bioMérieux Announces the Expansion of the CE Marking of Its Molecular Biology ARGENE® SARS-CoV-2 Diagnostic Test to Include...
16 November 2020 - 8:55AM
Business Wire
Regulatory News:
bioMérieux (Paris:BIM), a world leader in the field of in vitro
diagnostics, has announced the expansion of its ARGENE® range for
the detection of SARS-CoV-2. As a complement to nasopharyngeal swab
specimens, the singleplex SARS-CoV-2 R-GENE® real-time PCR test may
now be used on saliva and oropharyngeal (throat) swab specimens for
the detection of the virus that causes COVID-19. This development
helps optimize laboratory workflows.
The CE-marked SARS-CoV-2 R-GENE® test covers the above three
sample types. This allows bioMérieux to address the recommendation
issued by the French National Authority for Health (HAS) on
September 18, 2020, which encourages the preferential use of saliva
swabs to test symptomatic individuals for whom it is difficult or
impossible to use nasopharyngeal swabs.
“True to its commitment to combat the COVID-19 pandemic,
bioMérieux has taken into account the needs of laboratories.
Expanding the use of the ARGENE® molecular test to include saliva
swab specimens will make the test more acceptable to many patients,
and will make it easier to perform,” said François Lacoste,
Executive Vice President, R&D.
The expansion of the CE marking to include saliva and
oropharyngeal swab specimens has been declared to the French
National Agency of Medicines and Health Products Safety (ANSM).This
new type of specimen is now mentioned in the list of tests
authorized by the French Directorate General of Health.
Moreover, the Company will soon release a high-throughput test
for the simultaneous (multiplex) detection of influenza viruses A
and B and SARS-CoV-2, including a cellular control to check the
quality of the sample. It will be available in Europe and in
countries that recognize CE marking. This new test will be part of
the same test kit for the detection of two other disease agents
that often circulate during the winter months, RSV (human
respiratory syncytial virus) and HMPV (human metapneumovirus).
About the ARGENE® SARS-COV-2 R-GENE® test:
As for all tests in the ARGENE® range, the SARS-COV-2 R-GENE®
test is an open assay, meaning that it may be performed by any
laboratory using PCR technology on most commercially-available
nucleic acid extraction and amplification platforms. Results are
delivered in 4 to 5 hours, and a large number of patient samples
may be processed simultaneously. The entire ARGENE® range for the
detection of SARS-CoV-2 is produced in France at the bioMérieux
site in Verniolle (Ariège).
ABOUT BIOMÉRIEUX
Pioneering Diagnostics
A world leader in the field of in vitro diagnostics for over 55
years, bioMérieux is present in 44 countries and serves more than
160 countries with the support of a large network of distributors.
In 2019, revenues reached €2.7 billion, with over 90% of
international sales.
bioMérieux provides diagnostic solutions (systems, reagents,
software and services) which determine the source of disease and
contamination to improve patient health and ensure consumer safety.
Its products are mainly used for diagnosing infectious diseases.
They are also used for detecting microorganisms in agri-food,
pharmaceutical and cosmetic products.
bioMérieux is listed on the Euronext Paris
stock market.
Symbol: BIM – ISIN Code: FR0013280286
Reuters: BIOX.PA/Bloomberg: BIM.FP
Corporate website: www.biomerieux.com.
View source
version on businesswire.com: https://www.businesswire.com/news/home/20201115005191/en/
Investor Relations bioMérieux Franck Admant Tel.:
+ 33 4 78 87 20 00 investor.relations@biomerieux.com
Media Relations bioMérieux Olivier Rescaniere
Tel.: + 33 4 78 87 20 00 media@biomerieux.com
Image Sept Laurence Heilbronn Tel.: + 33 1 53 70 74 64
lheilbronn@image7.fr
Claire Doligez Tel.: + 33 1 53 70 74 48 cdoligez@image7.fr
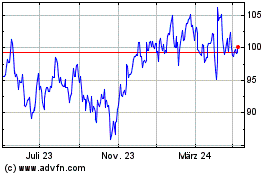
Biomerieux (EU:BIM)
Historical Stock Chart
Von Nov 2024 bis Dez 2024
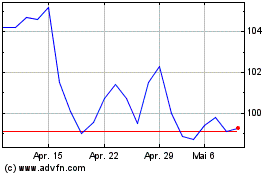
Biomerieux (EU:BIM)
Historical Stock Chart
Von Dez 2023 bis Dez 2024