symbol “MYNZ”. The following
table sets out the high and low bid price for our securities in each completed fiscal quarter since January 1, 2022 as quoted on
the Nasdaq Stock Market:
| | |
Ordinary Shares | |
| | |
High | | |
Low | |
| 2022 | |
| | |
| |
| Quarter Ended March 31 | |
$ | 1,110.40 | | |
$ | 412.00 | |
| Quarter Ended June 30 | |
$ | 620.00 | | |
$ | 356.40 | |
| Quarter Ended September 30 | |
$ | 462.40 | | |
$ | 244.40 | |
| Quarter Ended December 31 | |
$ | 375.60 | | |
$ | 247.20 | |
| | |
| | | |
| | |
| 2023 | |
| | | |
| | |
| Quarter Ended March 31 | |
$ | 294.00 | | |
$ | 243.20 | |
| Quarter Ended June 30 | |
$ | 260.00 | | |
$ | 128.00 | |
| Quarter Ended September 30 | |
$ | 196.00 | | |
$ | 115.60 | |
| Quarter Ended December 31 | |
$ | 127.60 | | |
$ | 40.80 | |
| | |
| | | |
| | |
| 2024 | |
| | | |
| | |
| Quarter Ended March 31 | |
$ | 48.00 | | |
$ | 35.20 | |
| Quarter Ended June 30 | |
$ | 45.20 | | |
$ | 15.20 | |
| Quarter Ended September 30 | |
$ | 21.20 | | |
$ | 8.00 | |
There
is no market for any of our pre-funded warrants, Class A Warrants or Class B Warrants, and we do not expect one to develop for any of
those securities. Upon completion of this offering, the ordinary units and the pre-funded units will automatically dissolve and separate
into their constituent parts without any further action required by the holder of the ordinary units or pre-funded units. Consequently,
no public market will ever develop for either the ordinary units or the pre-funded units.
SECURITIES
ELIGIBLE FOR FUTURE SALE
Ordinary
shares
As
of the date hereof, we have 2,001,500 ordinary shares outstanding, and upon completion of this offering, we will have 3,149,276 ordinary
shares outstanding assuming (i) the offering price per share is $6.97, which is the last reported sale price of our ordinary shares rounded
to the nearest whole cent on December 6, 2024 and (ii) that any pre-funded warrants sold hereby are immediately exercised. As of the
date hereof, there are 104,167 ordinary shares underlying warrants that are outstanding as of the date hereof, 69,679 ordinary shares
underlying options that we have granted as of the date hereof and 348,122 ordinary shares underlying principal under outstanding convertible
notes (assuming their conversion at the floor price contained in such notes). Upon the completion of this offering, there will be up
to 2,295,552 ordinary shares underlying the Class A Warrants and the Class B Warrants.
All of the ordinary shares sold in this offering
or to be issued upon the exercise of warrants sold in this offering will be freely transferable by persons other than by our “affiliates”
without restriction or further registration under the Securities Act. Sales of substantial amounts of our ordinary share in the public
market could adversely affect prevailing market prices of our ordinary share.
Rule 144
In
general, under Rule 144 as currently in effect, once we have been subject to public company reporting requirements for at least
90 days, a person who is not deemed to have been one of our affiliates for purposes of the Securities Act at any time during the
90 days preceding a sale and who has beneficially owned the shares proposed to be sold for at least six months, including the
holding period of any prior owner other than our affiliates, is entitled to sell those shares without complying with the manner of sale,
volume limitation or notice provisions of Rule 144, subject to compliance with the public information requirements of Rule 144.
If such a person has beneficially owned the shares proposed to be sold for at least one year, including the holding period of any prior
owner other than our affiliates, then that person is entitled to sell those shares without complying with any of the requirements of
Rule 144.
In
general, under Rule 144, as currently in effect, our affiliates or persons selling shares on behalf of our affiliates are entitled
to sell within any three-month period beginning 90 days after the date of this prospectus, a number of shares that does not exceed
the greater of:
| |
● |
1%
of the number of ordinary shares then outstanding, which will equal 31,493 shares immediately after this public offering, or |
| ● | the
average weekly trading volume of the ordinary shares during the four calendar weeks
preceding the filing of a notice on Form 144 with respect to such sale. |
Sales
under Rule 144 by our affiliates or persons selling shares on behalf of our affiliates are also subject to certain manner of sale
provisions and notice requirements and to the availability of current public information about us.
Rule 701
In
general, under Rule 701 as currently in effect, any of our employees, consultants or advisors who purchase shares from us in connection
with a compensatory stock or option plan or other written agreement in a transaction before the effective date of our initial public
offering that was completed in reliance on Rule 701 and complied with the requirements of Rule 701 will be eligible to resell
such shares 90 days after the date of this prospectus in reliance on Rule 144, but without compliance with certain restrictions,
including the holding period, contained in Rule 144.
ARTICLES
OF ASSOCIATION OF OUR COMPANY
The
following description of our Articles of Association, as amended by our Deed of Amendment on December 3, 2024 is intended as a summary
only and does not constitute legal advice regarding those matters and should not be regarded as such. The description is qualified in
its entirety by reference to the complete text of the Articles of Association.
Overview
We
were incorporated on March 8, 2021 as a private limited liability company (besloten vennootschap met beperkte aansprakelijkheid)
under Dutch law, and on November 9, 2021 we converted into a Dutch public company with limited liability (naamloze vennootschap).
We
are registered in the Commercial Register of the Chamber of Commerce (Kamer van Koophandel) in the Netherlands under number 82122571.
We have our corporate seat is in Amsterdam, the Netherlands and our registered office is at Robert-Koch Strasse 50, 55129 Mainz, Federal
Republic of Germany.
Our
ordinary shares are subject to, and have been created under, Dutch law. Set forth below is a summary of relevant information concerning
the material provisions of our articles of association and applicable Dutch law.
Board
of Directors
We
have a one-tier board structure. Our board of directors (the “Board of Directors”) consists of one executive director and
three non-executive directors. The Board of Directors shall consist of such number of executive Directors as the Board of Directors may
determine.
The
Board of Directors is charged with our management. In fulfilling their duties, our directors will serve our interest and the business
connected by us. The executive directors and the executive committee are charged with our day-to-day management. Supervision of the fulfilment
of duties by the executive directors and of the general course of our affairs and the business connected with us will primarily be carried
out by the non-executive directors. The executive directors must in due time provide the non-executive directors with the information
they need to carry out their duties.
Our
directors will be elected by the general meeting upon a binding nomination. The Board of Directors will be authorized to nominate one
or more director candidates for appointment at the general meeting. The general meeting may at all times overrule the binding nature
of each nomination by a resolution adopted by a majority of at least two thirds of the votes cast, representing more than half of the
issued share capital.
The
general meeting may at any time suspend and dismiss a non-executive director or executive director. The general meeting may only adopt
a resolution to suspend or dismiss a non-executive director or executive director by a majority of at least two thirds of the votes cast,
representing more than half of the issued share capital, unless the resolution is adopted on the basis of a proposal of the Board of
Directors.
The
following summary of the material terms of our securities is not intended to be a complete summary of the rights and preferences of such
securities and is qualified by reference to the Deed of Incorporation, the Articles of Association and the warrant-related documents
described herein, which are exhibits to the registration statement of which this prospectus is a part. We urge you to read each of the
Deed of Incorporation, the Articles of Association and the warrant-related documents described herein in their entirety for a complete
description of the rights and preferences of our securities.
Our
authorized share capital amounts to EUR 3,500,000.00 and consists of 7,875,000 ordinary shares with a nominal value of EUR 0.40 per share
and 875,000 preferred shares with a nominal value of EUR 0.40 per share. The preferred shares are divided into five series, each consisting
of 175,000 preferred shares. Currently there are no preferred shares outstanding. The Articles of Association contain a transitional
provision. If the number of issued ordinary shares first amounts to or exceeds 4,500,000, our authorized capital will amount to EUR 9,000,000.00
and consists of 20,250,000 ordinary shares with a nominal value of EUR 0.40 per share and 2,250,000 preferred shares with a nominal value
of EUR 0.40 per share. The preferred shares are divided into five series, each consisting of 450,000 preferred shares.
The
number of ordinary shares included in the authorized share capital may be decreased and the number of preferred shares included in the
authorized share capital may be increased pursuant to a resolution of the Board of Directors by a number not exceeding the number of
ordinary shares included in the authorized share capital which have not been issued and which are not subject to any rights to subscribe
for ordinary shares.
Shares
may be split into such number of fractional shares as the Board of Directors may determine and fractional shares together constituting
the nominal value of a share of a particular class, may be combined into one share of such class pursuant to a resolution of the Board
of Directors. The provisions of the Articles of Association on shares and shareholders shall apply by analogy to fractional shares and
holders of fractional shares, unless otherwise specified in the Articles of Association.
The
preferred shares may, at the request of the holder, be converted into ordinary shares. The conditions for conversion and the further
terms and conditions related to the preferred shares will be determined by our Board of Directors, subject to the prior approval of our
general meeting and the meeting of holders of the series of preferred shares concerned, if such series of preferred shares has been issued
and are held by persons other than us. The preceding sentence applies by analogy to any adjustment to the conditions.
Issuance
of shares
Under
Dutch law, shares are issued and rights to subscribe for shares are granted pursuant to a resolution of our general meeting. Our articles
of association provide that the general meeting may only resolve to issue shares upon the proposal of our Board of Directors. The general
meeting may authorize the Board of Directors to issue new ordinary shares or grant rights to subscribe for ordinary shares. The authorization
can be granted and extended, in each case for a period not exceeding five years. For as long as, and to the extent, that such authorization
is effective, our general meeting will not have the power to issue ordinary shares.
A
resolution of the general meeting has authorized our Board of Directors until November 9, 2026, to issue ordinary shares and preferred
shares up to the amount of the authorized share capital (from time to time).
Pre-emptive
Rights
Subject
to restrictions in our articles of association, holders of ordinary shares have pre-emptive rights in relation to newly issued ordinary
shares under Dutch law.
Under
our articles of association, the pre-emptive rights in respect of newly issued ordinary shares may be restricted or excluded by a resolution
of our general meeting, which resolution requires a two-thirds majority of the votes cast if less than half of the issued share capital
is present or represented at the meeting. The general meeting may authorize our Board of Directors to limit or exclude the pre-emptive
rights in respect of newly issued ordinary shares. Such authorization for our Board of Directors can be granted and extended, in each
case for a period not exceeding five years.
A
resolution of the general meeting has authorized our Board of Directors until November 9, 2026 to limit or exclude pre-emptive rights
on ordinary shares.
Pre-emptive
rights do not exist with respect (a) to the issue of ordinary shares or grant of rights to subscribe for ordinary shares to our
employees or a “group” company of ours, (b) the issue of ordinary shares against a contribution other than cash, and
(c) preferred shares to be issued. A holder of preferred shares has no pre-emptive right to acquire newly issued ordinary shares.
Transfer
of Ordinary Shares
Under
Dutch law, transfers of ordinary shares (other than in book-entry form) require a written deed of transfer and, unless we are a party
to the deed of transfer, and acknowledgement by or proper service upon us to be effective.
Our
articles of association provide that, if one or more ordinary shares or preferred shares are admitted to trading on Nasdaq or any other
regulated foreign stock exchange located in the United States the laws of the State of New York will apply to the property
law aspects of the ordinary shares and preferred shares included in the part of the register of shareholders kept by the relevant transfer
agent.
Form
of Ordinary Shares
The ordinary shares and preferred shares are
in registered form.
Purchase
and Repurchase of Ordinary Shares
Under
Dutch law, we may not subscribe for newly issued ordinary shares. We may acquire ordinary shares, subject to applicable provisions and
restrictions of Dutch law and our articles of association, to the extent that:
| |
● |
such ordinary shares are
fully paid-up; |
| |
|
|
| |
● |
such repurchase would not
cause our shareholders’ equity to fall below an amount equal to the sum of the paid-up and called-up part of the issued share
capital and the reserves we are required to maintain pursuant to Dutch law or our articles of association; and |
| |
|
|
| |
● |
immediately after the acquisition
of such ordinary shares, we and our subsidiaries would not hold, or would not hold as pledgees, shares having an aggregate nominal
value that exceeds 50% of our issued share capital. |
Other
than ordinary shares acquired for no valuable consideration or under universal title of succession (onder algemene titel) (e.g.,
through a merger or spin off) under statutory Dutch or other law, we may acquire ordinary shares only if our general meeting has authorized
our Board of Directors to do so. An authorization by our general meeting for the acquisition of ordinary shares can be granted for a
maximum period of 18 months. Such authorization must specify the number of ordinary shares that may be acquired, the manner in which
these shares may be acquired and the price range within which the shares may be acquired. No authorization of our general meeting is
required if ordinary shares are acquired by us on Nasdaq with the intention of transferring such ordinary shares to our employees or
employees of a group company pursuant to an arrangement applicable to them. For each annual general meeting, we expect that our Board
of Directors, will place on the agenda a proposal to re-authorize our Board of Directors to repurchase shares for a period of 18 months
from the date of the resolution. We cannot derive any right to any distribution from ordinary shares, or voting rights attached to ordinary
shares acquired by it.
A resolution of the general meeting, dated May
31, 2024, has authorized our Board of Directors until November 29, 2025 to acquire fully paid-up ordinary shares up to the maximum number
of ordinary shares permitted pursuant to the law and our articles of association from time to time, through privately negotiated repurchases,
in self-tender offers, or through accelerated repurchase arrangements, at prices ranging from the nominal value of the ordinary shares
up to one hundred and ten percent (110%) of the market price of ordinary shares, provided that (i) for open market or privately negotiated
repurchases, the market price will be the last closing price for ordinary shares on the Nasdaq Stock Market prior to the transaction,
(ii) for self-tender offers, the market price will be the volume weighted average price for the ordinary shares on the Nasdaq Capital
Market during a period, determined by the Board of Directors, of no less than one and no more than five consecutive trading days
immediately prior to the expiration of the tender offer, and (iii) for accelerated repurchase arrangements, the market price will
be the volume weighted average price of the ordinary shares on the Nasdaq Capital Market over the term of the arrangement. The volume
weighted average price for any number of trading days will be calculated as the arithmetic average of the daily volume weighted average
price on those trading days.
Pursuant
to a resolution of the general meeting, dated May 31, 2024, our Board of Directors is furthermore authorized until November 29, 2025
to acquire fully paid up preferred shares up to the maximum number of preferred shares permitted pursuant to the law and our articles
of association from time to time and that preferred shares may be acquired through privately negotiated repurchases, in self-tender offers,
or through accelerated repurchase arrangements, at prices ranging from the nominal value of the preferred shares up to the higher of
(i) the amount that would be paid by us upon cancellation of such preferred shares in accordance with the relevant provisions of
our articles of association and (ii) one hundred and ten percent (110%) of the market price of the ordinary shares into which the
preferred shares may be converted in accordance with the applicable provisions of our articles of association, whereby the market price
shall be determined in the manner as set out in our articles of association.
Capital
Reduction
At
a general meeting, our shareholders may resolve on the proposal of our Board of Directors to reduce our issued share capital by (i) cancelling
ordinary shares and preferred shares or (ii) reducing the nominal value of the ordinary shares and preferred shares by amending
our articles of association. In either case, this reduction would be subject to applicable statutory provisions. A resolution to cancel
shares may only relate to (i) shares held by us or in respect of which we hold the depository receipts, or (ii) all preferred
shares of a particular series. In order to be approved by our general meeting, a resolution to reduce the capital requires approval of
a majority of the votes cast at a general meeting if at least half of the issued share capital is represented at such meeting or at least
two thirds of the votes cast, if less than half of the issued share capital is represented at such meeting.
Reduction
of the nominal value of shares without repayment shall be effected proportionally to all ordinary shares and preferred shares. The requirement
of proportionality may be waived by agreement of all shareholders concerned.
A
resolution that would result in a reduction of capital requires approval by a majority of the votes cast of each group of shareholders
of the same class whose rights are prejudiced by the reduction. In addition, a reduction of capital involves a two-month waiting period
during which creditors have the right to object to a reduction of capital under specified circumstances.
General
Meeting
General
meetings are held in Amsterdam, Rotterdam, The Hague, Arnhem, Utrecht, or in the municipality of Haarlemmermeer (Schiphol Airport), the
Netherlands. All of our shareholders and others entitled to attend our general meetings are authorized to address the meeting and, in
so far as they have such right, to vote, either in person or by proxy.
We
will hold at least one general meeting each year, to be held within six months after the end of its financial year. A general meeting
will also be held within three months after our Board of Directors has determined it to be likely that our equity has decreased
to an amount equal to or lower than half of its paid up and called up capital, in order to discuss the measures to be taken if so required.
If our Board of Directors fails to hold such general meeting in a timely manner, each shareholder and other person entitled to attend
our general meeting may be authorized by the Dutch court to convene our general meeting.
Our
Board of Directors may convene additional extraordinary general meetings at its discretion, subject to the notice requirements described
below. Pursuant to Dutch law, one or more shareholders and/or others entitled to attend general meetings of shareholders, alone or jointly
representing at least 10% of our issued share capital, may on their application be authorized by the Dutch court to convene a general
meeting. The Dutch court will disallow the application if (i) the applicants have not previously requested in writing that our Board
of Directors convenes a shareholders’ meeting or (ii) our Board of Directors convenes a shareholders’ meeting or (iii) our
Board of Directors has not taken the necessary steps so that the shareholders’ meeting could be held within six weeks after
such request.
The
general meeting is convened by a notice, which includes an agenda stating the items to be discussed and the location and time of our
general meeting. For the annual general meeting the agenda will include, among other things, the adoption of our annual accounts, the
appropriation of its profits or losses and proposals relating to the composition of and filling of any vacancies on Board of Directors.
In addition, the agenda for a general meeting includes such additional items as determined by our Board of Directors. Pursuant to Dutch
law, one or more shareholders and/or others entitled to attend general meetings of shareholders, alone or jointly representing at least
3% of the issued share capital, have the right to request the inclusion of additional items on the agenda of shareholders’ meetings.
Such requests must be made in writing, and may include a proposal for a shareholder resolution, and must be received by us no later than
on the 60th day before the day the relevant shareholders’ meeting is held. Under our articles of association,
certain items can only be put on the agenda as a voting item by our Board of Directors. Shareholders meeting the relevant requirements
may still request the inclusion of such items on the agenda as a discussion item.
We
will give notice of each general meeting by publication on its website and, to the extent required by applicable law, in a Dutch daily
newspaper with national distribution, and in any other manner that we may be required to follow in order to comply with Dutch law and
applicable stock exchange and SEC requirements. We will observe the statutory minimum convening notice period for a general meeting.
Holders of registered shares may further be provided with notice of the meeting in writing at their addresses as stated in its shareholders’
register.
Pursuant
to our articles of association and Dutch law, our Board of Directors may determine a record date (registratiedatum) of 28 calendar days
prior to a general meeting to establish which shareholders and others with meeting rights are entitled to attend and, if applicable,
vote at our general meeting. The record date, if any, and the manner in which shareholders can register and exercise their rights will
be set out in the notice of our general meeting. Our articles of association provide that a shareholder must notify us in writing of
his or her intention to attend (or be represented at) our general meeting, such notice to be received by us on the date set by our Board
of Directors in accordance with our articles of association and as set forth in the convening notice.
Our
general meeting will be presided over by the chairman of our Board of Directors, who, nevertheless, may charge another person to preside
over the meeting in his place even if he or she is present at the meeting. If the chairman of our Board of Directors is absent and he
or she has not charged another person to preside over the meeting in his or her place, the directors present at the meeting will appoint
one of them to be chairman. In the absence of all directors, our general meeting will appoint its chairman.
Voting
Rights and Quorum
In
accordance with Dutch law and our articles of association, each ordinary share, irrespective of which class it concerns, confers the
right on the holder thereof to cast one vote at our general meeting. The voting rights attached to any ordinary shares held by us or
our direct or indirect subsidiaries are suspended, unless the ordinary shares were encumbered with a right of usufruct or a pledge in
favor of a party other than us or a direct or indirect subsidiary before such ordinary shares were acquired by us or such a subsidiary,
in which case, the other party may be entitled to exercise the voting rights on the ordinary shares. We may not exercise voting rights
for ordinary shares in respect of which its or a direct or indirect subsidiary has a right of usufruct or a pledge. Fractional shares
together constituting the nominal value of an ordinary share shall be put on par with such a share.
Voting
rights may be exercised by shareholders or by a duly appointed proxy holder (the written proxy being acceptable to the chairman of our
general meeting) of a shareholder, which proxy holder need not be a shareholder. The holder of a usufruct or pledge on shares will have
the voting rights attached thereto if so provided for when the usufruct or pledge was created.
Under
our articles of association, blank votes (votes where no choice has been made), abstentions and invalid votes will not be counted as
votes cast. However, shares in respect of which a blank vote or invalid vote has been cast and shares in respect of which the person
with meeting rights who is present or represented at the meeting has abstained from voting are counted when determining the part of the
issued share capital that is present or represented at a general meeting. The chairman of our general meeting will determine the manner
of voting and whether voting may take place by acclamation.
Resolutions
of the shareholders are adopted at a general meeting by an absolute majority of votes cast, except where Dutch law or our articles of
association provide for a special majority in relation to specified resolutions. Our articles of association do not provide for a quorum
requirement, subject to any provision of mandatory Dutch law.
Subject
to certain restrictions in our articles of association, the determination during our general meeting made by the chairman of that general
meeting with regard to the results of a vote will be decisive. Our Board of Directors will keep a record of the resolutions passed at
each general meeting.
Amendment
of Articles of Association
At
a general meeting, at the proposal of our Board of Directors, our general meeting may resolve to amend the articles of association. A
resolution by the shareholders to amend the articles of association requires an absolute majority of the votes cast.
Dissolution
and liquidation
Our
shareholders may at a general meeting, based on a proposal by our Board of Directors, by means of a resolution passed by an absolute
majority of the votes cast resolve that we will be dissolved. In the event of our dissolution, the liquidation will be effected by our
executive directors, under the supervision of our non-executive directors, unless our general meeting decides otherwise.
Certain
Other Major Transactions
Our
articles of association and Dutch law provide that resolutions of our Board of Directors concerning a material change in our identity,
character or business are subject to the approval of our general meeting. Such changes include:
| |
● |
a transfer of all or materially
all of its business to a third party; |
| |
|
|
| |
● |
the entry into or termination
of a long-lasting alliance of the company or of a subsidiary either with another entity or company, or as a fully liable partner
of a limited partnership or partnership, if this alliance or termination is of significant importance to the company; and |
| |
|
|
| |
● |
the acquisition or disposition
of an interest in the capital of a company by the company or by its subsidiary with a value of at least one third of the value of
our assets, according to the balance sheet with explanatory notes or, if the company prepares a consolidated balance sheet, according
to the consolidated balance sheet with explanatory notes in our most recently adopted annual accounts. |
Dividends
and Other Distributions
We
may only make distributions to its shareholders if our equity exceeds the aggregate amount of the issued share capital and the reserves
which must be maintained pursuant to Dutch law.
Under
our articles of association, any profits or distributable reserves must first be applied to pay a dividend on the preferred shares, if
outstanding. Any amount remaining out of distributable profits is added to our reserves as our Board of Directors determines. After reservation
by our Board of Directors of any distributable profits, our general meeting will be authorized to declare distributions on the proposal
of our Board of Directors. Our Board of Directors is permitted to declare interim dividends without the approval of the shareholders.
Interim dividends may be declared as provided in our articles of association and may be distributed to the extent that the shareholders’
equity, based on interim financial statements, exceeds the paid-up and called-up share capital and the reserves that must be maintained
under Dutch law or our articles of association. We may reclaim any distributions, whether interim or not interim, made in contravention
of certain restrictions of Dutch law from shareholders that knew or should have known that such distribution was not permissible. In
addition, on the basis of Dutch case law, if after a distribution we are not able to pay its due and collectable debts, then our shareholders
or directors who at the time of the distribution knew or reasonably should have foreseen that result may be liable to its creditors.
The
general meeting may determine that distributions will be made in whole or in part in the form of shares or a currency other than the
Euro, provided on the proposal of the Board of Directors. We shall announce any proposal for a distribution and the date when and the
place where the distribution will be payable to all shareholders by electronic means of communication with due observance of the applicable
law and stock exchange rules. Claims for payment of dividends and other distributions not made within five years from the date that
such dividends or distributions became payable will lapse, and any such amounts will be considered to have been forfeited to us (verjaring).
Transfer
Agent
We
have appointed Transhare Corporation as the transfer agent for our ordinary shares. Transhare Corporation’s telephone number and
address is (303) 662-1112 and 17755 US Hwy 19 N, Clearwater, FL 33764.
DESCRIPTION OF ORDINARY UNITS
We are offering up to 1,147,776 ordinary units
at an assumed offering price of $6.97 per ordinary unit, the closing price of our ordinary shares on the Nasdaq Capital Market on December
6, 2024, rounded to the nearest whole cent. Each ordinary unit consists of one ordinary share, one Class A Warrant and one Class B Warrant.
Upon completion of this offering, the ordinary units will dissolve and separate into their constituent parts without any further action
required by the holder of the ordinary units.
DESCRIPTION OF PRE-FUNDED UNITS
We are offering up to 1,147,776 pre-funded units at an assumed offering
price of $6.97 per pre-funded unit, the closing price of our ordinary shares on the Nasdaq Capital Market on December 6, 2024, rounded
to the nearest whole cent minus $0.0001. We are offering to each purchaser of ordinary units that would otherwise result in the purchaser’s
beneficial ownership exceeding 4.99% of our outstanding ordinary shares immediately following the consummation of this offering the opportunity
to purchase pre-funded units in lieu of ordinary units. Each pre-funded unit consists of one pre-funded warrant to purchase an ordinary
share, one Class A Warrant and one Class B Warrant. Upon completion of this offering, the pre-funded units will dissolve and separate
into their constituent parts without any further action required by the holder of the pre-funded units.
DESCRIPTION
OF PRE-FUNDED WARRANTS
The pre-funded warrants will be issued in physical
form directly by the Company to purchasers in this offering.
The following summary of certain terms and provisions
of the pre-funded warrants offered hereby is not complete and is subject to, and qualified in its entirety by, the provisions of the form
of pre-funded warrant, which is filed as an exhibit to the registration statement of which this prospectus is a part. Prospective investors
should carefully review the terms and provisions set forth in the form of pre-funded warrant.
Duration and Exercise Price
Each pre-funded warrant offered hereby will have
an initial exercise price per share equal to $0.001, will be immediately exercisable and can be exercised until all such pre-funded warrants
are exercised in full.
The exercise price and number of ordinary shares
issuable upon exercise of such pre-funded warrants are subject to appropriate adjustment in the event of stock dividends, stock splits,
reorganizations or similar events affecting our ordinary shares and the exercise price.
Exercisability
Each of the pre-funded warrants will be exercisable,
at the option of each holder of such pre-funded warrant, in whole or in part, by delivering a duly executed exercise notice accompanied
by payment in full for the number of our ordinary shares purchased upon such exercise (except in the case of a cashless exercise as discussed
below). A holder of such pre-funded warrant (together with its affiliates) may not exercise any portion of the pre-funded warrant to the
extent that the holder would own more than 4.99% (or at the election of the holder, 9.99%) of the outstanding ordinary shares immediately
after exercise, except that upon at least 61 days’ prior notice from the holder to us, the holder may increase the amount of ownership
of outstanding stock after exercising the holder’s pre-funded warrants. No fractional ordinary shares will be issued in connection
with the exercise of a pre-funded warrant. In lieu of fractional shares, the number of shares will be rounded down to the nearest whole
share.
Cashless Exercise
If, at the time a holder exercises its pre-funded
warrants, in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise
price, the holder thereof may elect instead to receive upon such exercise (either in whole or in part) the net number of pre-funded warrant
shares determined according to a formula set forth in such pre-funded warrant.
Fundamental Transaction
In the event of a fundamental transaction,
as described in the pre-funded warrants and generally including any reorganization, recapitalization or reclassification of our ordinary
shares, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger
with or into another person, the acquisition of more than 50% of our outstanding ordinary shares, or any person or group becoming the
beneficial owner of 50% of the voting power represented by our outstanding ordinary shares, the holders of the pre-funded warrants will
be entitled to receive upon exercise of such pre-funded warrants the kind and amount of securities, cash or other property that the holders
would have received had they exercised such pre-funded warrants immediately prior to such fundamental transaction.
Transferability
Subject to applicable laws, a pre-funded warrant
may be transferred at the option of the holder upon surrender of such pre-funded warrant together with the appropriate instruments of
transfer.
Exchange Listing
There is no established public trading market
for the pre-funded warrants, and we do not expect a market to develop. We do not intend to list the pre-funded warrants on any securities
exchange or nationally recognized trading system. Without an active trading market, the liquidity of the pre-funded warrants will be limited.
Right as a Shareholder
Except as otherwise provided in the pre-funded
warrants or by virtue of such holder’s ownership of our ordinary shares, the holders of the pre-funded warrants do not have the
rights or privileges of holders of our ordinary shares, including any voting rights, until they exercise their pre-funded warrants.
Amendment and Waiver
The pre-funded warrants may be modified or amended
or the provisions thereof waived with the written consent of the Company, and the holder of each such warrant.
Governing Law
The pre-funded warrants are governed by New York law.
DESCRIPTION OF CLASS A WARRANTS
The Class A Warrants will be issued in physical
form directly by the Company to purchasers in this offering.
The following summary of certain terms and
provisions of the Class A Warrants offered hereby is not complete and is subject to, and qualified in its entirety by, the provisions
of the form of Class A Warrant, which is filed as an exhibit to the registration statement of which this prospectus is a part. Prospective
investors should carefully review the terms and provisions set forth in the form of Class A Warrant.
Duration and Exercise Price
Each Class A Warrant offered hereby will have an initial exercise price
per share equal to the per ordinary unit offering price, which we have assumed to be $6.97 based on the closing price of our ordinary
shares rounded to the nearest whole cent on the Nasdaq Capital Market on December 6, 2024. Each Class A Warrant will be immediately exercisable
and may be exercised until the fifth anniversary of the date of issuance.
The exercise price and number of ordinary
shares issuable upon exercise of such Class A Warrants are subject to appropriate adjustment in the event of stock dividends, stock splits,
reorganizations or similar events affecting our ordinary shares and the exercise price.
Exercisability
Each of the Class A Warrants will be exercisable,
at the option of each holder of such warrant, in whole or in part, by delivering a duly executed exercise notice accompanied by payment
in full for the number of our ordinary shares purchased upon such exercise (except in the case of a cashless exercise as discussed below).
A holder of such Class A Warrant (together with its affiliates) may not exercise any portion of the Class A Warrant to the extent that
the holder would own more than 4.99% (or at the election of the holder, 9.99%) of the outstanding ordinary shares immediately after exercise,
except that upon at least 61 days’ prior notice from the holder to us, the holder may increase the amount of ownership of outstanding
stock after exercising the holder’s Class A Warrants. No fractional ordinary shares will be issued in connection with the exercise
of a Class A Warrant. In lieu of fractional shares, the number of shares will be rounded down to the nearest whole share.
Cashless Exercise
If, at the time a holder exercises its Class
A Warrants, in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate
exercise price, the holder thereof may elect instead to receive upon such exercise (either in whole or in part) the net number of Class
A Warrant shares determined according to a formula set forth in such Class A Warrant.
Fundamental Transaction
In the event of a fundamental transaction,
as described in the Class A Warrants and generally including any reorganization, recapitalization or reclassification of our ordinary
shares, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger
with or into another person, the acquisition of more than 50% of our outstanding ordinary shares, or any person or group becoming the
beneficial owner of 50% of the voting power represented by our outstanding ordinary shares, the holders of the Class A Warrants will
be entitled to receive upon exercise of such Class A Warrants the kind and amount of securities, cash or other property that the holders
would have received had they exercised such Class A Warrants immediately prior to such fundamental transaction. In the event of such
a fundamental transaction, the holders will have the option, which may be exercised within 30 days after the consummation of the fundamental
transaction, to require the company or the successor entity purchase such Class A Warrant from the holder by paying to the holder an
amount of cash equal to the Black Scholes Value (as defined in such Class A Warrant) of the remaining unexercised portion of such Class
A Warrant on the date of the consummation of such transaction. The consideration paid to the holder of such Class A Warrant shall be
the same type or form of consideration (and in the same proportion), valued at the Black Scholes Value of the unexercised portion of
such Class A Warrant, that is being offered and paid to the holders of Ordinary Shares of the Company in connection with such transaction,
whether that consideration be in the form of cash, stock or any combination thereof, or whether the holders of Ordinary Shares are given
the choice to receive from among alternative forms of consideration in connection with such fundamental transaction.
Transferability
Subject to applicable laws, a Class A Warrant
may be transferred at the option of the holder upon surrender of such Class A Warrant together with the appropriate instruments of transfer.
Exchange Listing
There is no established public trading market
for the Class A Warrants, and we do not expect a market to develop. We do not intend to list the Class A Warrants on any securities exchange
or nationally recognized trading system. Without an active trading market, the liquidity of the Class A Warrants will be limited.
Right as a Shareholder
Except as otherwise provided in the Class
A Warrants or by virtue of such holder’s ownership of our ordinary shares, the holders of the Class A Warrants do not have the
rights or privileges of holders of our ordinary shares, including any voting rights, until they exercise their Class A Warrants.
Amendment and Waiver
The Class A Warrants may be modified or amended
or the provisions thereof waived with the written consent of the Company, and the holder of each such warrant.
Governing Law
The Class A Warrants are governed by New York law.
DESCRIPTION OF CLASS B WARRANTS
The Class B Warrants will be issued in physical
form directly by the Company to purchasers in this offering.
The following summary of certain terms and
provisions of the Class B Warrants offered hereby is not complete and is subject to, and qualified in its entirety by, the provisions
of the form of Class B Warrant, which is filed as an exhibit to the registration statement of which this prospectus is a part. Prospective
investors should carefully review the terms and provisions set forth in the form of Class B Warrant.
Duration and Exercise Price
Each Class B Warrant offered hereby will have an initial exercise price
per share equal to the per ordinary unit offering price, which we have assumed to be $6.97 based on the closing price of our ordinary
shares rounded to the nearest whole cent on the Nasdaq Capital Market on December 6, 2024. Each Class B Warrant will be immediately exercisable
and may be exercised until the earlier of (i) first anniversary of the date of issuance or (ii) 30 days following the public disclosure
of results from the eAArly Detect 2 study.
The exercise price and number of ordinary
shares issuable upon exercise of such Class B Warrants are subject to appropriate adjustment in the event of stock dividends, stock splits,
reorganizations or similar events affecting our ordinary shares and the exercise price.
Exercisability
Each of the Class B Warrants will be exercisable,
at the option of each holder of such warrant, in whole or in part, by delivering a duly executed exercise notice accompanied by payment
in full for the number of our ordinary shares purchased upon such exercise (except in the case of a cashless exercise as discussed below).
A holder of such Class B Warrant (together with its affiliates) may not exercise any portion of the Class B Warrant to the extent that
the holder would own more than 4.99% (or at the election of the holder, 9.99%) of the outstanding ordinary shares immediately after exercise,
except that upon at least 61 days’ prior notice from the holder to us, the holder may increase the amount of ownership of outstanding
stock after exercising the holder’s Class B Warrants. No fractional ordinary shares will be issued in connection with the exercise
of a Class B Warrant. In lieu of fractional shares, the number of shares will be rounded down to the nearest whole share.
Cashless Exercise
If, at the time a holder exercises its Class
B Warrants, in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate
exercise price, the holder thereof may elect instead to receive upon such exercise (either in whole or in part) the net number of Class
B Warrant shares determined according to a formula set forth in such Class B Warrant.
Fundamental Transaction
In the event of a fundamental transaction, as described in the Class
B Warrants and generally including any reorganization, recapitalization or reclassification of our ordinary shares, the sale, transfer
or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into another person,
the acquisition of more than 50% of our outstanding ordinary shares, or any person or group becoming the beneficial owner of 50% of the
voting power represented by our outstanding ordinary shares, the holders of the Class B Warrants will be entitled to receive upon exercise
of such Class B Warrants the kind and amount of securities, cash or other property that the holders would have received had they exercised
such Class B Warrants immediately prior to such fundamental transaction. In the event of such a fundamental transaction, the holders will
have the option, which may be exercised within 30 days after the consummation of the fundamental transaction, to require the company or
the successor entity purchase such Class B Warrant from the holder by paying to the holder an amount of cash equal to the Black Scholes
Value (as defined in such Class B Warrant) of the remaining unexercised portion of such Class B Warrant on the date of the consummation
of such transaction. The consideration paid to the holder of such Class B Warrant shall be the same type or form of consideration (and
in the same proportion), valued at the Black Scholes Value of the unexercised portion of such Class B Warrant, that is being offered and
paid to the holders of Ordinary Shares of the Company in connection with such transaction, whether that consideration be in the form of
cash, stock or any combination thereof, or whether the holders of Ordinary Shares are given the choice to receive from among alternative
forms of consideration in connection with such fundamental transaction.
Transferability
Subject to applicable laws, a Class B Warrant
may be transferred at the option of the holder upon surrender of such Class B Warrant together with the appropriate instruments of transfer.
Exchange Listing
There is no established public trading market
for the Class B Warrants, and we do not expect a market to develop. We do not intend to list the Class B Warrants on any securities exchange
or nationally recognized trading system. Without an active trading market, the liquidity of the Class B Warrants will be limited.
Right as a Shareholder
Except as otherwise provided in the Class
B Warrants or by virtue of such holder’s ownership of our ordinary shares, the holders of the Class B Warrants do not have the
rights or privileges of holders of our ordinary shares, including any voting rights, until they exercise their Class B Warrants.
Amendment and Waiver
The Class B Warrants may be modified or amended
or the provisions thereof waived with the written consent of the Company, and the holder of each such warrant.
Governing Law
The Class B Warrants are governed by New York law.
MATERIAL
INCOME TAX INFORMATION
Material
Dutch Tax Income Tax Considerations
The
following are the material Dutch tax consequences of the acquisition, ownership, exercise and disposal of our securities. The term “securities”
as used in this discussion includes the ordinary shares, pre-funded warrants, Class A Warrants and Class B Warrants, as applicable. This
does not purport to set forth all possible tax considerations or consequences that may be relevant to all categories of investors, some
of which may be subject to special treatment under applicable law (such as trusts or other similar arrangements), and in view of its
general nature, it should be treated with corresponding caution. Holders or prospective holders of our securities should consult with
their tax advisors with regard to the tax consequences of investing in the securities in their particular circumstances.
Please
note that this section does not set forth the tax considerations for:
| |
● |
Holders of our securities
if such holders, and in the case of individuals, his/her partner or certain relatives by blood or marriage in the direct line (including
foster children), have a substantial interest (aanmerkelijk belang) or a deemed substantial interest (fictief aanmerkelijk
belang) in us under the Dutch Income Tax Act 2001 (Wet inkomstenbelasting 2001). A holder of an interest in a company
is considered to hold a substantial interest in such company if such holder alone or, in the case of individuals, together with his/her
partner (as defined in the Dutch Income Tax Act 2001), directly or indirectly holds (i) an interest of 5% or more of the
total issued and outstanding capital of that company or of 5% or more of the issued and outstanding capital of a certain class of
shares of that company; or (ii) rights to acquire, directly or indirectly, such interest; or (iii) certain profit sharing
rights in that company that relate to 5% or more of the company’s annual profits and/or to 5% or more of the company’s
liquidation proceeds. A deemed substantial interest may arise if a substantial interest (or part thereof) in a company has been disposed
of, or is deemed to have been disposed of, on a non-recognition basis; |
| |
● |
A holder of our securities
that is not an individual for which its shareholdings qualify or qualified as a participation (deelneming) for purposes of
the Dutch Corporate Income Tax Act 1969 (Wet op de vennootschapsbelasting 1969). A taxpayer’s shareholding of 5%
or more in a company’s nominal paid-up share capital or right to acquire such shareholding of 5% (or, in certain cases, in
voting rights) qualifies as a participation. A holder may also have a participation if such holder does not have a shareholding of
5% or more but a related entity (verbonden lichaam) has a participation or if the company in which the shares are held is
a related entity (verbonden lichaam); |
| |
● |
Holders of our securities
who are individuals for whom our securities or any benefit derived from our securities are a remuneration or deemed to be a remuneration
for (employment) activities performed by such holders or certain individuals related to such holders (as defined in the Dutch Income
Tax Act 2001); and |
| |
● |
Pension funds, investment
institutions (fiscale beleggingsinstellingen), exempt investment institutions (vrijgestelde beleggingsinstellingen)
and other entities that are, in whole or in part, not subject to or exempt from corporate income tax in the Netherlands, as well
as entities that are exempt from corporate income tax in their country of residence, such country of residence being another state
of the European Union, Norway, Liechtenstein, Iceland or any other state with which the Netherlands have agreed to exchange information
in line with international standards. |
Except
as otherwise indicated, this section only addresses Dutch national tax legislation and published regulations, whereby the Netherlands
and Dutch law means the part of the Kingdom of the Netherlands located in Europe and its law, respectively, as in effect on the date
hereof and as interpreted in published case law until this date, without prejudice to any amendment introduced (or to become effective)
at a later date and/or implemented with or without retroactive effect. The applicable tax laws or interpretations thereof may change,
or the relevant facts and circumstances may change, and such changes may affect the contents of this section, which will not be updated
to reflect any such changes.
Dividend
Withholding Tax
Holders
of ordinary shares are generally subject to Dutch dividend withholding tax at a rate of 15% on dividends distributed by us. We are required
to withhold such Dutch dividend withholding tax at source (which dividend withholding tax will not be borne by us but will be withheld
by us from the gross dividends paid on the ordinary shares). However, as long as we continue to have our place of effective management
in Germany, and not in the Netherlands, we will be considered to be solely tax resident in Germany for purposes of the Convention between
the Federal Republic of Germany and the Netherlands for the avoidance of double taxation and prevention of fiscal evasion with respect
to taxes on income (the “German-Dutch tax treaty”), and we will in principle not be required to withhold Dutch dividend withholding
tax. This exemption from withholding Dutch dividend withholding tax may not apply to dividends distributed by us to a holder who is resident
or deemed to be resident in the Netherlands for Dutch income tax purposes or Dutch corporate income tax purposes or to holders of ordinary
shares that are neither resident nor deemed to be resident of the Netherlands if the ordinary shares are attributable to a Dutch permanent
establishment of such non-resident holder, in which case the following paragraph applies.
Dividends
distributed by us to individuals and corporate legal entities who are resident or deemed to be resident in the Netherlands for Dutch
(corporate) income tax purposes (“Dutch Resident Individuals” and “Dutch Resident Entities,” as the case may
be) or to holders of ordinary shares that are neither resident nor deemed to be resident of the Netherlands if the ordinary shares are
attributable to a Dutch permanent establishment of such non-resident holder are generally subject to Dutch dividend withholding tax at
a rate of 15%. The expression “dividends distributed” include, but are not limited to:
| |
● |
Distributions in cash or
in kind, deemed and constructive distributions and repayments of paid-in capital not recognized for Dutch dividend withholding tax
purposes; |
| |
● |
Liquidation proceeds, proceeds
of redemption of shares, or proceeds of the repurchase of shares by us or one of our subsidiaries or other affiliated entities to
the extent such proceeds exceed the average paid-in capital of those shares as recognized for purposes of Dutch dividend withholding
tax, unless, in case of a repurchase, a particular statutory exemption applies; |
| |
● |
An amount equal to the
par value of shares issued or an increase of the par value of shares, to the extent that it does not appear that a contribution,
recognized for purposes of Dutch dividend withholding tax, has been made or will be made; and |
| |
● |
Partial repayment of the
paid-in capital, recognized for purposes of Dutch dividend withholding tax, if and to the extent that we have net profits (zuivere
winst), unless the holders of shares have resolved in advance at a general meeting to make such repayment and the par value of
the shares concerned has been reduced by an equal amount by way of an amendment of our articles of association. The term “net
profits” includes anticipated profits that have yet to be realized. |
Dutch
Resident Individuals and Dutch Resident Entities may credit the Dutch dividend withholding tax against their income tax or corporate
income tax liability (maximized to the amount of corporate income tax due in that financial year) or may under certain circumstances
be entitled to an exemption. The same applies to holders of ordinary shares that are neither resident nor deemed to be resident of the
Netherlands if the shares are attributable to a Dutch permanent establishment of such non-resident holder. Depending on their specific
circumstances, holders of ordinary shares that are resident in a country other than the Netherlands, may be entitled to exemptions from,
reduction of, or full or partial refund of, Dutch dividend withholding tax pursuant to Dutch law, EU law or treaties for avoidance of
double taxation.
As
of January 1, 2024, in addition to the (regular) Dutch dividend withholding tax, a conditional dividend withholding tax (the “Conditional
Dividend Withholding Tax”) is imposed on dividends paid to related entities in designated low-tax jurisdictions and in certain
abusive situations. If due, the Conditional Dividend Withholding Tax may be imposed at the highest Dutch corporate income tax rate in
effect at the time of the distribution (currently 25.8%), if the shareholder entitled to those dividend payments has such an interest
in us, possibly as part of a cooperating group, that such party can exert such influence on our decisions as to determine our activities,
while that shareholder is established in a jurisdiction that is included in the Regulation of low-taxing countries and non-cooperative
jurisdictions for tax purposes (Regeling laagbelastende staten en niet-coöperatieve rechtsgebieden voor belastingdoeleinden),
or has a relevant connection therewith. Any (regular) Dutch dividend withholding tax due in respect of the same dividend distribution
may be credited against the Conditional Dividend Withholding Tax.
However,
as long as we continue to have our place of effective management in Germany, and not in the Netherlands, we will be considered to be
solely tax resident in Germany for purposes of the German-Dutch tax treaty, and we will in principle not be required to withhold the
Conditional Dividend Withholding Tax.
Pursuant
to legislation to counteract “dividend stripping,” a reduction, exemption, credit or refund of Dutch dividend withholding
tax is not granted if the recipient of the dividend is not the beneficial owner (uiteindelijk gerechtigde) as described in the
Dutch Dividend Withholding Tax Act 1965 (Wet op de dividendbelasting 1965) of such dividends. This legislation targets situations
in which a shareholder retains its economic interest in shares but reduces the withholding tax costs on dividends by a transaction with
another party. It is not required for these rules to apply that the recipient of the dividends is aware that a dividend stripping transaction
took place. The Dutch State Secretary of Finance takes the position that the definition of beneficial ownership introduced by this legislation
will also apply in the context of a double taxation convention.
Pursuant
to additional measures introduced with effect from January 1, 2024, the burden of proof as regards the absence of dividend stripping
has been shifted to the taxpayer.
Taxes
on Income and Capital Gains
Dutch
Resident Individuals
If
a holder of our securities is a Dutch Resident Individual, any benefit derived or deemed to be derived from the securities is taxable
at the progressive income tax rates, if:
| |
(i) |
the securities are attributable
to an enterprise from which the Dutch Resident Individual derives a share of the profit, whether as an entrepreneur (ondernemer)
or as a person who has a co-entitlement to the net worth (medegerechtigd tot het vermogen) of such enterprise, without being
an entrepreneur or a shareholder in such enterprise, as defined in the Dutch Income Tax Act 2001; or |
| |
(ii) |
the
holder of our securities is considered to derive benefits from the securities that are taxable as
benefits from other activities (resultaat uit overige werkzaamheden), such as activities with
respect to the securities that go beyond ordinary asset management (normaal actief vermogensbeheer). |
If
the above-mentioned conditions (i) and (ii) do not apply to the individual holder of our securities, such Dutch Resident Individual
holder will be subject to an annual income tax imposed on a deemed return on the net value of the securities under the regime for savings
and investments (inkomen uit sparen en beleggen). Irrespective of the actual income and capital gains realized, the deemed annual
return of the Dutch Resident Individual’s net investment assets that are taxed under this regime, including the securities, is
set at variable percentages of the value of the investment assets and liabilities. For 2024, the variable percentages are set at 1.03%
for savings, at 6.04% for other investments (such as our securities) and at 2.47% for liabilities. Such fictitious annual return deemed
to be derived from our securities will be taxed at a flat rate of 36% in 2024.
The
net value of the investment assets for the year are the fair market value of the investment assets less the allowable liabilities on
January 1 of the relevant calendar year. The securities are included as investment assets. A tax-free allowance of €57,000
is available (2024). For the avoidance of doubt, actual income, capital gains or losses in respect of the ordinary shares are as such
not subject to Dutch income tax under the regime for savings and investments (inkomen uit sparen en beleggen). The deemed variable
return will be adjusted annually on the basis of historic market yields.
The
Dutch Government issued a draft legislative proposal for internet consultation on September 8, 2023 to introduce a new system regarding
the taxation of income from savings and investments as of the tax year 2027. Such new system will be based on actual returns realized
(such as dividends) and the value development of assets (such as a capital gain on shares or capital loss on shares). The Dutch cabinet
forwarded an amended legislative proposal to the Council of State for advice on June 14, 2024. At this stage, it remains uncertain whether
the authorities will be able to implement the new system by 2027.
On
June 6, 2024, the Dutch Supreme Court confirmed in several rulings that taxation under the current regime for savings and investments
violates Section 1 of the First Protocol to the European Convention on Human Rights in conjunction with Section 14 of the European Convention
on Human Rights, to the extent that the fictitious annual return to be recognized is higher than the actual return realized, calculated
in accordance with the rules set out in the recent decisions of the Dutch Supreme Court.
Dutch
resident individual holders of our securities are advised to consult their own tax advisor to ensure that the tax in respect of their
securities is levied in accordance with the applicable Dutch tax rules at the relevant time.
Dutch
Resident Entities
Any
benefit derived or deemed to be derived from the securities held by Dutch Resident Entities, including any capital gains realized on
the exercise or disposal thereof, will be subject to Dutch corporate income tax at a rate of 19% with respect to taxable profits up to
€200,000 and 25.8% with respect to taxable profits in excess of that amount (rates and brackets for 2024).
Non-residents
of the Netherlands
A
holder of our securities that is neither a Dutch Resident Individual nor a Dutch Resident Entity will not be subject to Dutch taxes on
income or on capital gains in respect of any payment under shares or any gain realized on the exercise, disposal or deemed disposal of
the securities or ordinary shares, provided that:
| ● | such
holder does not have an interest in an enterprise which, in whole or in part, is either effectively managed in the Netherlands or is
carried out through a permanent establishment, or a permanent representative in the Netherlands and to which enterprise or part of an
enterprise the securities are attributable; and |
| ● | in
the event such holder is an individual, such holder does not derive benefits from the securities that are taxable as benefits from other
activities in the Netherlands, such as activities in the Netherlands with respect to the shares that go beyond ordinary asset management. |
Under
certain specific circumstances, Dutch taxation rights may be restricted for a holder of our securities that is neither a Dutch Resident
Individual nor a Dutch Resident Entity pursuant to treaties for the avoidance of double taxation.
Gift
and Inheritance Taxes
Residents
of the Netherlands
Gift
or inheritance taxes will arise in the Netherlands with respect to a transfer of the securities by way of a gift by, or on the death
of, a holder of our securities who is resident or deemed to be resident in the Netherlands at the time of the gift or the holder’s
death.
Non-residents
of the Netherlands
No
Dutch gift or inheritance taxes will arise on the transfer of the securities by way of gift by, or on the death of, a holder of our securities
who is neither resident nor deemed to be resident in the Netherlands, unless:
| ● | in
the case of a gift of securities by an individual who at the date of the gift was neither resident nor deemed to be resident in the Netherlands,
such individual dies within 180 days after the date of the gift, while being resident or deemed to be resident in the Netherlands;
or |
| |
● |
the transfer is otherwise
construed as a gift, such as a gift that is made under a condition precedent, or inheritance made by, or on behalf of, a person who,
at the time of the gift or death, is or is deemed to be resident in the Netherlands. |
For
purposes of Dutch gift and inheritance taxes, a person that holds the Dutch nationality will be deemed to be resident in the Netherlands
if such person has been resident in the Netherlands at any time during the 10 years preceding the date of the gift or his/her death.
Additionally, for purposes of Dutch gift tax, any person, irrespective of his nationality will be deemed to be resident in the Netherlands
if such person has been resident in the Netherlands at any time during the 12 months preceding the date of the gift.
Other
Taxes and Duties
No
Dutch value-added tax (omzetbelasting) and no Dutch registration tax, stamp duty or any other similar documentary tax or duty
will be payable by a holder of our securities on any payment in consideration for the acquisition, ownership, exercise or disposal of
the securities.
Material
United States Tax Income Tax Considerations
Subject
to the limitations and qualifications stated herein, this discussion sets forth the material U.S. federal income tax considerations
relating to the acquisition, ownership and disposition by U.S. Holders (as defined below) of ordinary shares acquired pursuant to
this offering, the ownership, exercise and disposition of pre-funded warrants, Class A Warrants and Class B Warrants acquired pursuant
to this offering, and the ordinary shares received upon the exercise of such pre-funded warrants, Class A Warrants and Class B Warrants
(the “Warrant Shares”). The term “securities” as used in this discussion includes the ordinary shares, pre-funded warrants,
Class A Warrants, Class B Warrants and Warrant Shares, as applicable.
The
discussion is based on the U.S. Internal Revenue Code of 1986, as amended (the “Code”), its legislative history, existing
and proposed regulations thereunder, published rulings and court decisions, all as currently in effect and all subject to change at any
time, possibly with retroactive effect. This summary applies only to U.S. Holders and does not address tax consequences to a non-U.S. Holder
(as defined below) investing in securities.
This
discussion of a U.S. Holder’s tax consequences addresses only those persons that hold securities as capital assets and does
not address the tax consequences to any special class of holders, including without limitation, holders (directly, indirectly or constructively)
of 10% or more of our equity (based on value or voting power), dealers in securities or currencies, banks, tax-exempt organizations,
insurance companies, financial institutions, broker-dealers, regulated investment companies, real estate investment trusts, traders in
securities that elect the mark-to-market method of accounting for their securities holdings, persons that hold securities that are
a hedge or that are hedged against currency or interest rate risks or that are part of a straddle, conversion or “integrated”
transaction, persons required to accelerate the recognition of any item of gross income with respect to the ordinary shares as a result
of such income being recognized on an applicable financial statement, U.S. expatriates or former long-term residents of the
United States, partnerships or other pass-through entities for U.S. federal income tax purposes, U.S. Holders that
acquire securities in connection with the exercise of employee stock options or otherwise as compensation for services and U.S. Holders
whose functional currency for U.S. federal income tax purposes is not the U.S. dollar. This discussion does not address the
effect of the U.S. federal alternative minimum tax, U.S. federal estate and gift tax, alternative minimum tax, the 3.8% Medicare
contribution tax on net investment income or any state, local or non-U.S. tax laws applicable to a holder of securities. This discussion
does not take into account the individual facts and circumstances of any particular U.S. Holder that may affect the U.S. federal
income tax consequences to such U.S. Holder, including specific tax consequences to a U.S. Holder under an applicable tax treaty.
Accordingly, this summary is not intended to be, and should not be construed as, legal or U.S. federal income tax advice with respect
to any particular U.S. Holder. Each U.S. Holder should consult its own tax advisor regarding the U.S. federal, U.S. state
and local, U.S. federal estate and gift, alternative minimum, and non-U.S. tax consequences of the acquisition, ownership and
disposition of the securities.
For
purposes of this discussion, a “U.S. Holder” is a beneficial owner of securities acquired pursuant to this offering
that is for U.S. federal income tax purposes: (a) an individual who is a citizen or resident of the United States; (b) a
corporation (or other entity taxable as a corporation for U.S. federal income tax purposes) created or organized in or under the
laws of the United States, any state thereof or the District of Columbia; (c) an estate the income of which is subject to U.S. federal
income taxation regardless of its source; or (d) a trust (i) if a court within the United States can exercise primary
supervision over its administration, and one or more U.S. persons have the authority to control all of the substantial decisions
of that trust, or (ii) that has a valid election in effect under applicable Treasury regulations to be treated as a U.S. person.
The term “non-U.S. Holder” means any beneficial owner of securities acquired pursuant to this offering that is not a
U.S. Holder, a partnership (or an entity or arrangement that is treated as a partnership or other pass-through entity for U.S. federal
income tax purposes) or a person holding securities through such an entity or arrangement.
If
a partnership or an entity or arrangement that is treated as a partnership for U.S. federal income tax purposes holds securities,
the tax treatment of a partner generally will depend upon the status of the partner and the activities of the partnership. Partners in
partnerships that hold securities should consult their own tax advisors. You are urged to consult your own independent tax advisor
regarding the specific U.S. federal, state, local and non-U.S. income and other tax considerations relating to the acquisition,
ownership and disposition of securities.
U.S. Federal
Income Tax Consequences of the Acquisition, Ownership, and Disposition of Ordinary Shares, Pre-Funded Warrants, Class A Warrants, Class
B Warrants and Warrant Shares
The
following discussion is subject in its entirety to the rules described below under the heading “Passive Foreign Investment Company
Considerations.”
Cash
Dividends and Other Distributions
As
described in the section entitled “Dividend Policy” above, we currently intend to retain any future earnings to fund business
development and growth, and we do not expect to pay any dividends in the foreseeable future. However, to the extent there are any distributions
(including constructive distributions) made with respect to an ordinary share, pre-funded warrant, Class A Warrant, Class B Warrant
or Warrant Share, a U.S. Holder generally will be required to include the amount of such distribution in gross income (including
the amount of Dutch taxes withheld, if any) as dividend income to the extent of our current and accumulated earnings and profits (computed
using U.S. federal income tax principles). A dividend generally will be taxed to a U.S. Holder at ordinary income tax rates if the
Company is a PFIC for the tax year of such distribution or the preceding tax year. To the extent that a distribution exceeds our current
and accumulated “earnings and profits,” such distribution will be treated first as a non-taxable return of capital to
the extent of the holder’s adjusted tax basis in such securities and, thereafter, as gain from the sale or exchange of such securities
(see “Sale or Disposition” below). There can be no assurance that we will maintain calculations of our earnings and profits
in accordance with U.S. federal income tax accounting principles. U.S. Holders should therefore assume that any distribution
with respect to the securities will constitute ordinary dividend income. Dividends paid on such securities generally will not be eligible
for the dividends received deduction generally allowed to U.S. corporations.
Dividends
paid to a non-corporate U.S. Holder by a “qualified foreign corporation” may be subject to reduced rates of taxation
if certain holding period and other requirements are met. A qualified foreign corporation generally includes a foreign corporation (other
than a foreign corporation that is a PFIC in the taxable year in which the dividend is paid or the preceding taxable year) if (i) its
securities are readily tradable on an established securities market in the United States or (ii) it is eligible for benefits
under a comprehensive U.S. income tax treaty that includes an exchange of information program and which the U.S. Treasury Department
has determined is satisfactory for these purposes. Our ordinary shares (which would include Warrant Shares) are readily tradable on an
established securities market in the United States, the Nasdaq. However, none of the pre-funded warrants, Class A Warrants
or Class B Warrants is readily tradable on an established securities market.
Non-corporate U.S. Holders
will not be eligible for reduced rates of taxation on any dividends received from us if we are a PFIC in the taxable year in which such
dividends are paid or in the preceding taxable year.
A
U.S. Holder who pays (whether directly or through withholding) Dutch taxes with respect to dividends paid on our securities (or
with respect to any constructive dividend on the pre-funded warrants, Class A Warrants or Class B Warrants) may be entitled to receive,
at the election of such U.S. Holder, either a deduction or a foreign tax credit for such taxes paid. Complex limitations apply to the
foreign tax credit, including the general limitation that the credit cannot exceed the proportionate share of a U.S. Holder’s
U.S. federal income tax liability that such U.S. Holder’s “foreign source” taxable income bears to such U.S. Holder’s
worldwide taxable income. In applying this limitation, a U.S. Holder’s various items of income and deduction must be classified,
under complex rules, as either “foreign source” or “U.S. source.” In addition, this limitation is calculated
separately with respect to specific categories of income. Dividends paid by us generally will constitute “foreign source”
income and generally will be categorized as “passive category income.” However, if 50% or more of our equity (based on voting
power or value) is treated as held by U.S. persons, we will be treated as a “United States-owned foreign corporation,”
in which case dividends may be treated for foreign tax credit limitation purposes as “foreign source” income to the extent
attributable to our non-U.S. source earnings and profits and as “U.S. source” income to the extent attributable
to our U.S. source earnings and profits. Because the foreign tax credit rules are complex, each U.S. Holder should consult
its own tax advisor regarding the foreign tax credit rules.
Sale
or Disposition
Subject
to the PFIC rules discussed below, a U.S. Holder generally will recognize gain or loss on the taxable sale or exchange of its ordinary
shares, pre-funded warrants, Class A Warrants, Class B Warrants or Warrant Shares in an amount equal to the difference between the
U.S. dollar amount realized on such sale or exchange (determined in the case of securities sold or exchanged for currencies other
than U.S. dollars by reference to the spot exchange rate in effect on the date of the sale or exchange or, if the securities sold
or exchanged are traded on an established securities market and the U.S. Holder is a cash basis taxpayer or an electing accrual
basis taxpayer, the spot exchange rate in effect on the settlement date) and the U.S. Holder’s adjusted tax basis in the securities
sold or otherwise disposed of determined in U.S. dollars.
Assuming
we are not a PFIC and have not been treated as a PFIC during your holding period for our securities, such gain or loss will be capital
gain or loss and will be long-term gain or loss if the applicable securities have been held for more than one year. Under current
law, long-term capital gains of non-corporate U.S. Holders generally are eligible for reduced rates of taxation. The deductibility
of capital losses is subject to limitations. Capital gain or loss, if any, recognized by a U.S. Holder generally will be treated
as U.S. source income or loss for U.S. foreign tax credit purposes. Consequently, a U.S. Holder may not be able to use
the foreign tax credit arising from any Dutch tax imposed on the disposition of a security unless such credit can be applied (subject
to applicable limitations) against tax due on other income treated as derived from foreign sources. U.S. Holders are encouraged
to consult their own tax advisors regarding the availability of the U.S. foreign tax credit in their particular circumstances.
Passive
Foreign Investment Company Considerations
Status
as a PFIC
The
rules governing PFICs can have adverse tax effects on U.S. Holders. We generally will be classified as a PFIC for U.S. federal
income tax purposes if, for any taxable year, either: (1) 75% or more of our gross income consists of certain types of passive income,
or (2) the average value (determined on a quarterly basis), of our assets that produce, or are held for the production of, passive
income is 50% or more of the value of all of our assets.
For
purposes of the PFIC provisions, “gross income” generally means sales revenues less cost of goods sold, plus income from
investments and from incidental or outside operations or sources. Passive income generally includes dividends, interest, rents and royalties
(other than certain rents and royalties derived in the active conduct of a trade or business), annuities and gains from assets that produce
passive income. If a non-U.S. corporation owns at least 25% by value of the stock of another corporation, the non-U.S. corporation
is treated for purposes of the PFIC tests as owning its proportionate share of the assets of the other corporation and as receiving directly
its proportionate share of the other corporation’s income.
Additionally,
if we are classified as a PFIC in any taxable year with respect to which a U.S. Holder owns securities, we generally will continue
to be treated as a PFIC with respect to such U.S. Holder in all succeeding taxable years, regardless of whether we continue
to meet the tests described above, unless the U.S. Holder makes the “deemed sale election” described below.
We
do not believe that we are currently a PFIC, and we do not anticipate becoming a PFIC in the foreseeable future. Notwithstanding the
foregoing, the determination of whether we are a PFIC is made annually and depends on the particular facts and circumstances (such as
the valuation of our assets, including goodwill and other intangible assets) and also may be affected by the application of the PFIC
rules, which are subject to differing interpretations. The fair market value of our assets is expected to depend, in part, upon (a) the
market price of our ordinary shares, which is likely to fluctuate, and (b) the composition of our income and assets, which will
be affected by how, and how quickly, we spend any cash that is raised in any financing transaction, including this offering. In light
of the foregoing, no assurance can be provided that we are not currently a PFIC or that we will not become a PFIC in any future taxable
year. Prospective investors should consult their own tax advisors regarding our potential PFIC status.
U.S. Federal
Income Tax Treatment of a Shareholder of a PFIC
If
we are classified as a PFIC for any taxable year during which a U.S. Holder owns securities, the U.S. Holder, absent certain
elections (including the mark-to-market and QEF elections described below), generally will be subject to adverse rules (regardless
of whether we continue to be classified as a PFIC) with respect to (i) any “excess distributions” (generally, any distributions
received by the U.S. Holder on its securities in a taxable year that are greater than 125% of the average annual distributions received
by the U.S. Holder in the three preceding taxable years or, if shorter, the U.S. Holder’s holding period for its
securities) and (ii) any gain realized on the sale or other disposition, including a pledge, of its securities.
Under
these adverse rules (a) the excess distribution or gain will be allocated ratably over the U.S. Holder’s holding period,
(b) the amount allocated to the current taxable year and any taxable year prior to the first taxable year in which we are classified
as a PFIC will be taxed as ordinary income, (c) the amount allocated to each other taxable year during the U.S. Holder’s
holding period in which we were classified as a PFIC (i) will be subject to tax at the highest rate of tax in effect for the applicable
category of taxpayer for that year and (ii) will be subject to an interest charge at a statutory rate with respect to the resulting
tax attributable to each such other taxable year, and (d) loss recognized on the disposition of the securities will not be deductible.
If
we are classified as a PFIC, a U.S. Holder generally will be treated as owning a proportionate amount (by value) of stock or shares
owned by us in any direct or indirect subsidiaries that are also PFICs and will be subject to similar adverse rules with respect to any
distributions we receive from, and dispositions we make of, the stock or shares of such subsidiaries. You are urged to consult your tax
advisors about the application of the PFIC rules to any of our subsidiaries.
If
we are classified as a PFIC and then cease to be so classified, a U.S. Holder may make an election (a “deemed sale election”)
to be treated for U.S. federal income tax purposes as having sold such U.S. Holder’s ordinary shares, pre-funded warrants,
Class A Warrants, Class B Warrants or Warrant Shares on the last day our taxable year during which we were a PFIC. A U.S. Holder
that makes a deemed sale election with respect to such securities would then cease to be treated as owning stock in a PFIC by reason
of ownership of our ordinary shares, pre-funded warrants, Class A Warrants, Class B Warrants or Warrant Shares. However, gain recognized
as a result of making the deemed sale election would be subject to the adverse rules described above and loss would not be recognized.
PFIC
“Mark-to-Market” Election
In
certain circumstances, a U.S. Holder can avoid certain of the adverse rules described above by making a mark-to-market election
with respect to its ordinary shares and Warrant Shares, provided that such shares are “marketable.” The ordinary shares and Warrant
Shares generally will be marketable if they are “regularly traded” on certain U.S. stock exchanges or on a foreign stock
exchange that meets certain conditions. For these purposes, the ordinary shares and Warrant Shares will be considered regularly
traded during any calendar year during which they are traded, other than in de minimis quantities, on at least 15 days during each
calendar quarter. Any trades that have as their principal purpose meeting this requirement will be disregarded. Our ordinary shares (which
include Warrant Shares) are listed on the Nasdaq, which is a qualified exchange for these purposes. Consequently, if our ordinary
shares and Warrant Shares remain listed on the Nasdaq and are regularly traded, and you are a holder of ordinary shares or Warrant
Shares, we expect the mark-to-market election would be available to you if we are a PFIC. There can be no assurance that the shares
will be “regularly traded” in subsequent calendar quarters. You should consult your own tax advisor as to the whether
a mark-to-market election is available or advisable with respect to the ordinary shares and the Warrant Shares. A mark-to-market election
may not be available with respect to the pre-funded warrants, Class A Warrants or Class B Warrants.
A
U.S. Holder that makes a mark-to-market election must include in gross income, as ordinary income, for each taxable year that
we are a PFIC an amount equal to the excess, if any, of the fair market value of the U.S. Holder’s ordinary shares, pre-funded warrants,
Class A Warrants, Class B Warrants and any Warrant Shares at the close of the taxable year over the U.S. Holder’s adjusted
tax basis in such securities. An electing U.S. Holder may also claim an ordinary loss deduction for the excess, if any, of the U.S. Holder’s
adjusted tax basis in its ordinary shares, pre-funded warrants, Class A Warrants, Class B Warrants and any Pre-Funded Warrant
Shares over the fair market value of such securities at the close of the taxable year, but this deduction is allowable only to the extent
of any net mark-to-market gains previously included in income. A U.S. Holder that makes a mark-to-market election generally
will adjust such U.S. Holder’s tax basis in its ordinary shares, pre-funded warrants, Class A Warrants, Class B Warrants
and Pre-Funded Warrant Shares to reflect the amount included in gross income or allowed as a deduction because of such mark-to-market election.
Gains from an actual sale or other disposition of such securities in a year in which we are a PFIC will be treated as ordinary income,
and any losses incurred on a sale or other disposition of such securities will be treated as ordinary losses to the extent of any net
mark-to-market gains previously included in income.
If
we are classified as a PFIC for any taxable year in which a U.S. Holder owns securities but before a mark-to-market election
is made, the adverse PFIC rules described above will apply to any mark-to-market gain recognized in the year the election is made.
Otherwise, a mark-to-market election will be effective for the taxable year for which the election is made and all subsequent taxable years.
The election cannot be revoked without the consent of the IRS, unless the securities cease to be marketable, in which case the election
is automatically terminated.
A
U.S. Holder makes a mark-to-market election by attaching a completed IRS Form 8621 to a timely filed U.S. federal income tax return.
Each U.S. Holder should consult its own tax advisor regarding the availability of, and procedure for making, a mark-to-market election.
A
mark-to-market election is not permitted for the shares of any of our subsidiaries that are also classified as PFICs. Prospective
investors should consult their own tax advisors regarding the availability of, and the procedure for making, a mark-to-market election.
PFIC
“QEF” Election
In
some cases, a shareholder of a PFIC can avoid the interest charge and the other adverse PFIC consequences described above by obtaining
certain information from such PFIC and by making a QEF election to be taxed currently on its share of the PFIC’s undistributed
income. We do not, however, expect to provide the information regarding our income that would be necessary in order for a U.S. Holder
to make a QEF election with respect to securities if we are classified as a PFIC.
PFIC
Information Reporting Requirements
If
we are a PFIC in any year, a U.S. Holder of securities in such year will be required to file an annual information return on IRS
Form 8621 regarding distributions received on such securities and any gain realized on disposition of such securities. In addition,
if we are a PFIC, a U.S. Holder generally will be required to file an annual information return with the IRS (also on IRS Form 8621,
which PFIC shareholders are required to file with their U.S. federal income tax or information return) relating to their ownership
of securities. This new filing requirement is in addition to the pre-existing reporting requirements described above that apply
to a U.S. Holder’s interest in a PFIC (which this requirement does not affect).
NO
ASSURANCE CAN BE GIVEN THAT WE ARE NOT CURRENTLY A PFIC OR THAT WE WILL NOT BECOME A PFIC IN THE FUTURE. U.S. HOLDERS SHOULD
CONSULT THEIR OWN TAX ADVISORS WITH RESPECT TO THE OPERATION OF THE PFIC RULES AND RELATED REPORTING REQUIREMENTS IN LIGHT OF THEIR PARTICULAR
CIRCUMSTANCES, INCLUDING THE ADVISABILITY OF MAKING ANY ELECTION THAT MAY BE AVAILABLE.
Reporting
Requirements and Backup Withholding
Under
U.S. federal income tax law and applicable Treasury Regulations, certain categories of U.S. Holders must file information returns
with respect to their investment in, or involvement in, a non-U.S. corporation. For example, U.S. return disclosure obligations
(and related penalties) are imposed on U.S. Holders that hold certain specified foreign financial assets in excess of certain threshold
amounts. The definition of specified foreign financial assets includes not only financial accounts maintained in foreign financial institutions,
but also, unless held in accounts maintained by a financial institution, any stock or security issued by a non-U.S. person, any
financial instrument or contract held for investment that has an issuer or counterparty other than a U.S. person, and any interest
in a non-U.S. entity. U.S. Holders may be subject to these reporting requirements unless such U.S. Holder’s securities
are held in an account at certain financial institutions. Penalties for failure to file certain of these information returns are substantial.
Payments
made within the United States or by a U.S. payor or U.S. middleman of (a) distributions on the securities, and (b) proceeds
arising from the sale or other taxable disposition of securities generally may be subject to information reporting and backup withholding,
currently at the rate of 24%, if a U.S. Holder (a) fails to furnish such U.S. Holder’s correct U.S. taxpayer
identification number (generally on IRS Form W-9), (b) furnishes an incorrect U.S. taxpayer identification number, (c) is
notified by the IRS that such U.S. Holder has previously failed to properly report items subject to backup withholding, or (d) fails
to certify, under penalty of perjury, that such U.S. Holder has furnished its correct U.S. taxpayer identification number and
that the IRS has not notified such U.S. Holder that it is subject to backup withholding. However, certain exempt persons generally
are excluded from these information reporting and backup withholding rules. Any amounts withheld under the U.S. backup withholding
rules will be allowed as a credit against a U.S. Holder’s U.S. federal income tax liability, if any, or will be refunded,
if such U.S. Holder furnishes required information to the IRS in a timely manner.
THE
ABOVE DISCUSSION DOES NOT COVER ALL TAX MATTERS THAT MAY BE OF IMPORTANCE TO A PARTICULAR INVESTOR. YOU ARE STRONGLY URGED TO CONSULT
YOUR OWN TAX ADVISOR ABOUT THE TAX CONSEQUENCES TO YOU OF AN INVESTMENT IN THE SECURITIES.
PLAN
OF DISTRIBUTION
We
are offering on a “best efforts” basis up to 1,147,776 ordinary units, each consisting of one ordinary share, one Class A
Warrant and one Class B Warrant.
We
are also offering to each purchaser of ordinary units that would otherwise result in the purchaser’s beneficial ownership exceeding
4.99% of our outstanding ordinary shares immediately following the consummation of this offering the opportunity to purchase pre-funded
units in lieu of ordinary units, with each pre-funded unit consisting of one pre-funded warrant, one Class A Warrant and one Class B
Warrant. The purchase price of each pre-funded unit will be equal to the price per ordinary unit minus $0.0001. For each pre-funded unit
we sell (without regard to any limitation on exercise set forth therein), the number of ordinary units we are offering will be decreased
on a one-for-one basis.
Each
pre-funded warrant will be exercisable for one ordinary share, and the remaining exercise price of each pre-funded warrant will equal
$0.0001 per ordinary share. The pre-funded warrants will be immediately exercisable (subject to the beneficial ownership cap) and may
be exercised at any time until all of the pre-funded warrants are exercised in full.
Each Class A Warrant will be exercisable for one ordinary share. The
exercise price of each Class A Warrant will equal the per ordinary unit offering price hereunder, which we have assumed to be $6.97 based
on the closing price of our ordinary shares rounded to the nearest whole cent on the Nasdaq Capital Market on December 6, 2024. The Class
A Warrants will be immediately exercisable (subject to the beneficial ownership cap) and may be exercised until the fifth anniversary
of the date of issuance.
Each
Class B Warrant will be exercisable for one ordinary share. The exercise price of each Class B Warrant will equal the per ordinary unit
offering price hereunder, which we have assumed to be $6.97 based on the closing price of our ordinary shares rounded to the nearest
whole cent on the Nasdaq Capital Market on December 6, 2024. The Class B Warrants will be immediately exercisable (subject to the beneficial
ownership cap) and may be exercised until the earlier of (i) first anniversary of the date of issuance or (ii) 30 days following the
public disclosure of results from the eAArly Detect 2 study.
A
holder of pre-funded warrants, Class A Warrants or Class B Warrants will not have the right to exercise any portion of such warrants
if the holder, together with its affiliates, would beneficially own in excess of 4.99% (or, at the election of the holder, such limit
may be increased to up to 9.99%) of the number of ordinary shares outstanding immediately after giving effect to such exercise.
The
following table shows the public offering price, placement agent fees and proceeds, before expenses, to us.
| | |
Per
Ordinary
Unit | | |
Per
Pre-
Funded
Unit |
|
| Public offering price | |
$ | | | |
$ | |
|
| Placement agent fees | |
$ | | | |
$ | |
|
| Proceeds to us, before expenses | |
$ | | | |
$ | |
|
We
estimate that the total expenses of the offering, including registration, filing and listing fees, printing fees, legal and accounting
expenses, expenses for background ground checks, travel and lodging expenses associated with road show trips, but excluding the placement
agent fees, will be approximately $315,000, all of which are payable by us.
There
is no minimum amount of proceeds that is a condition to closing of this offering. The actual amount of gross proceeds, if any, in this
offering could vary substantially from the gross proceeds from the sale of the maximum amount of securities being offered in this prospectus.
Because this is a best-efforts offering, the
placement agent does not have an obligation to purchase any securities. We expect that the offering will end one trading day after we
first enter into a securities purchase agreement relating to the offering, provided that the offering will end fifteen days after the
date of this prospectus if if we have not sold any securities hereunder. The offering will settle delivery versus payment (“DVP”)/receipt
versus payment (“RVP”). Accordingly, we and the placement agent have not made any arrangements to place investor funds in
an escrow account or trust account since the placement agent will not receive investor funds in connection with the sale of the securities
offered hereunder.
Pursuant
to a placement agency agreement, dated as of ,
2024, we have engaged Maxim Group LLC to act as our exclusive placement agent to solicit offers to purchase the securities offered by
this prospectus. The placement agent is not purchasing or selling any securities, nor is it required to arrange for the purchase and
sale of any specific number or dollar amount of securities, other than to use its “reasonable best efforts” to arrange for
the sale of the securities by us. Therefore, we may not sell the entire amount of securities being offered. There is no minimum amount
of proceeds that is a condition to closing of this offering. We will enter into a securities purchase agreement directly with the investors,
at the investor’s option, who purchase our securities in this offering. Investors who do not enter into a securities purchase agreement
shall rely solely on this prospectus in connection with the purchase of our securities in this offering. The placement agent may engage
one or more subagents or selected dealers in connection with this offering.
Placement
Agent Fees, Commissions and Expenses
Upon
the closing of this offering, we will pay the placement agent a cash fee equal to seven percent (7%) of the aggregate gross cash proceeds
to us from the sale of the securities in the offering. Under the placement agency agreement, we will agree to reimburse the placement
agent for its legal fees, costs and expenses in connection with the offering, irrespective of whether the offering is consummated, (i)
up to US$100,000 (inclusive of any advance paid by us to the placement agent) in the event the offering is completed and (ii) up to US$50,000
if an offering is not consummated.
The
placement agency agreement provides that the placement agent’s obligations are subject to conditions contained in the placement
agency agreement.
We
will deliver the securities being issued to the investors upon receipt of investor funds for the purchase of the securities offered pursuant
to this prospectus. We expect to deliver the securities being offered pursuant to this prospectus on or about ,
2024.
Indemnification
We
have agreed to indemnify the placement agent against certain liabilities, including liabilities under the Securities Act and liabilities
arising from breaches of representations and warranties contained in the placement agency agreement, or to contribute to payments that
the placement agent may be required to make in respect of those liabilities.
Lock-Up
Agreements
We,
and each of our Directors, executive officers and certain holders of our outstanding ordinary shares as of the effective date of the
registration statement related to this offering have agreed to a ninety day “lock-up” period from the closing of this offering
with respect to the ordinary shares that they beneficially own. This means that, for a period of ninety (90) days following the closing
of the offering, such persons may not offer, issuer, sell, contract to sell, encumber, grant any option for the sale of or otherwise
dispose of any of our securities without the prior written consent of the placement agent, including the issuance of shares upon the
exercise of currently outstanding options approved by the placement agent. We have also agreed to similar restrictions on the issuance,
sale, disposal and registration (subject to certain exceptions) of our securities for ninety (90) days following the closing of this
offering, subject to certain customary exceptions, without the prior written consent of the placement agent.
The
placement agent has no present intention to waive or shorten the lock-up period; however, the terms of the lock-up agreements may be
waived at its discretion. In determining whether to waive the terms of the lock-up agreements, the placement agent may base its decision
on its assessment of the relative strengths of the securities markets and companies similar to ours in general, and the trading pattern
of, and demand for, our securities in general.
Other
Compensation
Upon
the closing of this offering, or if the engagement period as provided in the engagement letter between us and the placement agent ends
prior to a closing of an offering (other than a termination for cause), then if within six (6) months following such time, we complete
any financing of equity, equity-linked, convertible or debt or other capital-raising activity with, or receive any proceeds from, any
investors that were contacted, introduced or participated in this offering, then the Company shall pay to the placement agent a commission
as described in this section, in each case only with respect to the portion of such financing received from such investors.
Regulation
M
The
placement agent may be deemed to be an underwriter within the meaning of Section 2(a)(11) of the Securities Act, and any commissions
received by it and any profit realized on the resale of the securities sold by it while acting as principal might be deemed to be underwriting
discounts or commissions under the Securities Act. As an underwriter, the placement agent would be required to comply with the requirements
of the Securities Act and the Exchange Act, including, without limitation, Rule 10b-5 and Regulation M under the Exchange Act. These
rules and regulations may limit the timing of purchases and sales of our securities by the placement agent acting as principal. Under
these rules and regulations, the placement agent (i) may not engage in any stabilization activity in connection with our securities and
(ii) may not bid for or purchase any of our securities or attempt to induce any person to purchase any of our securities, other than
as permitted under the Exchange Act, until it has completed its participation in the distribution.
Certain
Relationships
The
placement agent and its affiliates have and may in the future provide, from time to time, investment banking and financial advisory services
to us in the ordinary course of business, for which they may receive customary fees and commissions.
Listing
Our
ordinary shares are currently listed on the Nasdaq Capital Market under the symbol “MYNZ”. We do not intend to list the pre-funded
warrants, the Class A Warrants or the Class B Warrants on any securities exchange or other trading market. Neither the ordinary units
nor the pre-funded units will survive the closing of this offering, and as a result neither of them will ever be listed.
Affiliations
The
placement agent and its respective affiliates are full service financial institutions engaged in various activities, which may include
securities trading, commercial and investment banking, financial advisory, investment management, investment research, principal investment,
hedging, financing and brokerage activities. The placement agent and its affiliates may from time to time in the future engage with us
and perform services for us or in the ordinary course of their business for which they will receive customary fees and expenses. In the
ordinary course of their various business activities, the placement agent and its respective affiliates may make or hold a broad array
of investments and actively trade debt and equity securities (or related derivative securities) and financial instruments (including
bank loans) for their own account and for the accounts of their customers, and such investment and securities activities may involve
securities and/or instruments of us. The placement agent and its respective affiliates may also make investment recommendations and/or
publish or express independent research views in respect of these securities or instruments and may at any time hold, or recommend to
clients that they acquire, long and/or short positions in these securities and instruments.
Electronic
Distribution
A
prospectus in electronic format may be made available on websites or through other online services maintained by the placement agent
of this offering, or by its affiliates. Other than the prospectus in electronic format, the information on the placement agent’s
website and any information contained in any other website maintained by the placement agent is not part of this prospectus or the registration
statement of which this prospectus forms a part, has not been approved and/or endorsed by us or the placement agent in its capacity as
the placement agent, and should not be relied upon by investors.
In
connection with this offering, the placement agent or certain securities dealers may distribute prospectuses by electronic means, such
as e-mail.
Selling
Restrictions Outside the United States
No
action may be taken in any jurisdiction other than the United States that would permit a public offering of our securities or the possession,
circulation, or distribution of this prospectus in any jurisdiction where action for that purpose is required. Accordingly, our securities
may not be offered or sold, directly or indirectly, and neither the prospectus nor any other offering material or advertisements in connection
with our securities may be distributed or published in or from any country or jurisdiction except under circumstances that will result
in compliance with any applicable laws, rules and regulations of any such country or jurisdiction. This prospectus does not constitute
an offer to sell or a solicitation of an offer to buy any of our securities offered by this prospectus in any jurisdiction in which such
an offer or a solicitation is unlawful.
Notice
to Prospective Investors in Canada
Securities
legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus
(including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by
the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser
should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory for particulars
of these rights or consult with a legal advisor.
Pursuant
to section 3A.3 (or, in the case of securities issued or guaranteed by the government of a non-Canadian jurisdiction, section 3A.4) of
National Instrument 33-105 Underwriting Conflicts (NI 33-105), the underwriters are not required to comply with the disclosure requirements
of NI 33-105 regarding underwriter conflicts of interest in connection with this offering. Our securities may be sold only to purchasers
purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined in National Instrument 45-106 Prospectus
Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted clients, as defined in National Instrument 31-103
Registration Requirements, Exemptions and Ongoing Registrant Obligations. Any resale of our securities must be made in accordance with
an exemption from, or in a transaction not subject to, the prospectus requirements of applicable securities laws.
Notice
to Prospective Investors in the United Kingdom
In
relation to each Member State of the European Economic Area which has implemented the Prospectus Directive (each, a “Relevant Member
State”) an offer to the public of any securities which are the subject of the offering contemplated by this prospectus may not be
made in that Relevant Member State except that an offer to the public in that Relevant Member State of any such securities may be made
at any time under the following exemptions under the Prospectus Directive, if they have been implemented in that Relevant Member State:
| |
(a) |
to
legal entities which are authorized or regulated to operate in the financial markets or, if not so authorized or regulated, whose
corporate purpose is solely to invest in securities; |
| |
(b) |
to
any legal entity which has two or more of (1) an average of at least 250 employees during the last financial year; (2) a total balance
sheet of more than €43,000,000 and (3) an annual net turnover of more than €50,000,000, as shown in its last annual or
consolidated accounts; |
| |
(c) |
by
the underwriters to fewer than 100 natural or legal persons (other than qualified investors as defined in the Prospectus Directive);
or |
| |
(d) |
in
any other circumstances falling within Article 3(2) of the Prospectus Directive, provided that no such offer of these securities
shall result in a requirement for the publication by the issuer or the underwriters of a prospectus pursuant to Article 3 of the
Prospectus Directive. |
For
the purposes of this provision, the expression an “offer to the public” in relation to any of the securities in any Relevant
Member State means the communication in any form and by any means of sufficient information on the terms of the offer and any such securities
to be offered so as to enable an investor to decide to purchase any such securities, as the same may be varied in that Member State by
any measure implementing the Prospectus Directive in that Member State and the expression “Prospectus Directive” means Directive
2003/71/EC and includes any relevant implementing measure in each Relevant Member State.
The
representative has represented, warranted and agreed that:
|
(a) |
it
has only communicated or caused to be communicated and will only communicate or cause to be communicated any invitation or inducement
to engage in investment activity (within the meaning of section 21 of the Financial Services and Markets Act 2000 (the FSMA)) received
by it in connection with the issue or sale of any of the securities in circumstances in which section 21(1) of the FSMA does not
apply to the issuer; and |
| |
(b) |
it
has complied with and will comply with all applicable provisions of the FSMA with respect to anything done by it in relation to the
securities in, from or otherwise involving the United Kingdom. |
Notice
to Prospective Investors in Singapore
This
prospectus has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, this prospectus and any other
document or material in connection with the offer or sale, or invitation for subscription or purchase, of our securities may not be circulated
or distributed, nor may our securities be offered or sold, or be made the subject of an invitation for subscription or purchase, whether
directly or indirectly, to any person in Singapore other than (i) to an institutional investor (as defined in Section 4A of the
Securities and Futures Act 2001 (the “SFA”)) pursuant to Section 274 of the SFA, (ii) to a relevant person (as
defined in Section 275(2) of the SFA) pursuant to Section 275(1) of the SFA, or any person pursuant to Section 275(1A) of the
SFA (where applicable) and Regulation 3 of the Securities and Futures (Classes of Investors) Regulations 2018 of Singapore, and in accordance
with the conditions specified in Section 275 of the SFA, or (iii) otherwise pursuant to, and in accordance with the conditions
of, any other applicable provision of the SFA.
Where
the offered securities are subscribed or purchased under Section 275 of the SFA by a relevant person which is:
| |
(a) |
a corporation (which is not an accredited
investor (as defined in Section 4A of the SFA)) the sole business of which is to hold investments and the entire share capital of
which is owned by one or more individuals, each of whom is an accredited investor; or |
| |
|
|
| |
(b) |
a
trust (where the trustee is not an accredited investor) whose sole purpose is to hold investments
and each beneficiary of the trust is an individual who is an accredited investor, securities or securities-based
derivatives contracts (each term as defined in Section 2(1) of the SFA) of that corporation
or the beneficiaries’ rights and interest (howsoever described) in that trust shall not be
transferred within six months after that corporation or that trust has acquired the offered securities
pursuant to an offer made under Section 275 of the SFA except: |
| (1) | to
an institutional investor or to a relevant person, or to any person arising from an offer
referred to in Section 275(1A) or Section 276(4)(i)(B) of the SFA; |
| (2) | where
no consideration is or will be given for the transfer; |
| (3) | where
the transfer is by operation of law; |
| (4) | as
specified in Section 276(7) of the SFA; or |
| (5) | as
specified in Regulation 37A of the Securities and Futures (Offers of Investments) (Securities
and Securities-based Derivatives Contracts) Regulations 2018 of Singapore. |
In
connection with Section 309B of the SFA and the Securities and Futures (Capital Markets Products)
Regulations 2018 (the “CMP Regulations 2018”), unless otherwise specified before an offer of the offered securities,
the Company has determined, and hereby notifies all relevant persons (as defined in Section 309A(1) of the SFA) (where applicable), that
the offered securities are “prescribed capital markets products” (as defined in the CMP Regulations 2018) and Excluded Investment
Products (as defined in MAS Notice SFA 04-N12: Notice on the Sale of Investment Products and MAS Notice FAA-N16: Notice on Recommendations
on Investment Products).
Notice
to Prospective Investors in the People’s Republic of China
This
prospectus may not be circulated or distributed in China and our securities may not be offered or sold, and will not offer or sell to
any person for re-offering or resale directly or indirectly to any resident of China except pursuant to applicable laws, rules and
regulations of China. For the purpose of this paragraph only, China does not include Taiwan and the special administrative regions of
Hong Kong and Macau.
Notice
to Prospective Investors in Hong Kong
Our securities may not be offered or sold in Hong Kong by means
of any document other than (i) in circumstances which do not constitute an offer to the public within the meaning of the Companies
Ordinance (Cap. 32, Laws of Hong Kong), (ii) to “professional investors” within the meaning of the Securities and Futures
Ordinance (Cap. 571, Laws of Hong Kong) and any rules made thereunder, or (iii) in other circumstances which do not result in the
document being a “prospectus” within the meaning of the Companies Ordinance (Cap. 32, Laws of Hong Kong) and no advertisement,
invitation or document relating to our securities be issued or may be in the possession of any person for the purpose of issue (in each
case whether in Hong Kong or elsewhere), which is directed at, or the contents of which are likely to be accessed or read by, the public
in Hong Kong (except if permitted to do so under the laws of Hong Kong) other than with respect to our securities which are or are intended
to be disposed of only to persons outside Hong Kong or only to “professional investors” within the meaning of the Securities
and Futures Ordinance (Cap. 571, Laws of Hong Kong) and any rules made thereunder.
Notice
to Prospective Investors in Israel
This
document does not constitute a prospectus under the Israeli Securities Law, 5728-1968, or the Securities Law, and has not been filed
with or approved by the Israel Securities Authority. In the State of Israel, this document is being distributed only to, and is directed
only at, and any offer of the offered securities is directed only at, investors listed in the first addendum, or the Addendum, to the
Israeli Securities Law, consisting primarily of joint investment in trust funds, provident funds, insurance companies, banks, portfolio
managers, investment advisors, members of the Tel Aviv Stock Exchange, underwriters, venture capital funds, entities with equity in excess
of NIS 50 million and “qualified individuals”, each as defined in the Addendum (as it may be amended from time to time), collectively
referred to as qualified investors (in each case purchasing for their own account or, where permitted under the Addendum, for the accounts
of their clients who are investors listed in the Addendum). Qualified investors will be required to submit written confirmation that
they fall within the scope of the Addendum, are aware of the meaning of same and agree to it.
Notice to Prospective Investors in Taiwan
Our securities have not
been and will not be registered with the Financial Supervisory Commission of Taiwan, pursuant to relevant securities laws and regulations
and may not be offered or sold in Taiwan through a public offering or in any manner which would constitute an offer within the meaning
of the Securities and Exchange Act of Taiwan or would otherwise require registration with or the approval of the Financial Supervisory
Commission of Taiwan.
Notice to Prospective Investors in the
Cayman Islands
No invitation, whether directly
or indirectly may be made to the public in the Cayman Islands to subscribe for our securities. This prospectus does not constitute a public
offer of our securities, whether by way of sale or subscription, in the Cayman Islands. Our securities have not been offered or sold,
and will not be offered or sold, directly or indirectly, in the Cayman Islands.
Notice to Prospective Investors in the European
Economic Area
In relation to each Member
State of the European Economic Area (each a “Member State”), none of our securities have been offered or will be offered pursuant
to the offering to the public in that Member State prior to the publication of a prospectus in relation to our securities which has been
approved by the competent authority in that Member State or, where appropriate, approved in another Member State and notified to the competent
authority in that Member State, all in accordance with the Prospectus Regulation, except that offers of our securities may be made to
the public in that Member State at any time under the following exemptions under the Prospectus Regulation:
| ● | to
any legal entity which is a qualified investor as defined under the Prospectus Regulation; |
| |
● |
to fewer than 150 natural or legal persons
(other than qualified investors as defined under the Prospectus Regulation), subject to obtaining the prior consent of the
underwriter for any such offer; or |
| |
● |
in any other circumstances falling within Article 1(4) of the Prospectus Regulation. |
provided that no such offer of our securities
shall require us or any of our representatives to publish a prospectus pursuant to Article 3 of the Prospectus Regulation or supplement
a prospectus pursuant to Article 23 of the Prospectus Regulation and each person who initially acquires any of our securities or to whom
any offer is made will be deemed to have represented, acknowledged and agreed to and with each of the representatives and us that it is
a “qualified investor” as defined in the Prospectus Regulation.
In the case of any of our
securities being offered to a financial intermediary as that term is used in Article 5 of the Prospectus Regulation, each such financial
intermediary will be deemed to have represented, acknowledged and agreed that our securities acquired by it in the offer have not been
acquired on a nondiscretionary basis on behalf of, nor have they been acquired with a view to their offer or resale to, persons in circumstances
which may give rise to an offer of any of our securities to the public other than their offer or resale in a Member State to qualified
investors as so defined or in circumstances in which the prior consent of the representatives has been obtained to each such proposed
offer or resale.
For the purposes of this provision,
the expression an “offer to the public” in relation to any of our securities in any Member State means the communication in
any form and by any means of sufficient information on the terms of the offer and any of our securities to be offered so as to enable
an investor to decide to purchase or subscribe for any of our securities, and the expression “Prospectus Regulation” means
Regulation (EU) 2017/1129 (as amended).
Stamp Taxes
If you purchase our securities
offered by this prospectus, you may be required to pay stamp taxes and other charges under the laws and practices of the country of purchase,
in addition to the public offering price listed on the cover page of this prospectus.
EXPENSES
RELATING TO THIS OFFERING
Set
forth below is an itemization of the total expenses, excluding placement discounts and commissions, the placement agent’s non-accountable
expenses and the placement agent fees, that we expect to incur in connection with this offering. With the exception of the SEC registration
fee and the FINRA filing fee listing fee, all amounts are estimates.
| Securities and Exchange Commission Registration
Fee | |
$ | 4,227 | |
| FINRA | |
$ | 1,880 | |
| Legal Fees and Expenses | |
$ | 280,000 | |
| Accounting Fees and Expenses | |
$ | 20,000 | |
| Printing and Engraving Expenses | |
$ | 3,000 | |
| Miscellaneous Expenses | |
$ | 5,893 | |
| Total Expenses | |
$ | 315,000 | |
LEGAL
MATTERS
Ortoli
Rosenstadt LLP is acting as counsel to our company regarding U.S. securities law matters. The current address of Ortoli Rosenstadt
LLP is 501 Madison Avenue, 14th Floor, New York, NY 10022. CMS Derks Star Busmann N.V. is acting as counsel to our
company regarding Dutch securities law matters. The current address of CMS Derks Star Busmann N.V. is Atrium, Parnassusweg 737, 1077
DG Amsterdam, Netherlands.
Pryor Cashman LLP, New York, NY, is acting as counsel to the placement
agent.
EXPERTS
The
financial statements of Mainz Biomed, N.V. as of December 31, 2023 and 2022 for the years respectively then ended incorporated
by reference into this prospectus and the registration statement have been so included in reliance on the report of Reliant CPA PC, an
independent registered public accounting firm, given on the authority of said firm as experts in accounting and auditing. Reliant CPA
PC has offices at 895 Dove Street, Suite 300, #300180, Newport Beach, CA 92660. Their telephone number is (949)558-7781.
INTERESTS
OF EXPERTS AND COUNSEL
None
of the named experts or legal counsel was employed on a contingent basis, owns an amount of ordinary shares in our company which is material
to that person, or has a material, direct or indirect economic interest in our company or that depends on the success of the offering.
DISCLOSURE
OF COMMISSION POSITION ON INDEMNIFICATION FOR
SECURITIES ACT LIABILITIES
Insofar
as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling the
registrant pursuant to the foregoing provisions, the registrant has been informed that in the opinion of the Securities and Exchange
Commission such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
ENFORCEABILITY
OF CIVIL LIABILITIES
We
are a corporation organized under the laws of the Netherlands, and the majority of our directors and officers reside outside of the United States.
Service of process upon such persons may be difficult or impossible to effect within the United States. Furthermore, because a substantial
portion of our assets, and substantially all the assets of our directors and officers and the experts named herein, are located outside
of the United States, any judgment obtained in the United States, including a judgment based upon the civil liability provisions
of United States federal securities laws, against us or any of such persons may not be collectible within the United States.
As
there is no treaty on the reciprocal recognition and enforcement of judgments other than arbitration awards in civil and commercial matters
between the United States and the Netherlands, courts in the Netherlands will not automatically recognize and enforce a final judgment
rendered by a U.S. court. In order to obtain a judgment enforceable in the Netherlands, claimants must litigate the relevant claim
again before a Dutch court of competent jurisdiction. Under current practice, however, a Dutch court will generally recognize and consider
as conclusive evidence a final and conclusive judgment for the payment of money rendered by a U.S. court and not rendered by default,
provided that the Dutch court finds that:
| ● | the
jurisdiction of the U.S. court has been based on grounds that are internationally acceptable; |
| ● | the
final judgment results from proceedings compatible with Dutch concepts of proper administration
of justice including sufficient safeguards (behoorlijke rechtspleging); |
| ● | the
final judgment does not contravene public policy (openbare orde) of the Netherlands; |
| ● | the
judgment by the U.S. court is not incompatible with a decision rendered between the
same parties by a Dutch court, or with a previous decision rendered between the same parties
by a foreign court in a dispute that concerns the same subject and is based on the same cause,
provided that the previous decision qualifies for acknowledgment in the Netherlands; and |
the
final judgment has not been rendered in proceedings of a penal, revenue or other public law nature. If a Dutch court upholds and regards
as conclusive evidence the final judgment, that court generally will grant the same judgment without litigating again on the merits.
Shareholders
may originate actions in the Netherlands based upon applicable Dutch laws.
Under
Dutch law, in the event that a third party is liable to us, only we ourselves can bring civil action against that party. The individual
shareholders do not have the right to bring an action on our behalf. Only in the event that the cause for the liability of a third party
to us also constitutes a tortious act directly against a shareholder does that shareholder have an individual right of action against
such third party in its own name. The Dutch Civil Code does provide for the possibility to initiate such actions collectively. A foundation
or an association whose objective is to protect the rights of a group of persons having similar interests can institute a collective
action. The collective action itself cannot result in an order for payment of monetary damages but may only result in a declaratory judgment
(verklaring voor recht). In order to obtain compensation for damages, the foundation or association and the defendant may reach — often
on the basis of such declaratory judgment — a settlement. A Dutch court may declare the settlement agreement binding
upon all the injured parties with an opt out choice for an individual injured party. An individual injured party may also itself institute
a civil claim for damages.
The
name and address of our agent for service of process in the United States is Vcorp Services, LLC, 25 Robert Pitt Drive, Suite 204,
Monsey, NY 10952.
WHERE
YOU CAN FIND MORE INFORMATION
We
have filed with the SEC a registration statement on Form F-1 under the Securities Act with respect to the offered securities. This
prospectus and the documents incorporated by reference herein do not contain all of the information set forth in the registration statement
and the exhibits thereto, to which reference is hereby made. With respect to each contract, agreement or other document filed as an exhibit
to the registration statement, reference is made to such exhibit for a more complete description of the matter involved. The registration
statement and the exhibits thereto filed by us with the SEC may be inspected at the public reference facility of the SEC listed below.
The
registration statement, reports and other information filed or to be filed with the SEC by us can be inspected and copied at the public
reference facilities maintained by the SEC at 100 F. Street NW, Washington, D.C. 20549. The SEC maintains a website at www.sec.gov
that contains reports, proxy and information statements, and other information regarding registrants that make electronic filings
with the SEC using its EDGAR system.
We
are subject to the information reporting requirements of the Exchange Act that are applicable to foreign private issuers, and under
those requirements are filing reports with the SEC. Those other reports or other information may be inspected without charge at
the locations described above. As a foreign private issuer, we are exempt from the rules under the Exchange Act related to the furnishing
and content of proxy statements, and our officers, directors and principal shareholders are exempt from the reporting and short-swing
profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we are not required under the Exchange Act
to file annual, quarterly and current reports and financial statements with the SEC as frequently or as promptly as U.S. companies
whose securities are registered under the Exchange Act. However, we will file with the SEC, within 120 days after the end of
each fiscal year, or such applicable time as required by the SEC, an annual report on Form 20-F containing financial statements
audited by an independent registered public accounting firm, and will submit to the SEC, on Form 6-K, unaudited quarterly financial
information.
INCORPORATION
OF DOCUMENTS BY REFERENCE
The
SEC allows us to incorporate by reference the information we file with them. This means that we can disclose important information to
you by referring you to those documents. Each document incorporated by reference is current only as of the date of such document, and
the incorporation by reference of such documents should not create any implication that there has been no change in our affairs since
such date. The information incorporated by reference is considered to be a part of this prospectus and should be read with the same care.
When we update the information contained in documents that have been incorporated by reference by making future filings with the SEC,
the information incorporated by reference in this prospectus is considered to be automatically updated and superseded. In other words,
in the case of a conflict or inconsistency between information contained in this prospectus and information incorporated by reference
into this prospectus, you should rely on the information contained in the document that was filed later.
We incorporate
by reference the documents listed below:
| ● | Form 6-K filed with the SEC on November 29, 2024; |
| |
● |
Form 6-K filed with
the SEC on November 15, 2024; |
| ● | Form
6-K filed with the SEC on November 12, 2024; |
| ● | Form
6-K filed with the SEC on October 21, 2024; |
| ● | Form
6-K filed with the SEC on October 18, 2024; |
| ● | Form
6-K filed with the SEC on October 9, 2024; |
| ● | Form
6-K filed with the SEC on October 3, 2024; |
| ● | Form
6-K filed with the SEC on May 31, 2024; |
| ● | Form
6-K filed with the SEC on April 24, 2024; |
| ● | Form
6-K filed with the SEC on April 19, 2024; and |
| ● | Our
Annual Report on Form
20-F for the year ended December 31, 2023 filed with the SEC on April 9, 2024. |
Any
statement contained herein or in a document, all or a portion of which is incorporated or deemed to be incorporated by reference herein,
shall be deemed to be modified or superseded for purposes of this prospectus to the extent that a statement contained herein or in any
other subsequently filed document which also is or is deemed to be incorporated by reference herein modifies or supersedes such statement.
Any such statement so modified or superseded shall not be deemed, except as so modified or amended, to constitute a part of this prospectus.
Our
filings with the SEC, and exhibits incorporated in and amendments to those reports, are available free of charge on our website (http://www.mainzbiomed.com)
as soon as reasonably practicable after they are filed with, or furnished to, the SEC. Our website and the information contained on that
site, or connected to that site, are not incorporated into and are not a part of this prospectus.
Upon
written or oral request, we will provide to each person to whom this prospectus is delivered, a copy of any or all of the reports or
documents that have been incorporated by reference into this prospectus at no cost. If you would like a copy of any of these documents,
at no cost, please write or call us at:
Mainz
Biomed N.V.
Robert Koch Strasse 50
55129 Mainz
Germany
Telephone: 0049 6131 5542860
Up to 1,147,776 Units
Each Unit Consisting of
One Ordinary Share,
One Class A Warrant to purchase One Ordinary
Share and
One Class B Warrant to purchase One Ordinary
Share
Up To 1,147,776 Pre-Funded Units
Each Unit Consisting of
One Pre-Funded Warrant to purchase One Ordinary
Share,
One Class A Warrant to purchase One Ordinary
Share and
One Class B Warrant to purchase One Ordinary
Share
Up To 3,443,328 Ordinary Shares underlying
the Class A Warrants,
the Class B Warrants, and the Pre-Funded
Warrants

MAINZ
BIOMED, N.V.
PROSPECTUS
Maxim
Group LLC
,
2024
Through
and including (the 25th day after the date of this
offering), all dealers effecting transactions in these securities, whether or not participating in this offering, may be required to
deliver a prospectus. This is in addition to a dealer’s obligation to deliver a prospectus when acting as an underwriter and with
respect to an unsold allotment or subscription.
PART II
INFORMATION
NOT REQUIRED IN PROSPECTUS
ITEM
6: INDEMNIFICATION OF DIRECTORS AND OFFICERS
Under
Dutch law, members of the board of directors may be liable to the registrant for damages in the event of improper or negligent performance
of their duties. They may be jointly and severally liable for damages to the registrant and third parties for infringement of our Articles
of Association or certain provisions of the Dutch Civil Code. In certain circumstances, they may also incur additional specific civil
and criminal liabilities.
Pursuant
to the registrant’s articles of association, to the fullest extent permitted by Dutch law, the following shall be reimbursed to
the indemnified officers:
| (a) | the
costs of conducting a defense against claims, also including claims by the Company and its
group companies, as a consequence of any acts or omissions in the fulfilment of their duties
or any other duties currently or previously performed by them at the company’s request; |
| (b) | any
damages or financial penalties payable by them as a result of any such acts or omissions; |
| (c) | any
amounts payable by them under settlement agreements entered into by them in connection with
any such acts or omissions; |
| (d) | the
costs of appearing in other legal proceedings in which they are involved as directors or
former directors, with the exception of proceedings primarily aimed at pursuing a claim on
their own behalf; |
| (e) | any
taxes payable by them as a result of any reimbursements in accordance with the articles of
association. |
An
indemnitee shall not be entitled to reimbursement if and to the extent that:
| (a) | it
has been adjudicated by a Dutch court or, in the case of arbitration, an arbitrator, in a
final and conclusive decision that the act or omission of the Indemnitee may be characterized
as intentional, deliberately reckless or grossly negligent conduct, unless Dutch law provides
otherwise or this would, in view of the circumstances of the case, be unacceptable according
to standards of reasonableness and fairness; or |
| (b) | the
costs or financial loss of the Indemnitee are covered by an insurance and the insurer has
paid out the costs or financial loss. |
The
description of indemnity herein is merely a summary of the provisions in the registrant’s articles of association described above,
and such description shall not limit or alter the mentioned provisions in the articles of association or other indemnification agreements.
Prior
to the public offering of the securities being registered by this registration statement, we intend to enter into a directors’
and officers’ liability insurance policy to cover the liability of members of the board of directors and members.
The
placement agency agreement the registrant will enter into in connection with the offering being registered hereby provides that the placement
agent will indemnify, under certain conditions, the registrant’s board of directors and its officers against certain liabilities
arising in connection with this offering.
ITEM
7. RECENT SALES OF UNREGISTERED SECURITIES
In
the past three years, we have issued and sold the securities described below without registering the securities under the Securities
Act. None of these transactions involved the placement agent fees or any public offering. We believe that each of the following issuances
was exempt from registration under the Securities Act in reliance on Regulation S promulgated under the Securities Act regarding
sales by an issuer in offshore transactions, Regulation D under the Securities Act, Rule 701 under the Securities Act or pursuant
to Section 4(a)(2) of the Securities Act regarding transactions not involving a public offering.
During calendar 2022, 20,537 ordinary
shares were issued upon the exercise of warrants that were issued in 2021 at an exercise price of $120 per share.
During calendar 2022, 1,825 ordinary
shares were issued to consultants for services rendered, valued at an average price of $496.80 per share.
During calendar 2023, 7,645 ordinary
shares were issued on the exercise of warrants that were issued in 2021 at an exercise price of $120 per share.
On June 28, 2023, 1,361 ordinary
shares were issued as a commitment fee related to a pre-paid advance Agreement entered into as of the same date, valued at $183.60 per
share.
On February 15, 2023, 7,500 ordinary
shares were issued under an intellectual property asset purchase agreement, valued at $274 per share.
During calendar 2023, 3,570 ordinary
shares were issued to consultants for services rendered, valued at an average price of $153.60 per share.
On September 3, 2023, 30,000 ordinary
shares issued to a consultant for services rendered, valued at an average price of $14 per share
In October 2024, 191,013 ordinary
shares were issued for the conversion of debt valued at $1,734,345 for an average price of $9.08 per share.
From November 1, 2024 to the date
hereof, 46,149 ordinary shares were issued for the conversion of debt valued at $373,622 for an average price of $8.10 per share.
ITEM
8. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
The
following exhibits are filed with this registration statement:
| 1.1 |
Form of Placement Agency Agreement between the Company and Maxim Group LLC** |
| 2.1 |
Description of Securities registered under Section 12 of the Exchange Act** |
| 3.1 |
Unofficial English translation of Deed of Conversion** |
| 3.2 |
Unofficial English translation of Deed of Amendment, dated July 19, 2024** |
| 3.3 |
Unofficial English translation of Articles of Association, dated December 3, 2024** |
| 4.1 |
Share Certificate—Ordinary Shares** |
| 4.2 |
Form of Pre-Funded Warrant** |
| 4.3 |
Form of Class A Ordinary Share Purchase Warrant** |
| 4.4 |
Form of Class B Ordinary Share Purchase Warrant** |
| 5.1 |
Opinion of CMS Derks Star Busmann N.V.** |
| 5.2 |
Opinion of Ortoli Rosenstadt LLP** |
| 10.1 |
Management Services Agreement, dated July 1, 2020, between the Company and Guido Baechler** |
| 10.2 |
Amendment to Management Services Agreement, dated October 2021, between Guido Baechler and the Company** |
| 10.3 |
Amendment to Management Services Agreement, dated October 2024, between Guido Baechler and the Company** |
| 10.4 |
Consulting Agreement, dated July 16, 2021, between the Company and William Caragol** |
| 10.5 |
Amendment to Consultant Agreement, dated October 2021, between William Caragol and the Company** |
| 10.6 |
Amendment to Consultant Agreement, dated October 2024, between William Caragol and the Company** |
| 10.7 |
Form of Silent Partnership Agreements** |
| 10.8 |
Mainz Biomed N.V. 2021 Omnibus Incentive Plan** |
| 10.9 |
Mainz Biomed N.V. Amended and Restated 2022 Omnibus Incentive Plan** |
| 10.10 |
Technology Rights Agreement, dated January 4, 2022, between the Company and Socpra Sciences Santé Et Humaines S.E.C. ** |
| 10.11 |
Employment Contract with William Caragol, dated April 29, 2022** |
| 10.12 |
Intellectual Property Asset Purchase Agreement, dated February 15, 2023, with Uni Targeting Research AS** |
| 10.13 |
Assignment Agreement, dated February 15, 2023, with SOCPRA Sciences Santé et Humaines S.E.C. ** |
| 10.14 |
Mainz Biomed USA, Inc. Carve-Out Plan** |
| 10.15 |
Pre-Paid Advance Agreement (the “PPA”), dated June 28, 2023, between the Company and YA II PN, Ltd.** |
| 10.16 |
Form of Promissory Note to be issued under the PPA**
|
| 10.17 |
Supplemental Agreement to PPA, dated April 18, 2024** |
| 10.18 |
Second Supplemental Agreement to PPA, dated October 8, 2024** |
| 10.19 |
Amendment Agreement, dated December 6, 2024, between the Company and YA II PN, Ltd.** |
| 10.20 |
Form of Securities Purchase Agreement** |
| 10.21 |
Form of Lock-Up Agreement** |
| 10.22 |
Form of Warrant Agent Agreement** |
| 11.1 |
Insider Trading Policy** |
| 11.2 |
Code of Ethics and Business Conduct** |
| 23.1 |
Consent of Reliant CPA PC** |
| 23.2 |
Consent of CMS Derks Star Busmann N.V. (contained in Exhibit 5.1)** |
| 23.3 |
Consent of Ortoli Rosenstadt LLP (contained in Exhibit 5.2)** |
| 24.1 |
Power of Attorney (included
on signature page to the initial filing of this registration statement). |
| 97.1 |
Executive Compensation Clawback Policy** |
| 107 |
Filing Fee Table** |
ITEM
9. UNDERTAKINGS
The
undersigned Registrant hereby undertakes:
| (1) | To
file, during any period in which offers or sales of securities are being made, a post-effective
amendment to this registration statement to: |
| (i) | Include
any prospectus required by Section 10(a)(3) of the Securities Act of 1933; |
| (ii) | Reflect
in the prospectus any facts or events arising after the effective date of the registration
statement (or the most recent post-effective amendment thereof) which, individually or in
the aggregate, represent a fundamental change in the information set forth in the registration
statement. Notwithstanding the foregoing, any increase or decrease in volume of securities
offered (if the total dollar value of securities offered would not exceed that which was
registered) and any deviation from the low or high end of the estimated maximum offering
range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if,
in the aggregate, the changes in volume and price represent no more than 20% change in the
maximum aggregate offering price set forth in the “Calculation of Registration Fee”
table in the effective registration statement; and |
| (iii) | Include
any material information with respect to the plan of distribution not previously disclosed
in the registration statement or any material change to such information in the registration
statement. |
| (2) | That,
for the purpose of determining any liability under the Securities Act of 1933,
each such post- effective amendment shall be deemed to be a new registration statement relating
to the securities offered therein, and the offering of such securities at that time shall
be deemed to be the initial bona fide offering thereof. |
| (3) | To
remove from registration by means of a post-effective amendment any of the securities being
registered which remain unsold at the termination of the offering. |
| (4) | To
file a post-effective amendment to the registration statement to include any financial statements
required by Item 8.A. of Form 20-F at the start of any delayed offering or throughout
a continuous offering. Financial statements and information otherwise required by Section 10(a)(3) of
the Act need not be furnished, provided that the Registrant includes in the prospectus, by
means of a post- effective amendment, financial statements required pursuant to this paragraph
(4) and other information necessary to ensure that all other information in the prospectus
is at least as current as the date of those financial statements. Notwithstanding the foregoing,
with respect to registration statements on Form F-3, a post-effective amendment need
not be filed to include financial statements and information required by Section 10(a)(3) of
the Act or Rule 3-19 of Regulation S- X if such financial statements and information
are contained in periodic reports filed with or furnished to the Commission by the Registrant
pursuant to section 13 or section 15(d) of the Securities Exchange Act of 1934
that are incorporated by reference in the Form F-3. |
| (5) | Insofar
as indemnification for liabilities arising under the Securities Act of 1933 may
be permitted to directors, officers and controlling persons of the registrant pursuant to
the provisions described herein, or otherwise, the registrant has been advised that in the
opinion of the Securities and Exchange Commission such indemnification is against public
policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim
for indemnification against such liabilities (other than the payment by the registrant of
expenses incurred or paid by a director, officer or controlling person of the registrant
in the successful defense of any action, suit or proceeding) is asserted by such director,
officer or controlling person in connection with the securities being registered, the registrant
will, unless in the opinion of its counsel the matter has been settled by controlling precedent,
submit to a court of appropriate jurisdiction the question whether such indemnification by
it is against public policy as expressed in the Act and will be governed by the final adjudication
of such issue. |
| (6) | Each
prospectus filed pursuant to Rule 424(b) as part of a registration statement relating
to an offering, other than registration statements relying on Rule 430B or other than
prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included
in the registration statement as of the date it is first used after effectiveness. Provided,
however, that no statement made in a registration statement or prospectus that is part of
the registration statement or made in a document incorporated or deemed incorporated by reference
into the registration statement or prospectus that is part of the registration statement
will, as to a purchaser with a time of contract of sale prior to such first use, supersede
or modify any statement that was made in the registration statement or prospectus that was
part of the registration statement or made in any such document immediately prior to such
date of first use. |
SIGNATURES
Pursuant
to the requirements of the Securities Act of 1933, the Registrant certifies that it has reasonable grounds to believe it meets
all of the requirements for filing on Form F-1 and has duly caused this Registration Statement to be signed on its behalf by the
undersigned, thereunto duly authorized on December 11, 2024.
| |
MAINZ BIOMED
N.V. |
| |
(Registrant) |
| |
|
| |
By: |
/s/
Guido Baechler |
| |
|
Guido Baechler, Chief Executive Officer
(Principal Executive Officer) |
Pursuant
to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in
the capacities and on the dates indicated.
| Signature |
|
Title |
|
Date |
| |
|
|
|
|
| /s/ Guido Baechler |
|
Chief Executive Officer (Principal Executive Officer), |
|
December 11, 2024 |
| Guido Baechler |
|
Executive Director |
|
|
| |
|
|
|
|
| /s/ William Caragol |
|
Chief Financial Officer (Principal Financial Officer and |
|
December 11, 2024 |
| William Caragol |
|
Principal Accounting Officer) |
|
|
| |
|
|
|
|
| /s/ Dr. Heiner Dreismann* |
|
Director |
|
December 11, 2024 |
| Dr. Heiner Dreismann |
|
|
|
|
| |
|
|
|
|
| /s/ Gregory Tibbits* |
|
Director |
|
December 11, 2024 |
| Gregory Tibbits |
|
|
|
|
| |
|
|
|
|
| /s/ Hans Hekland* |
|
Director |
|
December 11, 2024 |
| Hans Hekland |
|
|
|
|
* Pursuant to power of attorney
SIGNATURE
OF AUTHORIZED REPRESENTATIVE IN THE UNITED STATES
Pursuant
to the Securities Act of 1933, the undersigned, the duly authorized representative in the United States of Mainz Biomed
N.V., has signed this registration statement or amendment thereto in New York, New York, on December 11, 2024.
| |
Ortoli Rosenstadt
LLP |
| |
|
| |
By: |
/s/
William S. Rosenstadt |
| |
Name: |
William S. Rosenstadt |
| |
Title: |
Managing Partner |
II-6
Mainz BioMed NV (NASDAQ:MYNZ)
Historical Stock Chart
Von Nov 2024 bis Dez 2024
Mainz BioMed NV (NASDAQ:MYNZ)
Historical Stock Chart
Von Dez 2023 bis Dez 2024