false
0001346830
0001346830
2024-12-17
2024-12-17
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K/A
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):
December
17, 2024
CARA THERAPEUTICS, INC.
(Exact name of registrant as specified in its
charter)
| Delaware |
|
001-36279 |
|
75-3175693 |
(state or other jurisdiction
of incorporation) |
|
(Commission
File Number) |
|
(I.R.S. Employer
Identification No.) |
| |
|
|
|
|
|
400 Atlantic Street
Suite 500
Stamford, CT |
|
|
|
06901 |
| (Address of principal executive offices) |
|
|
|
(Zip Code) |
| |
|
|
|
|
| Registrant's telephone number, including area code: (203) 406-3700 |
Not applicable
(Former name or former address, if changed since
last report.)
Check the appropriate box below if the Form 8-K filing is intended
to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2.
below):
| x |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ¨ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ¨ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ¨ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title
of each class |
|
Trading
Symbol |
|
Name
of each exchange
on which registered |
| Common Stock, $0.001 par value per share |
|
CARA |
|
The Nasdaq Capital Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ¨
If an emerging growth company, indicate by check
mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting
standards provided pursuant to Section 13(a) of the Exchange Act. ¨
This Amendment No. 1 to the
Form 8-K (this “Amendment”) amends the Form 8-K (the “Original Form 8-K”) of Cara Therapeutics, Inc. (the “Company”),
which was filed with the Securities and Exchange Commission (the “SEC”) on December 18, 2024. This Amendment is being filed
solely to correct formatting errors and omitted text resulting from the conversion to the Electronic Data Gathering, Analysis, and Retrieval
system (“EDGAR”), of the investor presentation attached as Exhibit 99.2 to the Original Form 8-K.
Except as described above,
this Amendment does not update or modify any other information presented in the Original Form 8-K and does not reflect events occurring
after the Original Form 8-K’s filing date of December 18, 2024.
Cautionary Statement Regarding Forward-Looking
Statements
Certain statements contained
in this Current Report on Form 8-K regarding matters that are not historical facts are "forward-looking statements" within the
meaning of the Private Securities Litigation Reform Act of 1995. Examples of these forward-looking statements include statements concerning
the anticipated completion and effects of the proposed Merger and Asset Disposition and related timing, Tvardi’s and the combined
company’s planned clinical programs, including planned clinical trials and the timing for anticipated trial results, the potential
of Tvardi’s product candidates, the expected trading of the combined company’s stock on the Nasdaq Capital Market, management
of the combined company and other statements regarding management’s intentions, plans, beliefs, expectations or forecasts for the
future, and, therefore, you are cautioned not to place undue reliance on them.
Because such statements are
subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements.
These forward-looking statements are subject to a number of risks, including, among other things: the risk that the conditions to the
closing of the Merger are not satisfied, including that the approval of the stockholders of Cara is not obtained on the timeline expected,
if at all; uncertainties as to the timing of the closing of the Merger and the ability of each of Tvardi and Cara to consummate the Merger;
risks related to the ability of Tvardi and Cara to correctly estimate and manage their respective operating expenses and expenses associated
with the Merger pending the closing of the Merger; risks associated with the possible failure to realize certain anticipated benefits
of the Merger, including with respect to future financial and operating results; the potential for the occurrence of any event, change
or other circumstance or condition that could give rise to the termination of the Merger and any agreements entered into in connection
therewith; the possible effect of the announcement, pendency or completion of the Merger on Tvardi’s or Cara’s business relationships,
operating results and business generally; the risk that as a result of adjustments to the exchange ratio, Tvardi stockholders and Cara
stockholders could own more or less of the combined company than is currently anticipated; risks related to the market price of Cara’s
common stock relative to the value suggested by the exchange ratio; unexpected costs, charges or expenses resulting from the Merger; the
uncertainties associated with Tvardi’s product candidates, as well as risks associated with the clinical development and regulatory
approval of product candidates, including potential delays in the completion of clinical trials; the significant net losses each of Cara
and Tvardi has incurred since inception; the combined company’s ability to initiate and complete ongoing and planned preclinical
studies and clinical trials and advance its product candidates through clinical development; the timing of the availability of data from
the combined company’s clinical trials; the outcome of preclinical testing and clinical trials of the combined company’s product
candidates, including the ability of those trials to satisfy relevant governmental or regulatory requirements; the combined company’s
plans to research, develop and commercialize its current and future product candidates; the clinical utility, potential benefits and market
acceptance of the combined company’s product candidates; the requirement for additional capital to continue to advance these product
candidates, which may not be available on favorable terms or at all; the combined company’s ability to attract, hire, and retain
skilled executive officers and employees; the combined company’s ability to protect its intellectual property and proprietary technologies;
the combined company’s reliance on third parties, contract manufacturers, and contract research organizations; the possibility that
Tvardi, Cara or the combined company may be adversely affected by other economic, business, or competitive factors; risks associated with
changes in applicable laws or regulations; those factors discussed in Cara’s filings with the Securities and Exchange Commission,
including the “Risk Factors” section of the Company’s Annual Report on Form 10-K for the year ending December 31, 2023,
and its other documents subsequently filed with or furnished to the Securities and Exchange Commission, including its Form 10-Q for the
quarter ended September 30, 2024. All forward-looking statements contained in this Current Report on Form 8-K speak only as of the date
on which they were made. Cara undertakes no obligation to update such statements to reflect events that occur or circumstances that exist
after the date on which they were made, except as required by law.
Additional Information and Where to Find It
In connection with the proposed
transaction between Cara and Tvardi, Cara intends to file relevant materials with the SEC, including a registration statement on Form
S-4 that will contain a proxy statement and prospectus. CARA URGES INVESTORS AND STOCKHOLDERS TO READ THESE MATERIALS CAREFULLY AND IN
THEIR ENTIRETY WHEN THEY BECOME AVAILABLE BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION ABOUT CARA, TVARDI, THE PROPOSED TRANSACTION
AND RELATED MATTERS. Stockholders will be able to obtain free copies of the proxy statement, prospectus and other documents filed by Cara
with the SEC (when they become available) through the website maintained by the SEC at www.sec.gov. In addition, stockholders will be
able to obtain free copies of the proxy statement, prospectus and other documents filed by Cara with the SEC by contacting Investor Relations
by email at investor@caratherapeutics.com. Stockholders are urged to read the proxy statement, prospectus and the other relevant materials
when they become available before making any voting or investment decision with respect to the proposed transaction.
Participants in the Solicitation
Cara and Tvardi, and each
of their respective directors and executive officers and certain of their other members of management and employees, may be deemed to
be participants in the solicitation of proxies in connection with the proposed transaction. Information about Cara’s directors and
executive officers, consisting of Helen M. Boudreau, Jeffrey L. Ives, Ph.D., Christopher Posner, Susan Shiff, Ph.D., Martin Vogelbaum,
Lisa von Moltke, M.D., Ryan Maynard and Scott Terrillion, including a description of their interests in Cara, by security holdings or
otherwise, can be found under the captions, “Security Ownership of Certain Beneficial Owners and Management,” “Executive
Compensation” and “Director Compensation” contained in the definitive proxy statement on Schedule 14A for Cara’s
2024 annual meeting of stockholders, filed with the SEC on April 22, 2024 (the “2024 Cara Proxy Statement”). To the extent
that Cara’s directors and executive officers and their respective affiliates have acquired or disposed of security holdings since
the applicable “as of” date disclosed in the 2024 Cara Proxy Statement, such transactions have been or will be reflected on
Statements of Change in Beneficial Ownership on Form 4 filed with the SEC. Additional information regarding the persons who may be deemed
participants in the proxy solicitation, including the information about the directors and executive officers of Tvardi, and a description
of their direct and indirect interests, by security holdings or otherwise, will also be included in a registration statement filed on
Form S-4 that will contain a proxy statement (and prospectus and other relevant materials) to be filed with the SEC when they become available.
Investors should read the registration statement, proxy statement/prospectus and the other relevant materials when they become available
before making any voting or investment decision with respect to the proposed transaction. These documents can be obtained free of charge
from the sources indicated above.
Non-Solicitation
This Current Report on Form
8-K shall not constitute an offer to sell or the solicitation of an offer to sell or the solicitation of an offer to buy any securities,
nor shall there be any sale of securities in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration
or qualification under the securities laws of any such jurisdiction. No public offer of securities shall be made except by means of a
prospectus meeting the requirements of Section 10 of the Securities Act of 1933, as amended.
| Item 9.01 |
Financial Statements and Exhibits. |
(d) Exhibits.
Exhibit
No. |
|
Description |
| 2.1* |
|
Agreement and Plan of Merger and Reorganization, dated December 17, 2024, by and among Cara Therapeutics, Inc., CT Convergence Merger Sub, Inc. and Tvardi Therapeutics, Inc. (incorporated by reference to Exhibit 2.1 to the Original Form 8-K) |
| 10.1 |
|
Form of Cara Therapeutics, Inc. Stockholder Support Agreement, dated December 17, 2024 (incorporated by reference to Exhibit 10.1 to the Original Form 8-K) |
| 10.2 |
|
Form of Tvardi Therapeutics, Inc. Stockholder Support Agreement, dated December 17, 2024 (incorporated by reference to Exhibit 10.2 to the Original Form 8-K) |
| 10.3 |
|
Form of Lock-Up Agreement, dated December 17, 2024 (incorporated by reference to Exhibit 10.3 to the Original Form 8-K) |
| 10.4* |
|
Asset Purchase Agreement, dated December 17, 2024, by and among Cara Therapeutics, Inc. Cara Royalty Sub, LLC and Vifor Fresenius Medical Care Renal Pharma, Ltd. (incorporated by reference to Exhibit 10.4 to the Original Form 8-K) |
| 99.1 |
|
Joint Press Release of Cara Therapeutics, Inc. and Tvardi Therapeutics, Inc. issued on December 18, 2024 (incorporated by reference to Exhibit 99.1 to the Original Form 8-K) |
| 99.2 |
|
Investor Presentation |
| 104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL document) |
* Exhibits and/or schedules have been omitted
pursuant to Item 601(a)(5) or 601(b)(2) of Regulation S-K, as applicable. The registrant hereby undertakes to furnish supplementally copies
of any of the omitted exhibits and schedules upon request by the SEC; provided, however, that the registrant may request confidential
treatment pursuant to Rule 24b-2 under the Exchange Act for any exhibits or schedules so furnished.
SIGNATURES
Pursuant to the requirements of the Securities
Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| CARA THERAPEUTICS, INC. |
|
| |
|
| By: |
/s/ Ryan Maynard |
|
| |
Ryan Maynard |
|
| |
Chief Financial Officer |
|
Dated: December 20, 2024
Exhibit 99.2

| 1
Proposed
Merger
Overview
December 18, 2024 |

| 2
Disclaimer and Forward-Looking Statements
This presentation and any accompanying oral commentary have been prepared by Tvardi Therapeutics, Inc. (“Tvardi”) and Cara Therapeutics, Inc. (“Cara”) for informational purposes only and to assist
such parties in making their own evaluation with respect to the potential combination (the “Proposed Merger”) of a wholly-owned subsidiary of Cara with and into Tvardi and related transactions and not
for any other purpose. All statements contained in this presentation and the accompanying oral commentary, other than statements of historical facts, are forward-looking statements, including:
statements about the combined company’s expectations regarding the potential benefits, activity, effectiveness, and safety of its product candidates; the combined company’s expectations with regard
to the design and results of its research and development programs, preclinical studies, and clinical trials, including the timing and availability of data from such studies and trials; the combined
company’s preclinical, clinical, and regulatory development plans for its product candidates, including the timing or likelihood of regulatory filings and approvals for the combined company’s product
candidates; the combined company’s expectations with regard to its ability to license, acquire, discover, and develop additional products candidates and advance such product candidates into, and
successfully complete, preclinical studies and clinical trials; the potential market size and size of the potential patient populations for the combined company’s product candidates and any future product
candidates; ability to maintain existing, and establish new, strategic collaborations, licensing, or other arrangements; the scope of protection the combined company is able to establish and maintain for
intellectual property rights covering its initial product candidate and any future product candidates; the combined company’s business strategy; the combined company’s future results of operations and
financial position; the combined company’s expectations with respect to future performance and anticipated financial impacts of the Proposed Merger; the satisfaction of closing conditions to the
Proposed Merger and the timing of the completion of the Proposed Merger, including obtaining the approval of the Proposed Merger and issuance of shares contemplated thereby by Cara’s
stockholders. These statements involve substantial known and unknown risks, uncertainties and other factors that may cause the combined company’s actual results, timing of results, levels of activity,
performance, or achievements to be materially different from the information expressed or implied by these forward-looking statements. New risks emerge from time to time. It is not possible for Tvardi’s
and Cara’s management to predict all risks, nor can they assess the impact of all factors on the combined company’s business or the extent to which any factor, or combination of factors, may cause
actual results to differ materially and adversely from those anticipated or implied in the forward-looking statements.
Tvardi and Cara may not actually achieve the plans, intentions, or expectations disclosed in their forward-looking statements, and you should not place undue reliance on such forward-looking
statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements Tvardi and Cara make. The forward-looking
statements in this presentation represent Tvardi’s and Cara’s views as of the date of this presentation. Tvardi and Cara anticipate that subsequent events and developments will cause their views to
change. However, while Tvardi and Cara may elect to update these forward-looking statements at some point in the future, they have no current intention of doing so except to the extent required by
applicable law. Except as required by law, neither Tvardi and Cara nor any other person assumes responsibility for the accuracy and completeness of the forward-looking statements in this presentation
and the accompanying oral commentary. You should, therefore, not rely on these forward-looking statements as representing Tvardi’s or Cara’s views as of any date subsequent to the date of this
presentation.
This presentation also contains estimates and other statistical data made by independent parties and by Tvardi and Cara relating to market size and growth and other data about the combined
company’s industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions, and estimates
of the combined company’s future performance and the future performance of the markets in which it will operate are necessarily subject to a high degree of uncertainty and risk.
This presentation contains trademarks, service marks, trade names and copyrights of Tvardi, Cara and other companies which are the property of their respective owners.
This presentation shall not constitute an offer to sell or the solicitation of an offer to buy these securities, nor shall there be any sale of these securities in any state or jurisdiction in which such offer,
solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction. |

| 3
C O N F I D E N T I A L
Chris Posner
CEO, Cara Therapeutics |
| 4
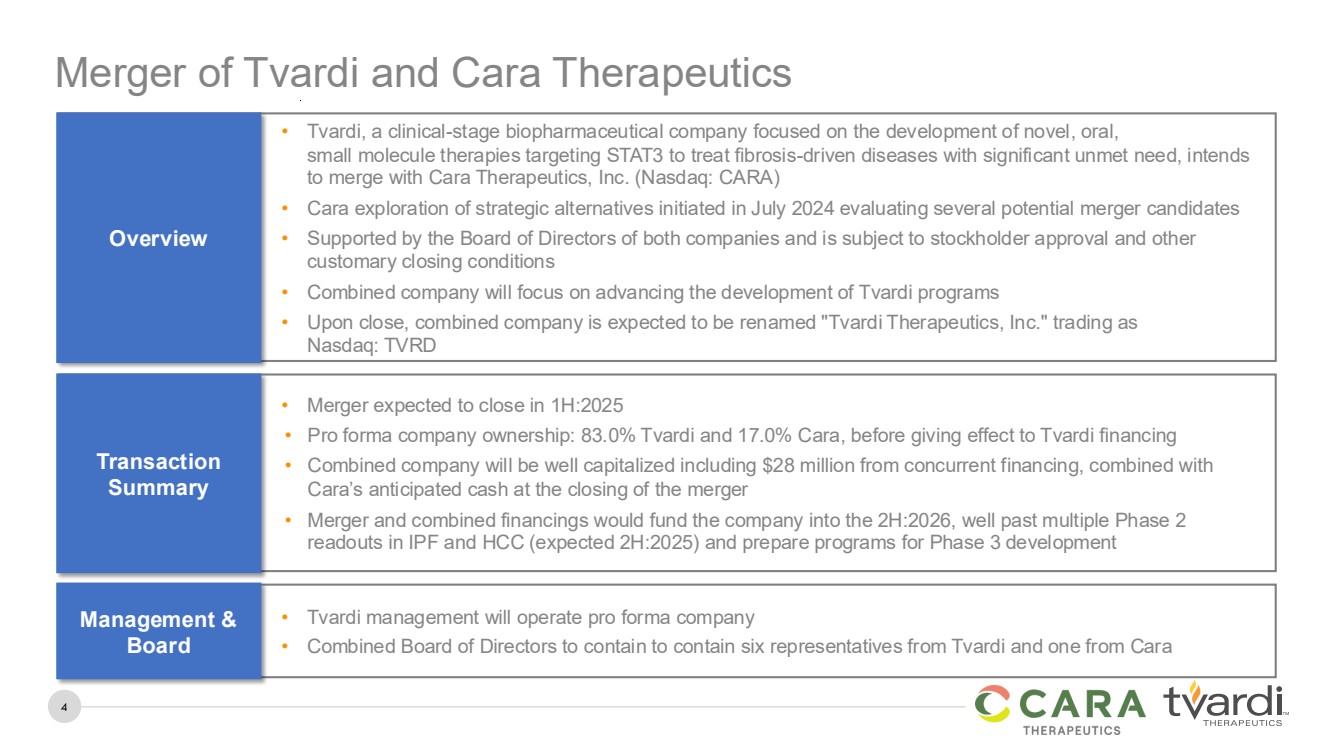
Merger of Tvardi and Cara Therapeutics
• Tvardi, a clinical-stage biopharmaceutical company focused on the development of novel, oral,
small molecule therapies targeting STAT3 to treat fibrosis-driven diseases with significant unmet need, intends
to merge with Cara Therapeutics, Inc. (Nasdaq: CARA)
• Cara exploration of strategic alternatives initiated in July 2024 evaluating several potential merger candidates
• Supported by the Board of Directors of both companies and is subject to stockholder approval and other
customary closing conditions
• Combined company will focus on advancing the development of Tvardi programs
• Upon close, combined company is expected to be renamed "Tvardi Therapeutics, Inc." trading as
Nasdaq: TVRD
Overview
• Merger expected to close in 1H:2025
• Pro forma company ownership: 83.0% Tvardi and 17.0% Cara, before giving effect to Tvardi financing
• Combined company will be well capitalized including $28 million from concurrent financing, combined with
Cara’s anticipated cash at the closing of the merger
• Merger and combined financings would fund the company into the 2H:2026, well past multiple Phase 2
readouts in IPF and HCC (expected 2H:2025) and prepare programs for Phase 3 development
Transaction
Summary
• Tvardi management will operate pro forma company
• Combined Board of Directors to contain to contain six representatives from Tvardi and one from Cara
Management &
Board |

| 5
C O N F I D E N T I A L
Imran Alibhai, PhD
CEO, Tvardi Therapeutics |
| 6
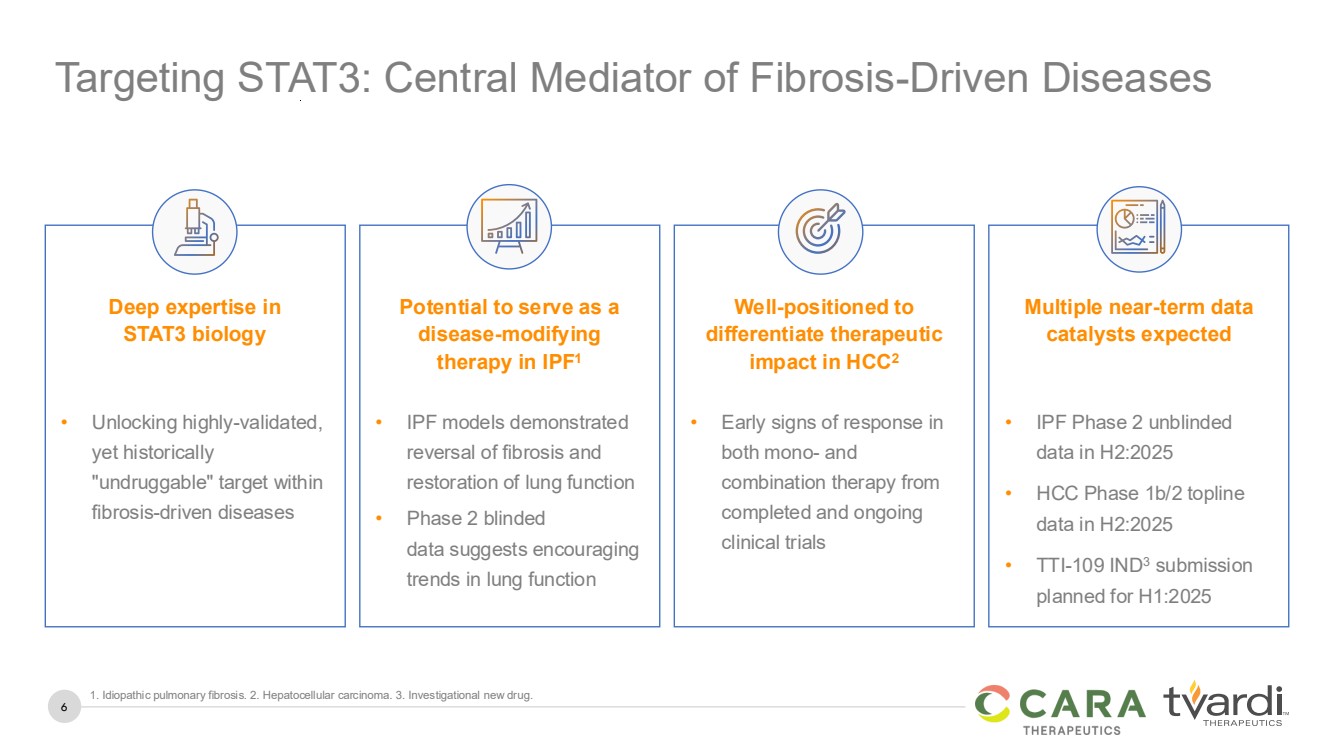
Targeting STAT3: Central Mediator of Fibrosis-Driven Diseases
Deep expertise in
STAT3 biology
Potential to serve as a
disease-modifying
therapy in IPF1
Well-positioned to
differentiate therapeutic
impact in HCC2
Multiple near-term data
catalysts expected
• Unlocking highly-validated,
yet historically
"undruggable" target within
fibrosis-driven diseases
• IPF models demonstrated
reversal of fibrosis and
restoration of lung function
• Phase 2 blinded
data suggests encouraging
trends in lung function
• Early signs of response in
both mono- and
combination therapy from
completed and ongoing
clinical trials
• IPF Phase 2 unblinded
data in H2:2025
• HCC Phase 1b/2 topline
data in H2:2025
• TTI-109 IND3 submission
planned for H1:2025
1. Idiopathic pulmonary fibrosis. 2. Hepatocellular carcinoma. 3. Investigational new drug. |
| 7
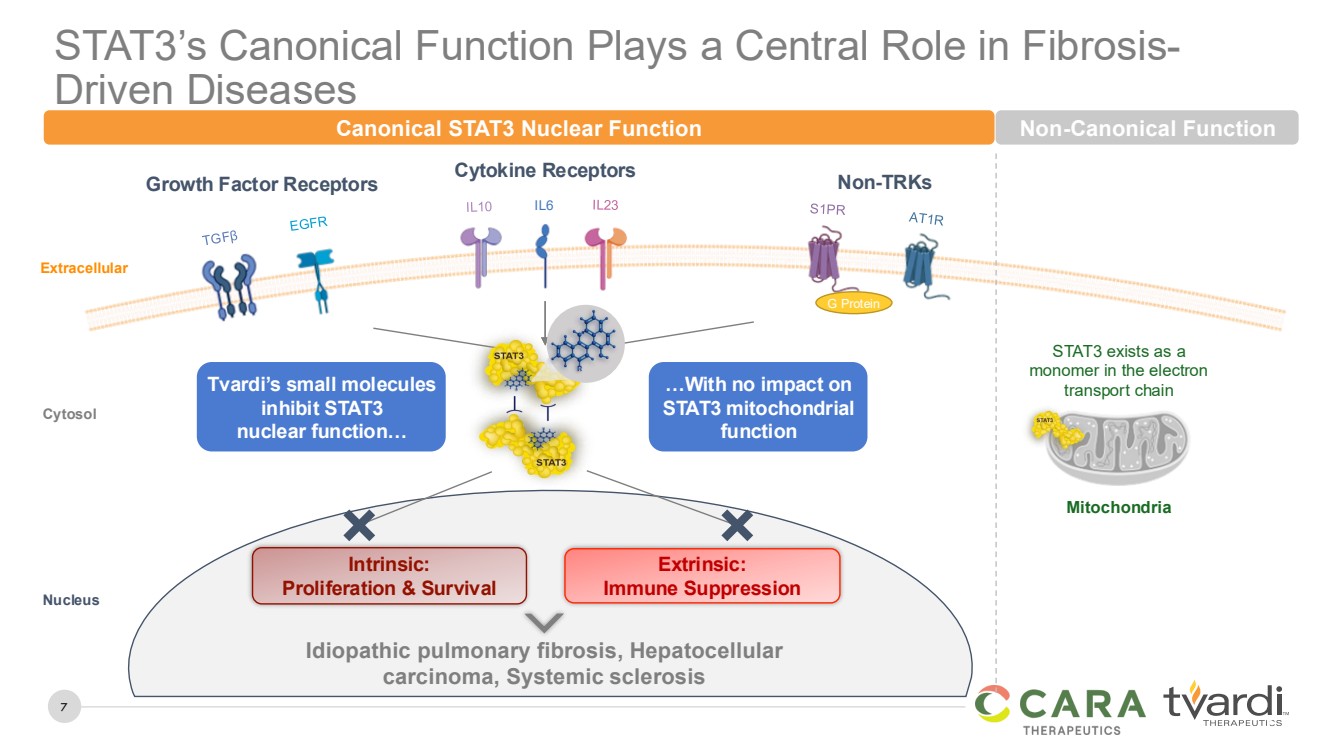
STAT3’s Canonical Function Plays a Central Role in Fibrosis-Driven Diseases
CONFIDENTIAL 7
Mitochondria
STAT3 exists as a
monomer in the electron
transport chain
IL6
Growth Factor Receptors Cytokine Receptors Non-TRKs
Extracellular
Idiopathic pulmonary fibrosis, Hepatocellular
carcinoma, Systemic sclerosis
Cytosol
Nucleus
G Protein
Tvardi’s small molecules
inhibit STAT3
nuclear function…
…With no impact on
STAT3 mitochondrial
function
Canonical STAT3 Nuclear Function Non-Canonical Function
Intrinsic:
Proliferation & Survival
Extrinsic:
Immune Suppression |
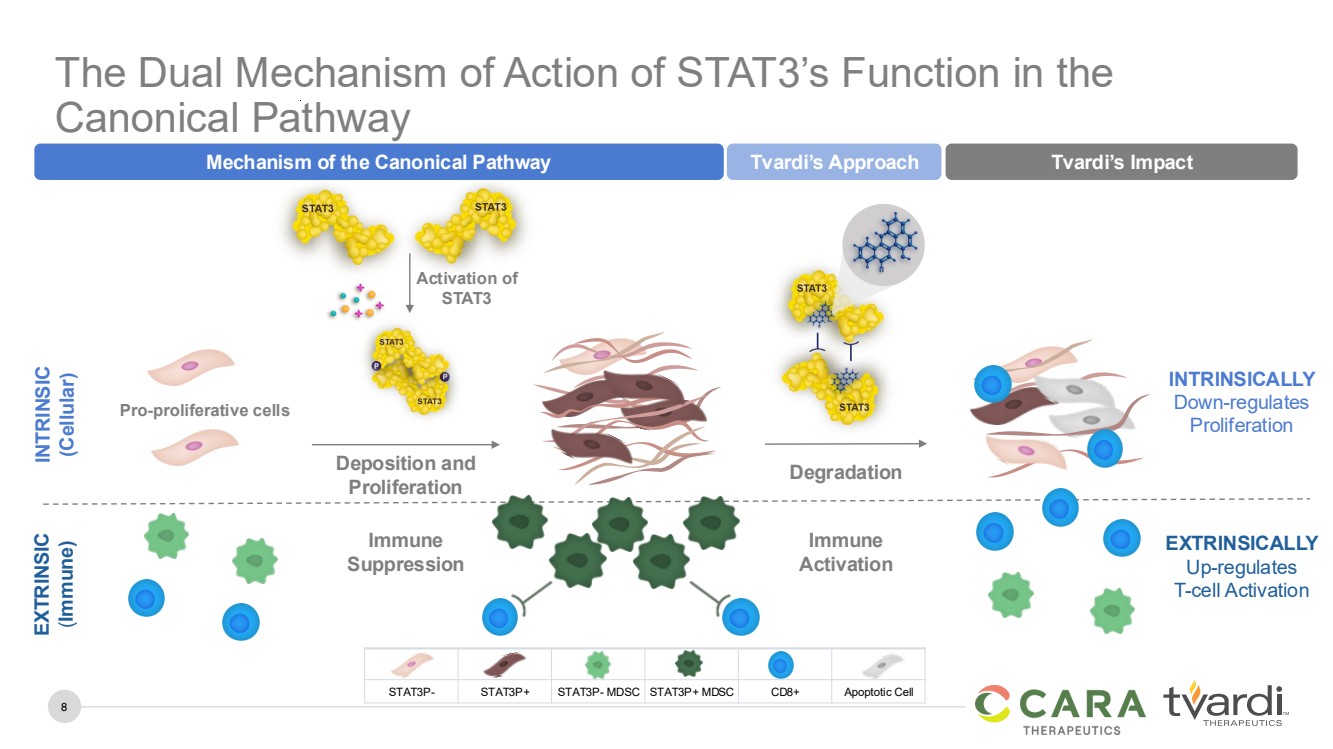
| 8
The Dual Mechanism of Action of STAT3’s Function in the
Canonical Pathway
EXTRINSICALLY
Up-regulates
T-cell Activation
Immune
Activation
Degradation
Tvardi’s Approach Tvardi’s Impact
INTRINSICALLY
Down-regulates
Proliferation
INTRINSIC
(Cellular)
EXTRINSIC
(Immune)
Immune
Suppression
Pro-proliferative cells
Activation of
STAT3
Deposition and
Proliferation
Mechanism of the Canonical Pathway
STAT3P- STAT3P+ STAT3P- MDSC STAT3P+ MDSC CD8+ Apoptotic Cell |

| 9
Sujal Shah Chairman
Michael Wyzga Director
Shaheen Wirk, MD Director
Wallace Hall Director
Cara Representative Director
Seasoned Leadership: Deep R&D and Operational Expertise &
Strong Existing Support
Imran Alibhai, PhD CEO & Director Dan Conn, JD, MBA CFO John Kauh, MD CMO
David Tweardy, MD Founder & Advisor
Ron DePinho, MD Founder & Advisor
Keith Flaherty, MD Advisor (Oncology)
Lisa Lancaster, MD Advisor (IPF)
Jeff Swigris, DO Advisor (IPF)
Management Team
Scientific Advisory Board
Existing Investors
Board of Directors
BioMatrix Partners |
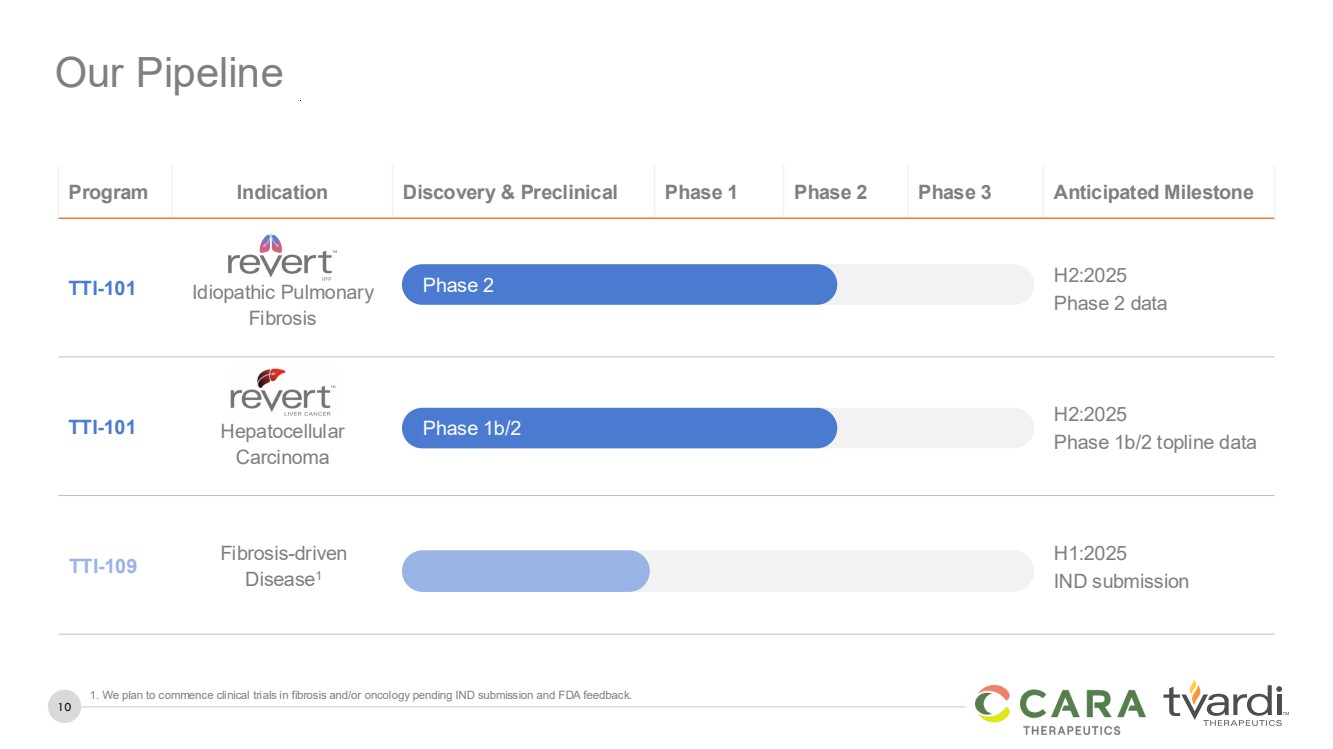
| 10
Our Pipeline
Program Indication Discovery & Preclinical Phase 1 Phase 2 Phase 3 Anticipated Milestone
TTI-101 Idiopathic Pulmonary
Fibrosis
H2:2025
Phase 2 data
TTI-101 Hepatocellular
Carcinoma
H2:2025
Phase 1b/2 topline data
TTI-109 Fibrosis-driven
Disease1
H1:2025
IND submission
Phase 2
Phase 1b/2
1. We plan to commence clinical trials in fibrosis and/or oncology pending IND submission and FDA feedback. |

| 11
CONFIDENTIAL
TTI-101 in IPF |
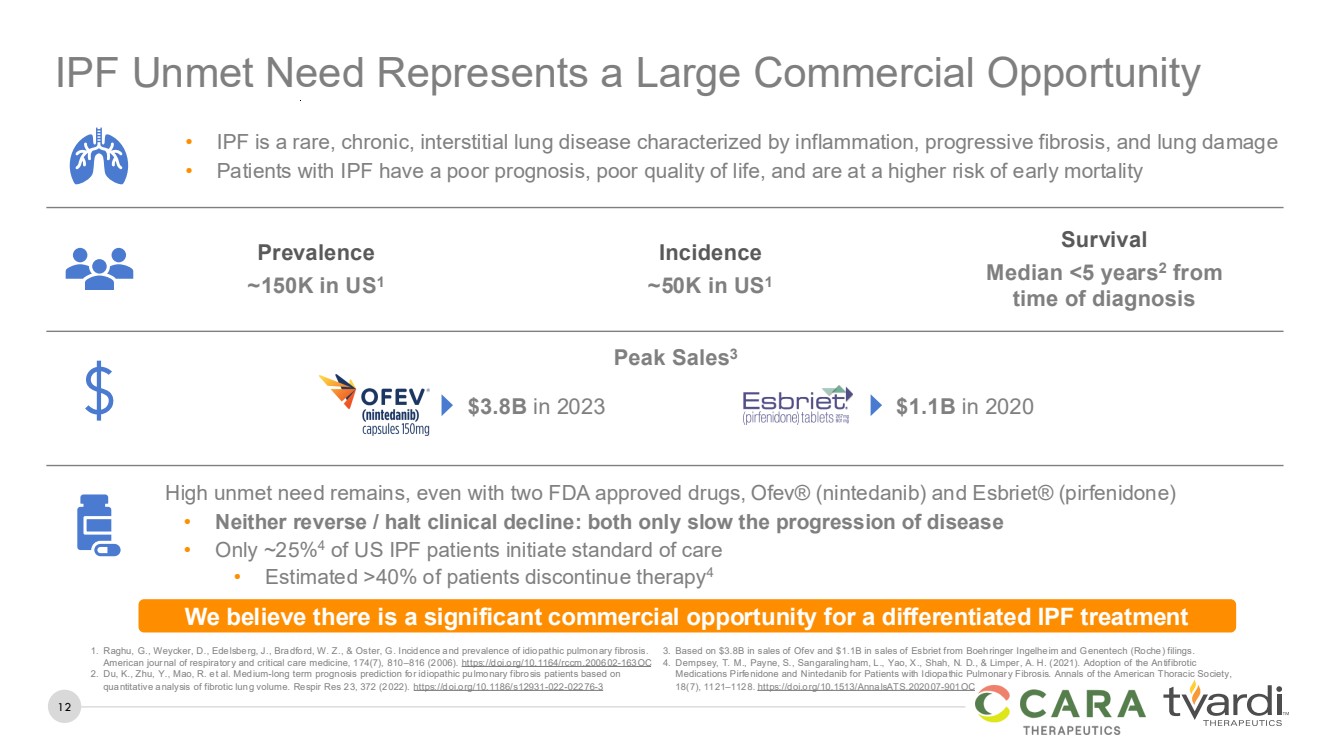
| 12
IPF Unmet Need Represents a Large Commercial Opportunity
• IPF is a rare, chronic, interstitial lung disease characterized by inflammation, progressive fibrosis, and lung damage
• Patients with IPF have a poor prognosis, poor quality of life, and are at a higher risk of early mortality
We believe there is a significant commercial opportunity for a differentiated IPF treatment
Prevalence
~150K in US1
Incidence
~50K in US1
Survival
Median <5 years2
from
time of diagnosis
High unmet need remains, even with two FDA approved drugs, Ofev® (nintedanib) and Esbriet® (pirfenidone)
• Neither reverse / halt clinical decline: both only slow the progression of disease
• Only ~25%4 of US IPF patients initiate standard of care
• Estimated >40% of patients discontinue therapy4
Peak Sales3
$3.8B in 2023 $1.1B in 2020
1. Raghu, G., Weycker, D., Edelsberg, J., Bradford, W. Z., & Oster, G. Incidence and prevalence of idiopathic pulmonary fibrosis.
American journal of respiratory and critical care medicine, 174(7), 810–816 (2006). https://doi.org/10.1164/rccm.200602-163OC
2. Du, K., Zhu, Y., Mao, R. et al. Medium-long term prognosis prediction for idiopathic pulmonary fibrosis patients based on
quantitative analysis of fibrotic lung volume. Respir Res 23, 372 (2022). https://doi.org/10.1186/s12931-022-02276-3
3. Based on $3.8B in sales of Ofev and $1.1B in sales of Esbriet from Boehringer Ingelheim and Genentech (Roche) filings.
4. Dempsey, T. M., Payne, S., Sangaralingham, L., Yao, X., Shah, N. D., & Limper, A. H. (2021). Adoption of the Antifibrotic
Medications Pirfenidone and Nintedanib for Patients with Idiopathic Pulmonary Fibrosis. Annals of the American Thoracic Society,
18(7), 1121–1128. https://doi.org/10.1513/AnnalsATS.202007-901OC |
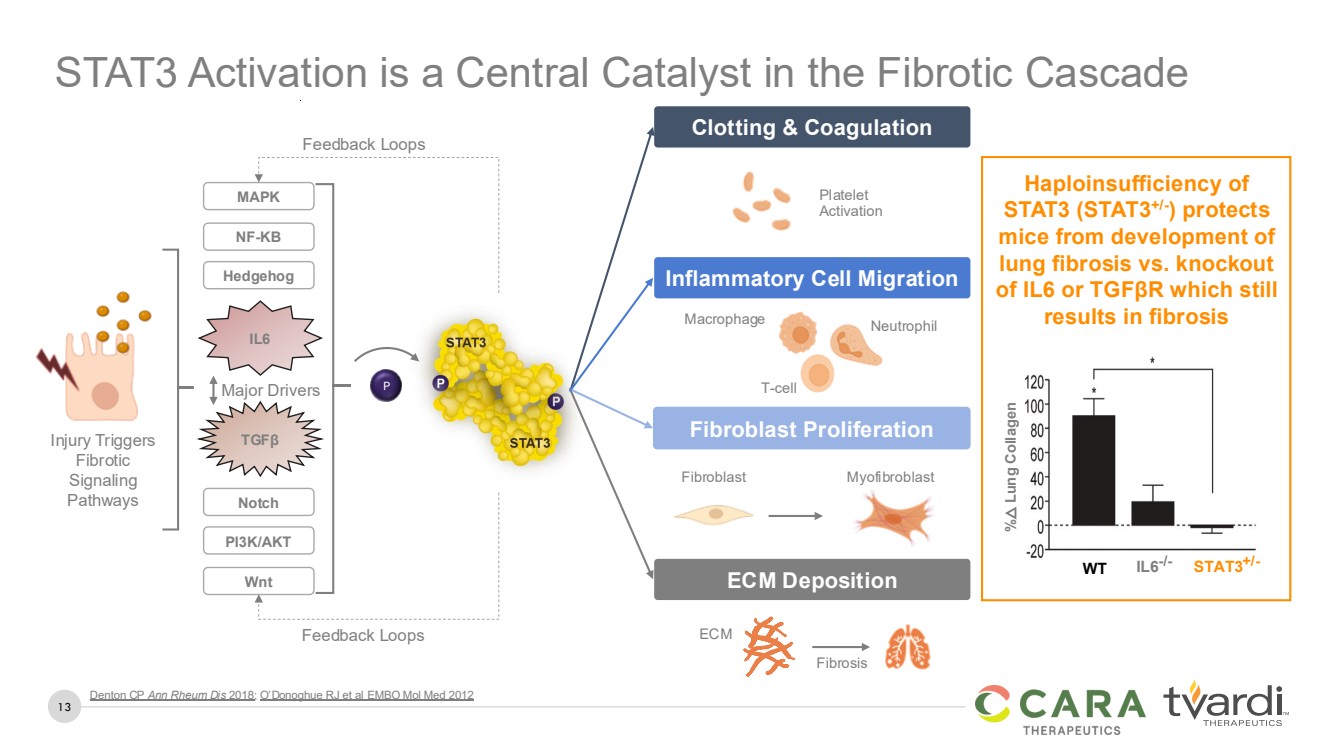
| 13
STAT3 Activation is a Central Catalyst in the Fibrotic Cascade
IL6-/- STAT3+/- WT
%
△ Lung Collagen
Denton CP Ann Rheum Dis 2018; O’Donoghue RJ et al EMBO Mol Med 2012
Notch
IL6
TGFβ
NF-KB
MAPK
Hedgehog
PI3K/AKT
Wnt
Injury Triggers
Fibrotic
Signaling
Pathways
Haploinsufficiency of
STAT3 (STAT3+/-
) protects
mice from development of
lung fibrosis vs. knockout
of IL6 or TGFβR which still
results in fibrosis
Clotting & Coagulation
Fibroblast Proliferation
ECM Deposition
Macrophage Neutrophil
T-cell
Platelet
Activation
ECM
Fibrosis
Fibroblast Myofibroblast
Inflammatory Cell Migration
Major Drivers P
Feedback Loops
Feedback Loops |
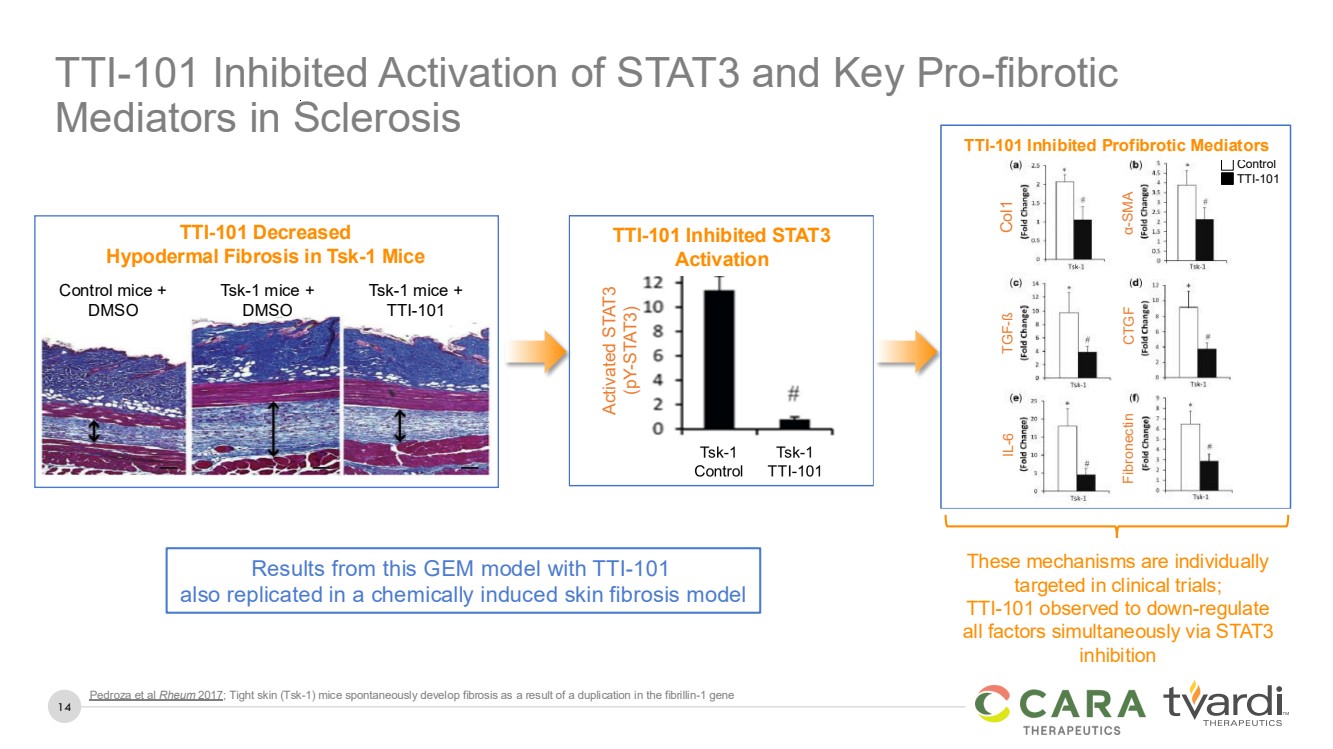
| 14
Pedroza et al Rheum 2017; Tight skin (Tsk-1) mice spontaneously develop fibrosis as a result of a duplication in the fibrillin-1 gene
TTI-101 Inhibited Activation of STAT3 and Key Pro-fibrotic
Mediators in Sclerosis
TTI-101 Decreased
Hypodermal Fibrosis in Tsk-1 Mice
Control mice +
DMSO
Tsk-1 mice +
DMSO
Tsk-1 mice +
TTI-101
These mechanisms are individually
targeted in clinical trials;
TTI-101 observed to down-regulate
all factors simultaneously via STAT3
inhibition
Results from this GEM model with TTI-101
also replicated in a chemically induced skin fibrosis model
TTI-101 Inhibits STAT3 Activation
Activated STAT3
(pY-STAT3)
TTI-101 Inhibited STAT3
Activation
TTI-101 Inhibits Pro-fibrotic Mediators
Control
TTI-101
Col1 TGF-ß IL-6
α-SMA CTGF Fibronectin
TTI-101 Inhibited Profibrotic Mediators |
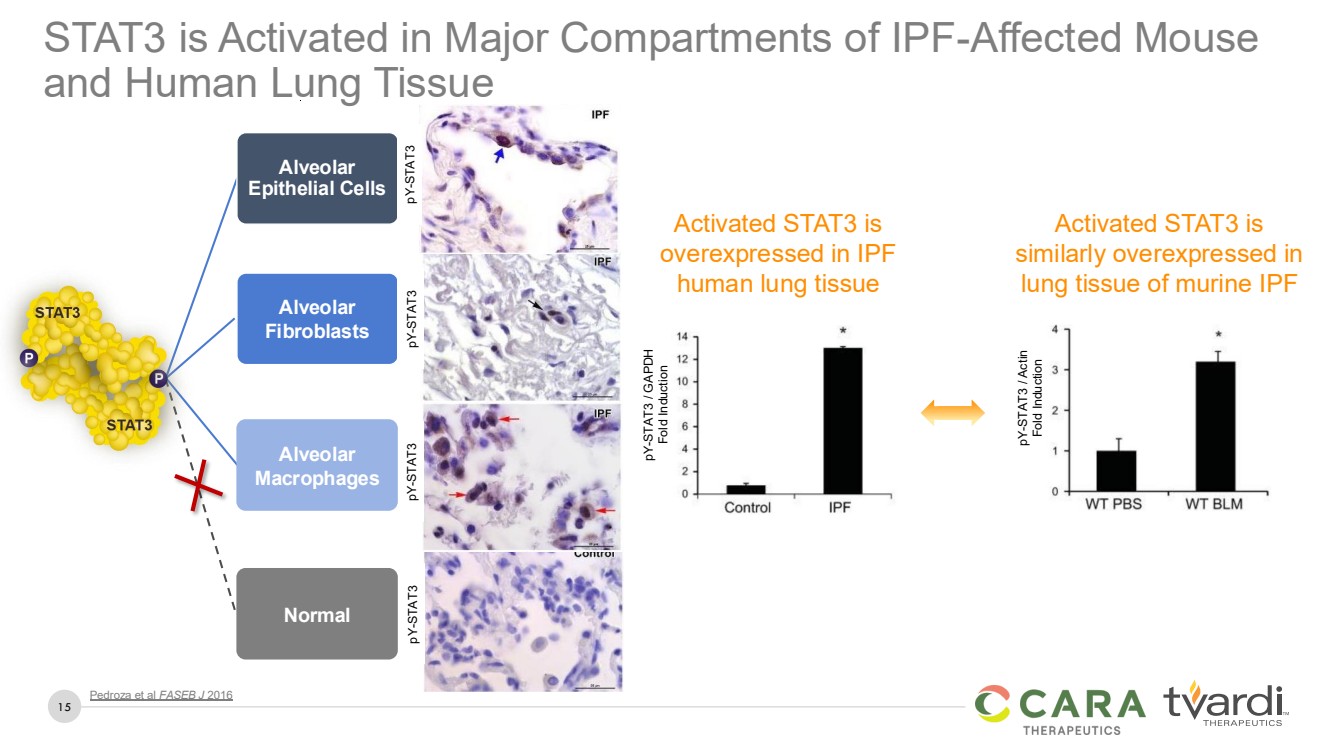
| 15
STAT3 is Activated in Major Compartments of IPF-Affected Mouse
and Human Lung Tissue
Pedroza et al FASEB J 2016
Activated STAT3 is
overexpressed in IPF
human lung tissue
Activated STAT3 is
similarly overexpressed in
lung tissue of murine IPF
pY-STAT3 / GAPDH
Fold Induction
pY-STAT3 / Actin
Fold Induction
Alveolar
Epithelial Cells
Alveolar
Fibroblasts
Alveolar
Macrophages
Normal
pY-STAT3 pY-STAT3 pY-STAT3 pY-STAT3 |
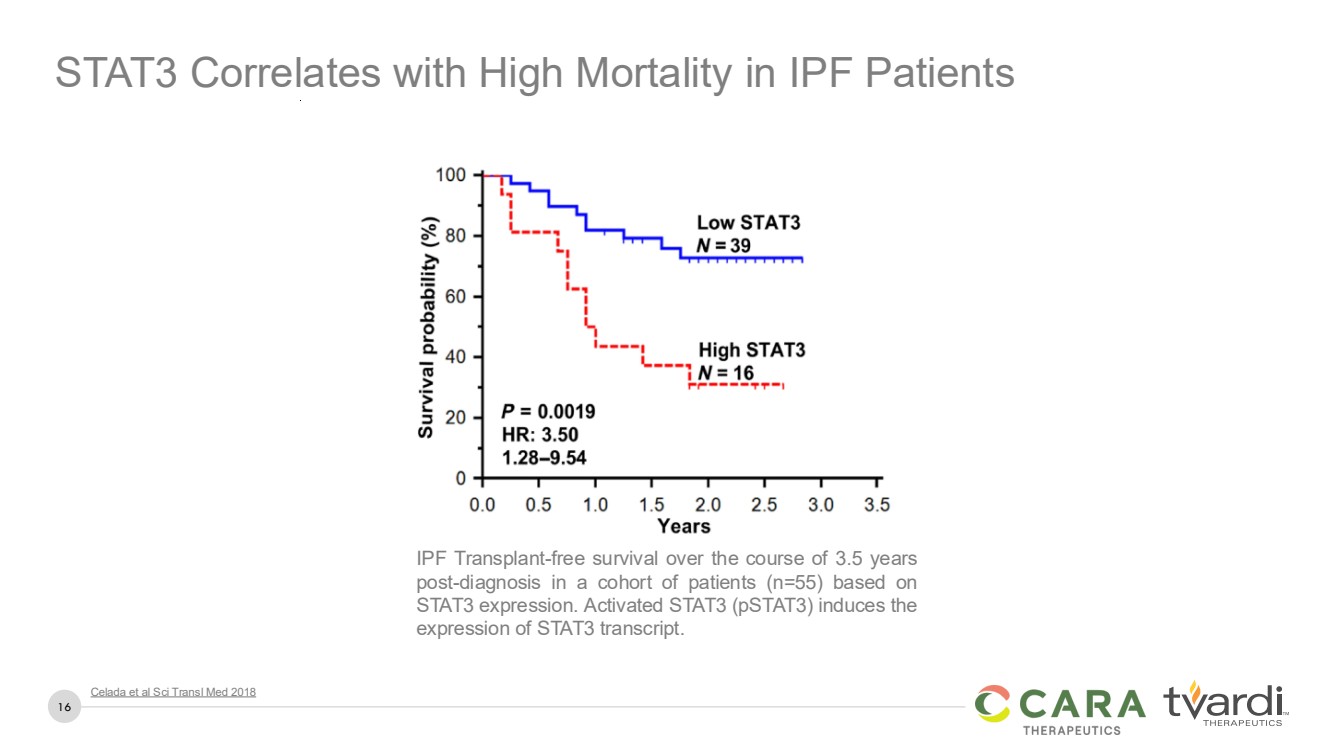
| 16
IPF Transplant-free survival over the course of 3.5 years
post-diagnosis in a cohort of patients (n=55) based on
STAT3 expression. Activated STAT3 (pSTAT3) induces the
expression of STAT3 transcript.
Celada et al Sci Transl Med 2018
STAT3 Correlates with High Mortality in IPF Patients |
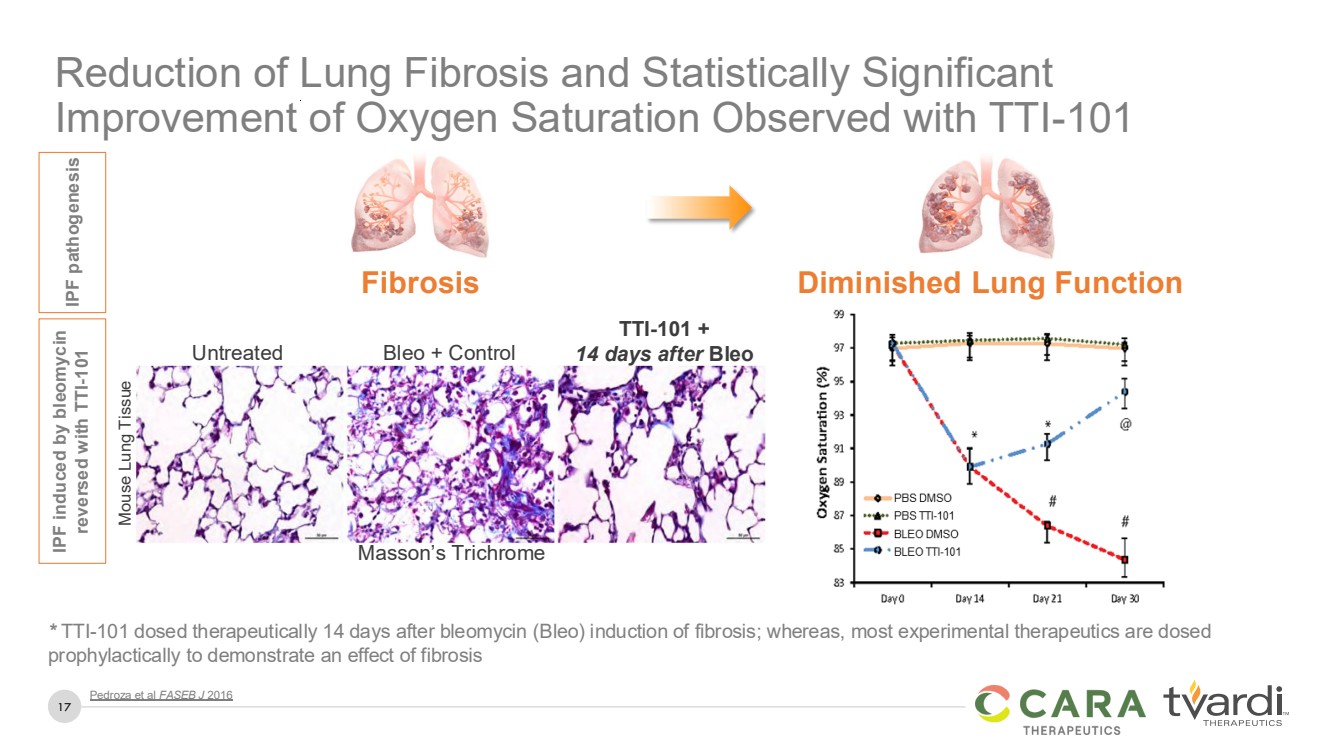
| 17
Reduction of Lung Fibrosis and Statistically Significant
Improvement of Oxygen Saturation Observed with TTI-101
* TTI-101 dosed therapeutically 14 days after bleomycin (Bleo) induction of fibrosis; whereas, most experimental therapeutics are dosed
prophylactically to demonstrate an effect of fibrosis
Pedroza et al FASEB J 2016
Untreated Bleo + Control
TTI-101 +
14 days after Bleo
IPF pathogenesis IPF induced by bleomycin reversed with TTI-101
Fibrosis Diminished Lung Function
Masson’s Trichrome
Mouse Lung Tissue |
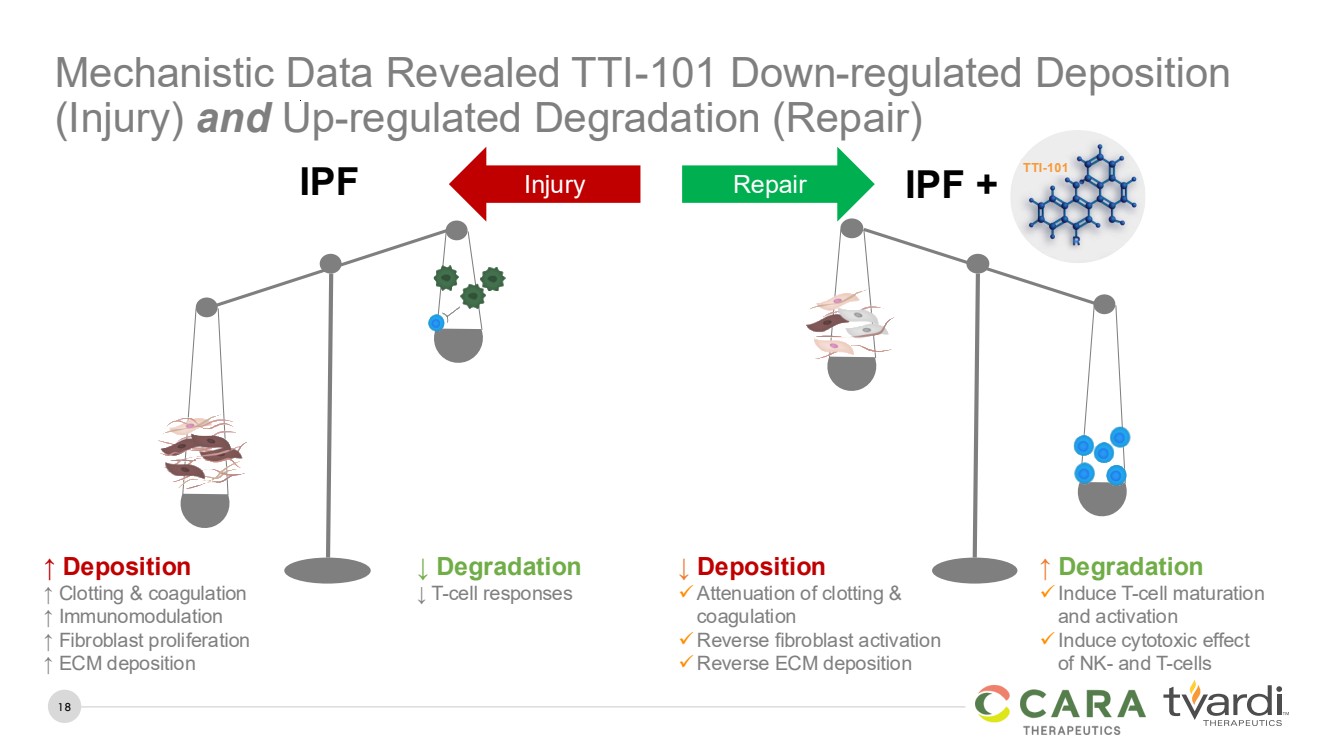
| 18
Mechanistic Data Revealed TTI-101 Down-regulated Deposition
(Injury) and Up-regulated Degradation (Repair)
↑ Deposition
↑ Clotting & coagulation
↑ Immunomodulation
↑ Fibroblast proliferation
↑ ECM deposition
IPF
↓ Degradation
↓ T-cell responses
↓ Deposition
✓Attenuation of clotting &
coagulation
✓Reverse fibroblast activation
✓Reverse ECM deposition
IPF +
↑ Degradation
✓Induce T-cell maturation
and activation
✓Induce cytotoxic effect
of NK- and T-cells
Injury Repair
TUMORIGENESIS &
DRUG RESISTANCE
TTI-101 |
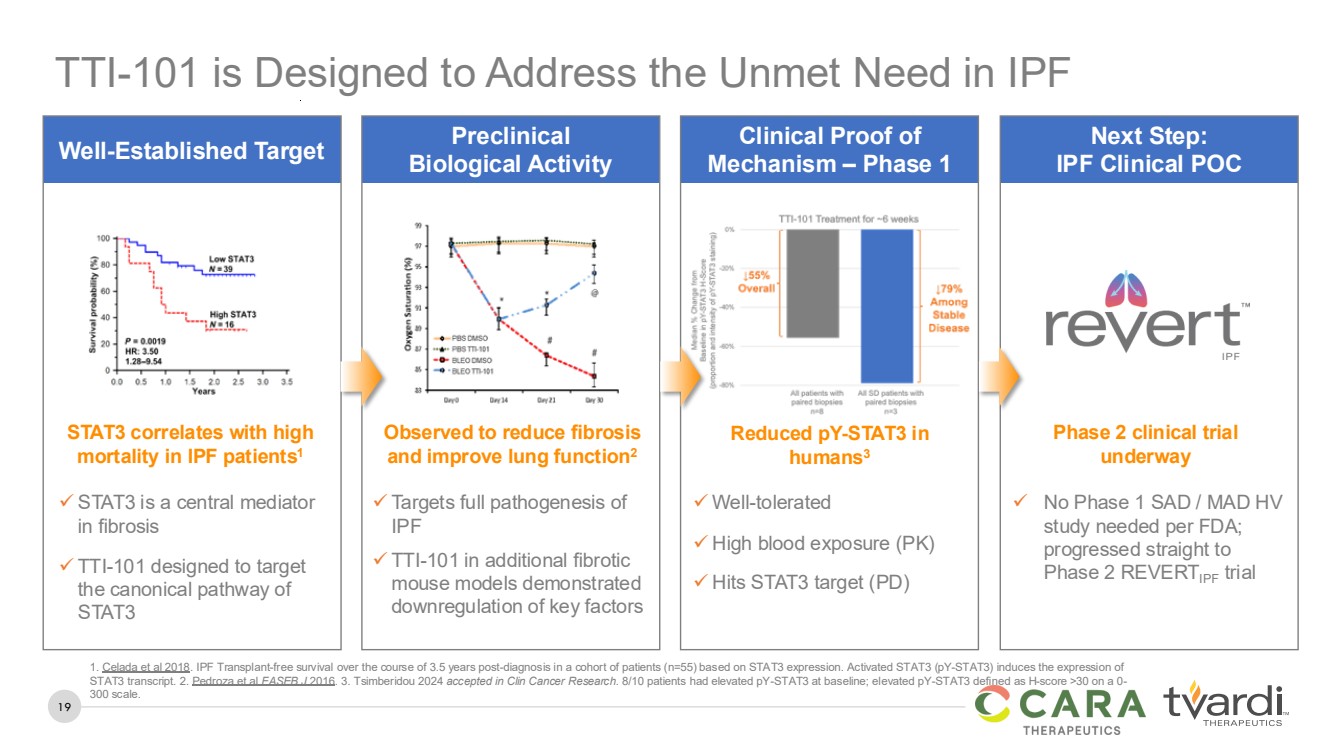
| 19
Well-Established Target Preclinical
Biological Activity
Clinical Proof of
Mechanism – Phase 1
Next Step:
IPF Clinical POC
✓ STAT3 is a central mediator
in fibrosis
✓ TTI-101 designed to target
the canonical pathway of
STAT3
STAT3 correlates with high
mortality in IPF patients1
✓ Well-tolerated
✓ High blood exposure (PK)
✓ Hits STAT3 target (PD)
Reduced pY-STAT3 in
humans3
✓ No Phase 1 SAD / MAD HV
study needed per FDA;
progressed straight to
Phase 2 REVERTIPF trial
Phase 2 clinical trial
underway
✓ Targets full pathogenesis of
IPF
✓ TTI-101 in additional fibrotic
mouse models demonstrated
downregulation of key factors
Observed to reduce fibrosis
and improve lung function2
TTI-101 is Designed to Address the Unmet Need in IPF
1. Celada et al 2018. IPF Transplant-free survival over the course of 3.5 years post-diagnosis in a cohort of patients (n=55) based on STAT3 expression. Activated STAT3 (pY-STAT3) induces the expression of
STAT3 transcript. 2. Pedroza et al FASEB J 2016. 3. Tsimberidou 2024 accepted in Clin Cancer Research. 8/10 patients had elevated pY-STAT3 at baseline; elevated pY-STAT3 defined as H-score >30 on a 0-
300 scale. |
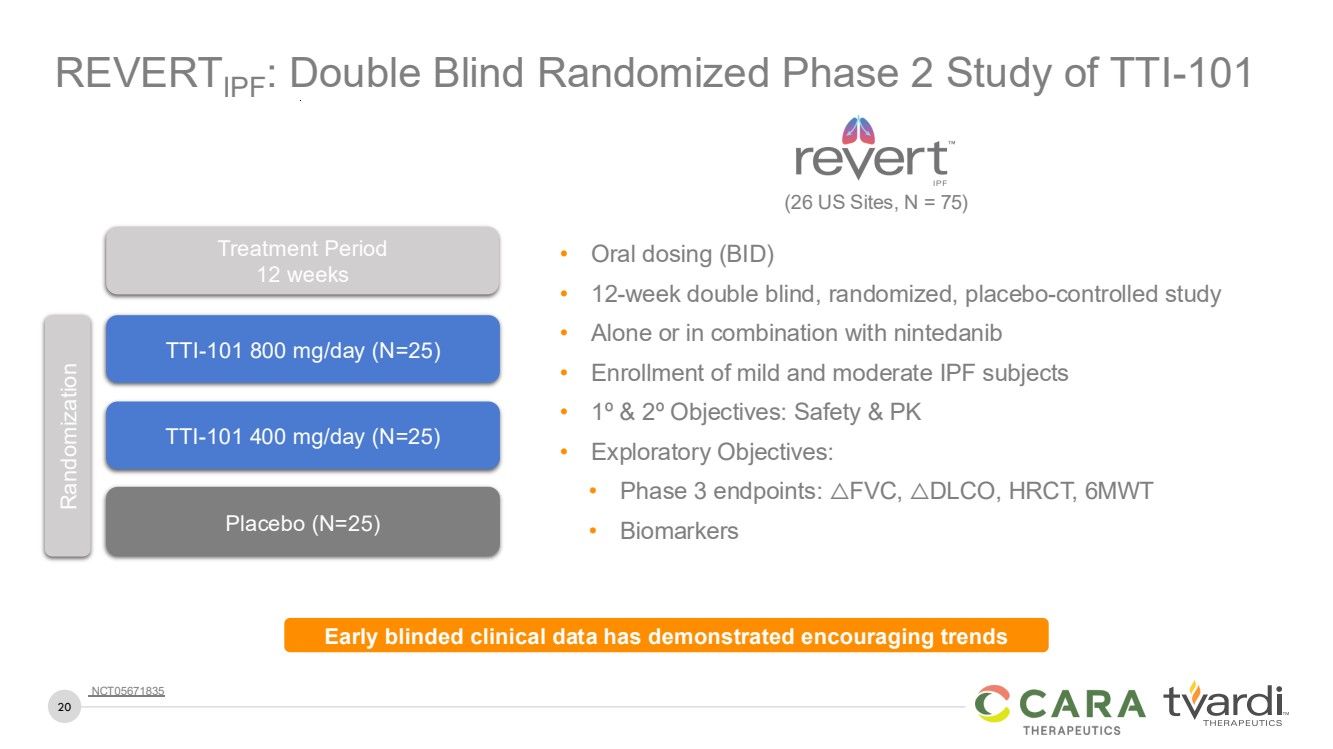
| 20
REVERTIPF: Double Blind Randomized Phase 2 Study of TTI-101
• Oral dosing (BID)
• 12-week double blind, randomized, placebo-controlled study
• Alone or in combination with nintedanib
• Enrollment of mild and moderate IPF subjects
• 1º & 2º Objectives: Safety & PK
• Exploratory Objectives:
• Phase 3 endpoints: △FVC, △DLCO, HRCT, 6MWT
• Biomarkers
TTI-101 800 mg/day (N=25)
TTI-101 400 mg/day (N=25)
Placebo (N=25)
Randomization
Treatment Period
12 weeks
Early blinded clinical data has demonstrated encouraging trends
(26 US Sites, N = 75)
NCT05671835 |

| 21
Driving inhibition of STAT3
activation to address both
IPF disease pathologies
(downregulating deposition
and upregulating
degradation)
REVERTIPF Phase 2 trial
ongoing with clinically relevant
endpoints and collection of
STAT3-mediated biomarkers
Results from ongoing
Phase 2 REVERTIPF trial
expected in H2:2025
Compelling and validated
target → central mediator
in fibrosis
STAT3: Well-Established Biology
Differentiated
Approach
Clinical PoC
Underway
Near-Term Clinical
Milestones
Key Takeaways: TTI-101 in IPF |

| 22
CONFIDENTIAL
TTI-101 in HCC |
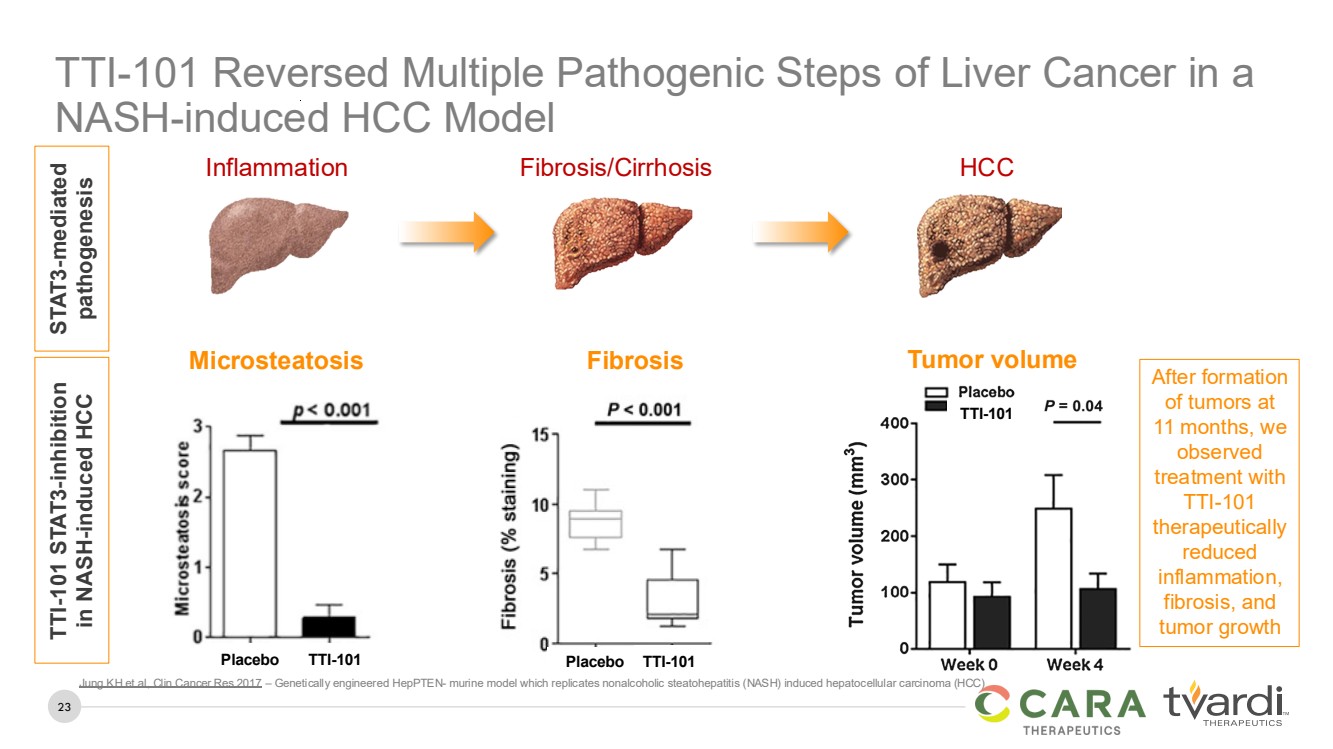
| 23
TTI-101 Reversed Multiple Pathogenic Steps of Liver Cancer in a
NASH-induced HCC Model
Inflammation Fibrosis/Cirrhosis HCC
STAT3-mediated
pathogenesis
TTI-101 STAT3-inhibition
in NASH-induced HCC
TTI-101
Microsteatosis Tumor volume
Placebo TTI-101 TTI-101
Fibrosis
Placebo TTI-101
After formation
of tumors at
11 months, we
observed
treatment with
TTI-101
therapeutically
reduced
inflammation,
fibrosis, and
tumor growth
Jung KH et al, Clin Cancer Res 2017 – Genetically engineered HepPTEN- murine model which replicates nonalcoholic steatohepatitis (NASH) induced hepatocellular carcinoma (HCC) |
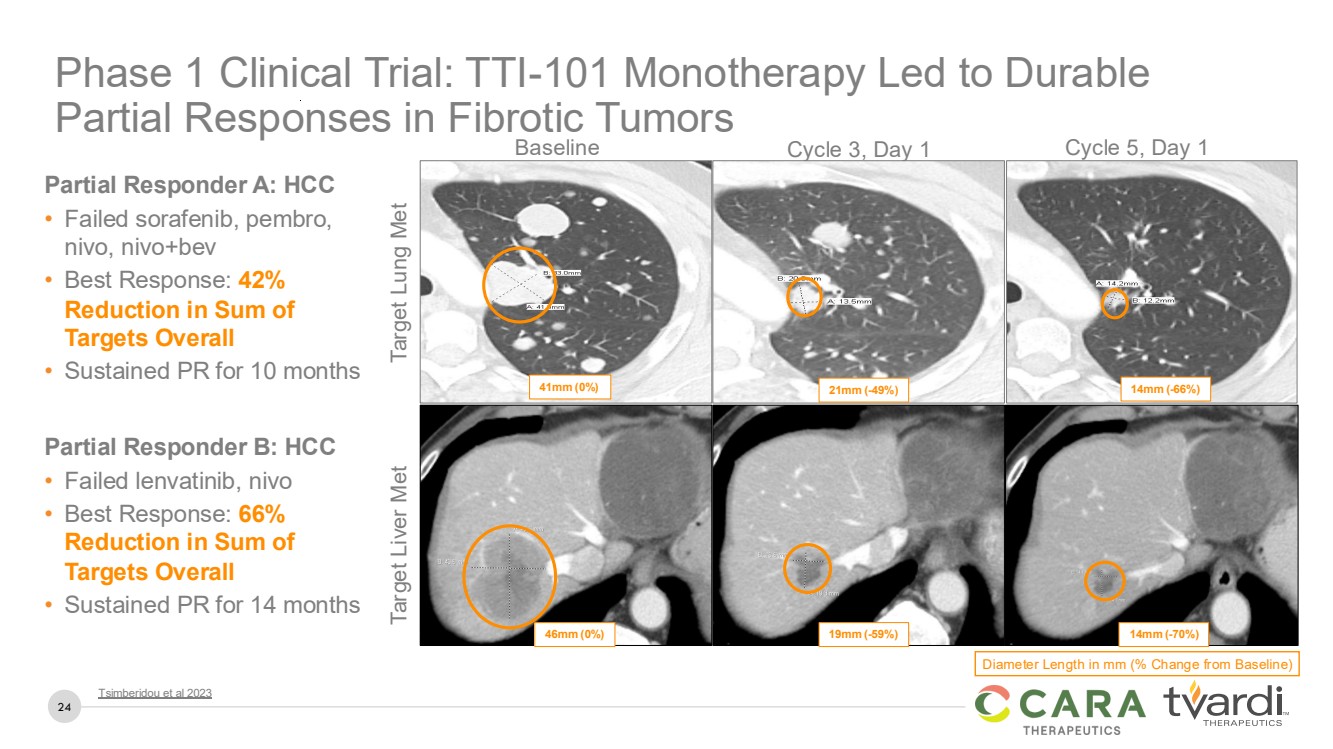
| 24
Phase 1 Clinical Trial: TTI-101 Monotherapy Led to Durable
Partial Responses in Fibrotic Tumors
Baseline Cycle 3, Day 1 Cycle 5, Day 1
Diameter Length in mm (% Change from Baseline)
41mm (0%) 21mm (-49%) 14mm (-66%)
46mm (0%) 19mm (-59%) 14mm (-70%)
Target Lung Met Target Liver Met
Partial Responder A: HCC
• Failed sorafenib, pembro,
nivo, nivo+bev
• Best Response: 42%
Reduction in Sum of
Targets Overall
• Sustained PR for 10 months
Partial Responder B: HCC
• Failed lenvatinib, nivo
• Best Response: 66%
Reduction in Sum of
Targets Overall
• Sustained PR for 14 months
Tsimberidou et al 2023 |
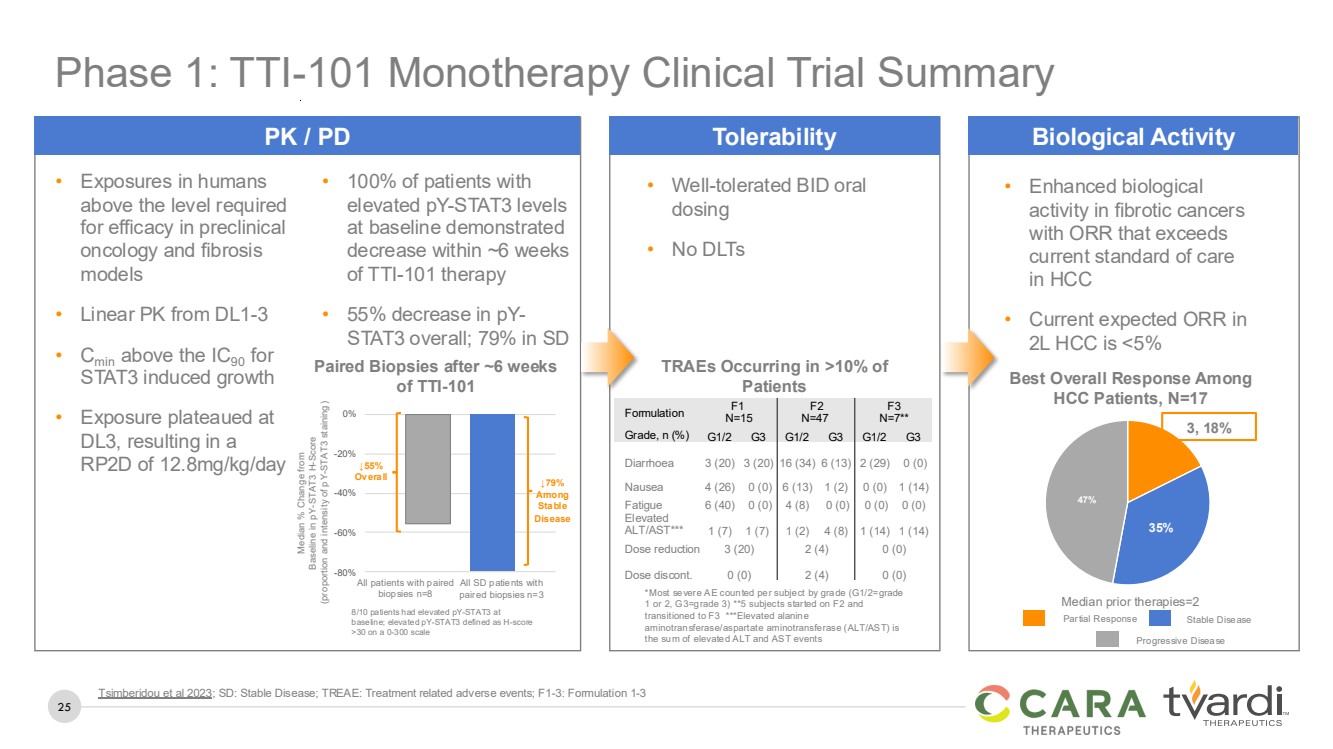
| 25
• Well-tolerated BID oral
dosing
• No DLTs
*Most severe AE counted per subject by grade (G1/2=grade
1 or 2, G3=grade 3) **5 subjects started on F2 and
transitioned to F3 ***Elevated alanine
aminotransferase/aspartate aminotransferase (ALT/AST) is
the sum of elevated ALT and AST events
Tolerability
TRAEs Occurring in >10% of
Patients
• Exposures in humans
above the level required
for efficacy in preclinical
oncology and fibrosis
models
• Linear PK from DL1-3
• Cmin above the IC90 for
STAT3 induced growth
• Exposure plateaued at
DL3, resulting in a
RP2D of 12.8mg/kg/day
Median % Change from
Baseline in pY-STAT3 H-Score
(proportion and intensity of pY-STAT3 staining)
All patients with paired
biopsies n=8
All SD patients with
paired biopsies n=3
-80%
-60%
-40%
-20%
0%
↓79%
Among
Stable
Disease
8/10 patients had elevated pY-STAT3 at
baseline; elevated pY-STAT3 defined as H-score
>30 on a 0-300 scale
↓55%
Overall
• 100% of patients with
elevated pY-STAT3 levels
at baseline demonstrated
decrease within ~6 weeks
of TTI-101 therapy
• 55% decrease in pY-STAT3 overall; 79% in SD
PK / PD
• Enhanced biological
activity in fibrotic cancers
with ORR that exceeds
current standard of care
in HCC
• Current expected ORR in
2L HCC is <5%
35%
47%
3, 18%
Best Overall Response Among
HCC Patients, N=17
Median prior therapies=2
Biological Activity
Paired Biopsies after ~6 weeks
of TTI-101
Partial Response Stable Disease
Progressive Disease
Phase 1: TTI-101 Monotherapy Clinical Trial Summary
Formulation F1
N=15
F2
N=47
F3
N=7**
Grade, n (%) G1/2 G3 G1/2 G3 G1/2 G3
Diarrhoea 3 (20) 3 (20) 16 (34) 6 (13) 2 (29) 0 (0)
Nausea 4 (26) 0 (0) 6 (13) 1 (2) 0 (0) 1 (14)
Fatigue 6 (40) 0 (0) 4 (8) 0 (0) 0 (0) 0 (0)
Elevated
ALT/AST*** 1 (7) 1 (7) 1 (2) 4 (8) 1 (14) 1 (14)
Dose reduction 3 (20) 2 (4) 0 (0)
Dose discont. 0 (0) 2 (4) 0 (0)
Tsimberidou et al 2023; SD: Stable Disease; TREAE: Treatment related adverse events; F1-3: Formulation 1-3 |
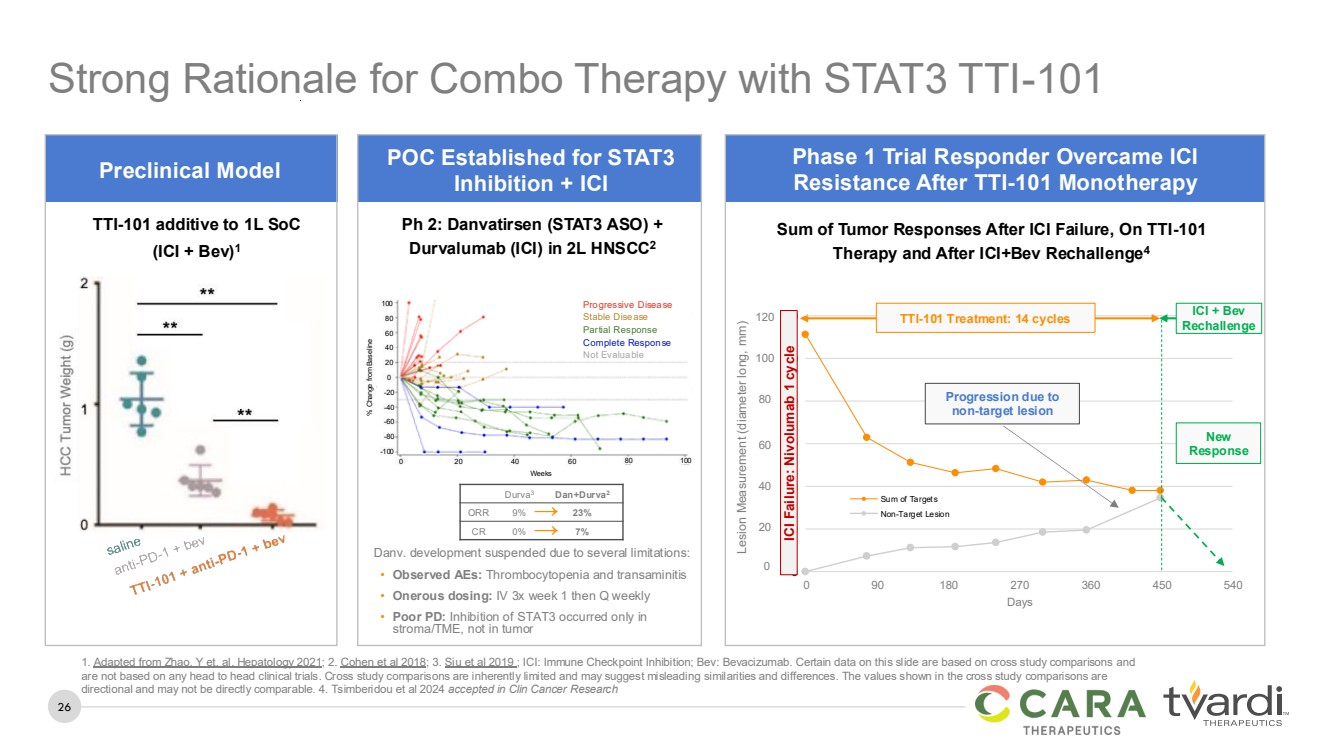
| 26
→
→
Ph 2: Danvatirsen (STAT3 ASO) +
Durvalumab (ICI) in 2L HNSCC2
% Change from Baseline
0
-20
-40
-60
-80
-100
20
40
60
80
100
0 20 40 60 80 100
Weeks
Danv. development suspended due to several limitations:
• Observed AEs: Thrombocytopenia and transaminitis
• Onerous dosing: IV 3x week 1 then Q weekly
• Poor PD: Inhibition of STAT3 occurred only in
stroma/TME, not in tumor
Progressive Disease
Stable Disease
Partial Response
Complete Response
Not Evaluable
POC Established for STAT3
Inhibition + ICI
Strong Rationale for Combo Therapy with STAT3 TTI-101
Durva3 Dan+Durva2
ORR 9% 23%
CR 0% 7%
TTI-101 additive to 1L SoC
(ICI + Bev)1
Preclinical Model
0
20
40
60
80
100
120
0 90 180 270 360 450 540
Days
Sum of Targets
Non-Target Lesion
Progression due to
non-target lesion
New
Response
ICI + Bev
Rechallenge
Sum of Tumor Responses After ICI Failure, On TTI-101
Therapy and After ICI+Bev Rechallenge4
0
20
40
60
80
100
120
ICI Failure: Nivolumab 1 cycle
Lesion Measurement (diameter long, mm)
Phase 1 Trial Responder Overcame ICI
Resistance After TTI-101 Monotherapy
1. Adapted from Zhao, Y et. al. Hepatology 2021; 2. Cohen et al 2018; 3. Siu et al 2019 ; ICI: Immune Checkpoint Inhibition; Bev: Bevacizumab. Certain data on this slide are based on cross study comparisons and
are not based on any head to head clinical trials. Cross study comparisons are inherently limited and may suggest misleading similarities and differences. The values shown in the cross study comparisons are
directional and may not be directly comparable. 4. Tsimberidou et al 2024 accepted in Clin Cancer Research
TTI-101 Treatment: 14 cycles |
| 27
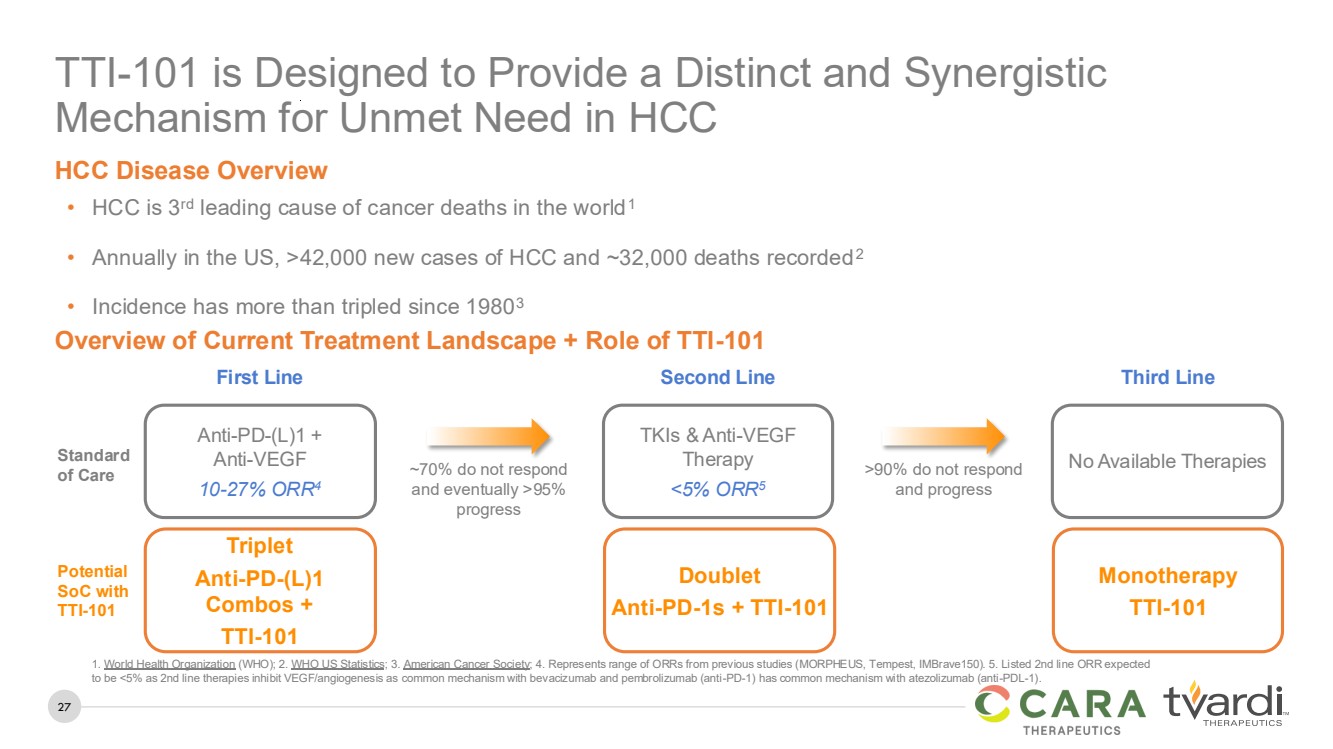
Overview of Current Treatment Landscape + Role of TTI-101
TTI-101 is Designed to Provide a Distinct and Synergistic
Mechanism for Unmet Need in HCC
• HCC is 3rd leading cause of cancer deaths in the world1
• Annually in the US, >42,000 new cases of HCC and ~32,000 deaths recorded2
• Incidence has more than tripled since 19803
HCC Disease Overview
Triplet
Anti-PD-(L)1
Combos +
TTI-101
Potential
SoC with
TTI-101
Doublet
Anti-PD-1s + TTI-101
Monotherapy
TTI-101
1. World Health Organization (WHO); 2. WHO US Statistics; 3. American Cancer Society; 4. Represents range of ORRs from previous studies (MORPHEUS, Tempest, IMBrave150). 5. Listed 2nd line ORR expected
to be <5% as 2nd line therapies inhibit VEGF/angiogenesis as common mechanism with bevacizumab and pembrolizumab (anti-PD-1) has common mechanism with atezolizumab (anti-PDL-1).
Anti-PD-(L)1 +
Anti-VEGF
10-27% ORR4
Standard
of Care
First Line
TKIs & Anti-VEGF
Therapy
<5% ORR5
Second Line
No Available Therapies
Third Line
~70% do not respond
and eventually >95%
progress
>90% do not respond
and progress |
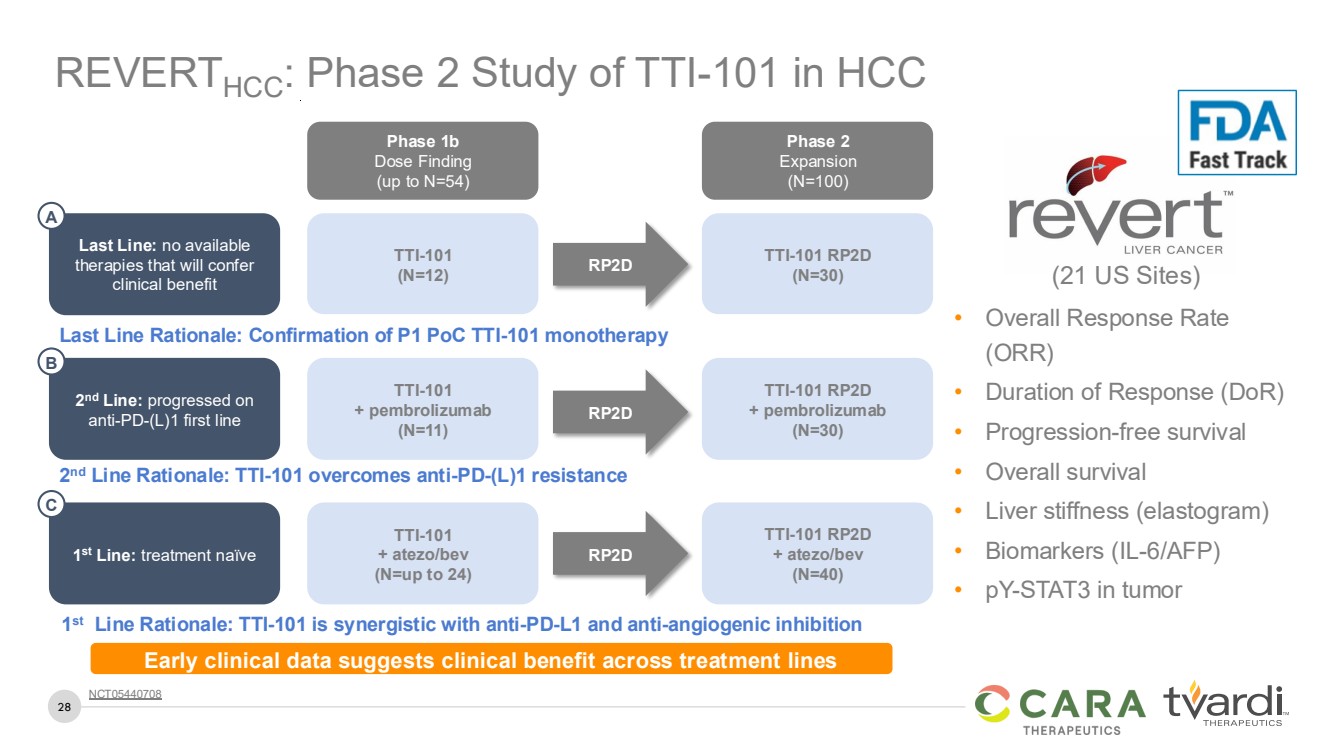
| 28
REVERTHCC: Phase 2 Study of TTI-101 in HCC
• Overall Response Rate
(ORR)
• Duration of Response (DoR)
• Progression-free survival
• Overall survival
• Liver stiffness (elastogram)
• Biomarkers (IL-6/AFP)
• pY-STAT3 in tumor
(21 US Sites)
Phase 1b
Dose Finding
(up to N=54)
Phase 2
Expansion
(N=100)
TTI-101 RP2D
(N=30)
TTI-101
(N=12)
Last Line: no available
therapies that will confer
clinical benefit
RP2D
Last Line Rationale: Confirmation of P1 PoC TTI-101 monotherapy
A
TTI-101 RP2D
+ pembrolizumab
(N=30)
TTI-101
+ pembrolizumab
(N=11)
RP2D
2
nd Line Rationale: TTI-101 overcomes anti-PD-(L)1 resistance
2
nd Line: progressed on
anti-PD-(L)1 first line
B
TTI-101 RP2D
+ atezo/bev
(N=40)
TTI-101
+ atezo/bev
(N=up to 24)
RP2D
1
st
Line Rationale: TTI-101 is synergistic with anti-PD-L1 and anti-angiogenic inhibition
1
st Line: treatment naïve
C
NCT05440708
Early clinical data suggests clinical benefit across treatment lines |

| 29
Inhibition of STAT3
activation to have dual
therapeutic effect on cancer
cells – overcoming
tumorigenesis and immune
suppression
REVERTHCC trial Phase 2
assessing activity in both
monotherapy and combination
therapy in areas of unmet
need
Topline results from
ongoing Phase 2
REVERTHCC trial expected
in H2:2025
STAT3 long recognized as
prime target in oncology;
>95% of patients with HCC
have activated STAT3 in
their tumors
STAT3: Well-Established Biology
Differentiated
Approach
Clinical PoC
Underway
Near-Term Clinical
Milestones
Key Takeaways: TTI-101 in HCC |
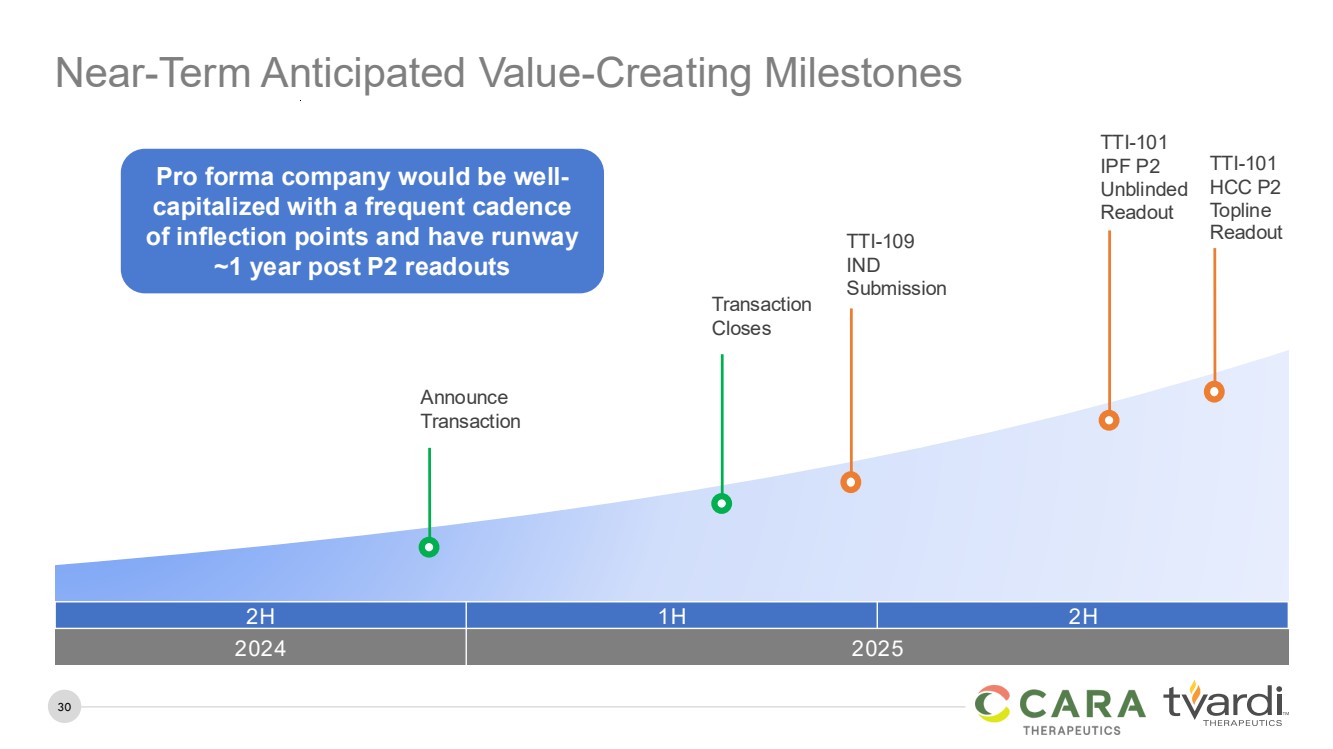
| 30
Near-Term Anticipated Value-Creating Milestones
TTI-101
HCC P2
Topline
Readout
TTI-101
IPF P2
Unblinded
Readout
Announce
Transaction
TTI-109
IND
Submission
Transaction
Closes
2H 1H 2H
2024 2025
Pro forma company would be well-capitalized with a frequent cadence
of inflection points and have runway
~1 year post P2 readouts |
| 31
Targeting STAT3: Central Mediator of Fibrosis-Driven Diseases
Deep expertise in
STAT3 biology
Potential to serve as a
disease-modifying
therapy in IPF1
Well-positioned to
differentiate therapeutic
impact in HCC2
Multiple near-term data
catalysts expected
• Unlocking highly-validated,
yet historically
"undruggable" target within
fibrosis-driven diseases
• IPF models demonstrated
reversal of fibrosis and
restoration of lung function
• Phase 2 Clinical PoC
ongoing
• Evaluating both mono- and
combination therapy from
an ongoing clinical trial
• IPF Phase 2 unblinded
data in H2:2025
• HCC Phase 1b/2 topline
data in H2:2025
• TTI-109 IND3 submission
planned for H1:2025
1. Idiopathic pulmonary fibrosis. 2. Hepatocellular carcinoma. 3. Investigational new drug. |
Cover
|
Dec. 17, 2024 |
| Cover [Abstract] |
|
| Document Type |
8-K/A
|
| Amendment Flag |
false
|
| Document Period End Date |
Dec. 17, 2024
|
| Entity File Number |
001-36279
|
| Entity Registrant Name |
CARA THERAPEUTICS, INC.
|
| Entity Central Index Key |
0001346830
|
| Entity Tax Identification Number |
75-3175693
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Address, Address Line One |
400 Atlantic Street
|
| Entity Address, Address Line Two |
Suite 500
|
| Entity Address, City or Town |
Stamford
|
| Entity Address, State or Province |
CT
|
| Entity Address, Postal Zip Code |
06901
|
| City Area Code |
203
|
| Local Phone Number |
406-3700
|
| Written Communications |
true
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Common Stock, $0.001 par value per share
|
| Trading Symbol |
CARA
|
| Security Exchange Name |
NASDAQ
|
| Entity Emerging Growth Company |
false
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
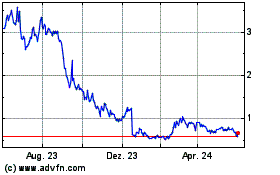
Cara Therapeutics (NASDAQ:CARA)
Historical Stock Chart
Von Nov 2024 bis Dez 2024
Cara Therapeutics (NASDAQ:CARA)
Historical Stock Chart
Von Dez 2023 bis Dez 2024