false
0001430306
0001430306
2024-01-08
2024-01-08
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities
Exchange Act of 1934
Date of report (date of earliest event reported):
January 8, 2024
TONIX PHARMACEUTICALS HOLDING CORP.
(Exact name of registrant as specified in its charter)
| Nevada |
001-36019 |
26-1434750 |
|
(State or Other Jurisdiction
of Incorporation) |
(Commission
File Number) |
(IRS Employer
Identification No.) |
26 Main Street, Chatham, New Jersey 07928
(Address of principal executive offices) (Zip Code)
Registrant’s telephone number, including area
code: (862) 904-8182
Check the appropriate box below if the Form 8-K filing
is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction
A.2. below):
☐
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant
to Section 12(b) of the Act:
| Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered |
| Common Stock |
TNXP |
The NASDAQ Capital Market |
Indicate by check mark whether
the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or
Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company,
indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial
accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01 |
Regulation FD Disclosure. |
On January 8, 2024, Tonix Pharmaceuticals Holding
Corp. (the “Company”) announced additional data from the Phase 3 RESILIENT study evaluating its TNX-102 SL (cyclobenzaprine
HCl sublingual tablets) product candidate for the management of fibromyalgia in a presentation at the 2024 Biotech Showcase Conference
(the “Presentation”). A copy of the Presentation, which may contain nonpublic information, is filed as Exhibit 99.01 hereto
and incorporated herein by reference.
The information
in this Item 7.01 of this Current Report on Form 8-K, including Exhibit 99.01 attached hereto, shall not be deemed “filed”
for purposes of Section 18 of the United States Securities Exchange Act of 1934 (the “Exchange Act”) or otherwise subject
to the liabilities of that section, nor shall they be deemed incorporated by reference in any filing under the United States Securities
Act of 1933 or the Exchange Act, except as shall be expressly set forth by specific reference in such a filing.
On
January 8, 2024, the Company announced that data from the Phase 3 RESILIENT study demonstrated that TNX-102-SL was not associated with
an increase in systolic or diastolic blood pressure or body weight. In addition, when systematically investigated using the Changes in
Sexual Functioning Questionnaire short form (“CSFQ-14”), female participants in the RESILIENT study who received TNX-102 SL
had a higher CSQ-14 score than female participants who received placebo, consistent with an improvement with sexual functioning.
Forward-
Looking Statements
This
Current Report on Form 8-K contains certain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933
and Section 21E of the Securities Exchange Act of 1934 and Private Securities Litigation Reform Act, as amended, including those relating
to the Company’s product development, clinical trials, clinical and regulatory timelines, market opportunity, competitive position,
possible or assumed future results of operations, business strategies, potential growth opportunities and other statement that are predictive
in nature. These forward-looking statements are based on current expectations, estimates, forecasts and projections about the industry
and markets in which we operate and management’s current beliefs and assumptions.
These
statements may be identified by the use of forward-looking expressions, including, but not limited to, “expect,” “anticipate,”
“intend,” “plan,” “believe,” “estimate,” “potential,” “predict,”
“project,” “should,” “would” and similar expressions and the negatives of those terms. These statements
relate to future events or our financial performance and involve known and unknown risks, uncertainties, and other factors which may cause
actual results, performance or achievements to be materially different from any future results, performance or achievements expressed
or implied by the forward-looking statements. Such factors include those set forth in the Company’s filings with the SEC. Prospective
investors are cautioned not to place undue reliance on such forward-looking statements, which speak only as of the date of this press
release. The Company undertakes no obligation to publicly update any forward-looking statement, whether as a result of new information,
future events or otherwise.
| Item 9.01 |
Financial Statements and Exhibits. |
| (d) |
|
Exhibit
No. |
|
Description. |
| |
|
99.01
104 |
|
Biotech Showcase Presentation
Cover Page Interactive Data File (embedded within the Inline XBRL document) |
SIGNATURE
Pursuant to the requirement of
the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto
duly authorized.
| |
TONIX PHARMACEUTICALS HOLDING CORP. |
| |
|
| Date: January 8, 2024 |
By: |
/s/ Bradley Saenger |
|
| |
|
Bradley Saenger |
| |
|
Chief Financial Officer |
Tonix Pharmaceuticals Holding Corp. 8-K
Exhibit 99.01

© 2024 Tonix Pharmaceuticals Holding Corp. Biotech Showcase Focus on: TNX - 102 SL in Development for the Management of Fibromyalgia January 2024 NASDAQ: TNXP Version P0519 January 5 , 2024 (Doc 1365 )

2 © 2024 Tonix Pharmaceuticals Holding Corp. Cautionary Note on Forward - Looking Statements Certain statements in this presentation regarding strategic plans, expectations and objectives for future operations or results are “forward - looking statements” as defined by the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of forward - looking words such as “anticipate,” “believe,” “forecast,” “estimate” and “intend,” among others. These forward - looking statements are based on Tonix’s current expectations and actual results could differ materially. There are a number of factors that could cause actual events to differ materially from those indicated by such forward - looking statements. These factors include, but are not limited to, the risks related to failure to obtain FDA clearances or approvals and noncompliance with FDA regulations; risks related to the failure to successfully market any of our products; risks related to the timing and progress of clinical development of our product candidates; our need for additional financing; uncertainties of patent protection and litigation; uncertainties of government or third party payor reimbursement; limited research and development efforts and dependence upon third parties; and substantial competition. As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products. The forward - looking statements in this presentation are made as of the date of this presentation, even if subsequently made available by Tonix on its website or otherwise. Tonix does not undertake an obligation to update or revise any forward - looking statement, except as required by law. Investors should read the risk factors set forth in the Annual Report on Form 10 - K for the year ended December 31, 2022, as filed with the Securities and Exchange Commission (the “SEC”) on March 13, 2023, and periodic reports and current reports filed with the SEC on or after the date thereof. All of Tonix's forward - looking statements are expressly qualified by all such risk factors and other cautionary statements.

3 © 2024 Tonix Pharmaceuticals Holding Corp. CNS - Focused Biopharma with Preclinical, Clinical and Commercial Stage Products Marketed Products • Zembrace ® and Tosymra ® indicated for the treatment of acute migraine TNX - 102 SL for Fibromyalgia: Preparing New Drug Application (NDA) • Two positive Phase 3 trials completed • NDA filing expected 2H’24 • FDA decision on NDA approval expected 2H’25 Internal Capabilities • Commercial prescription drug sales • R&D and clinical - trial scale manufacturing Strategic Partnerships • With government institutions, world - class academic & research organizations Pipeline • Phase 2 biologic cocaine antidote, FDA “Breakthrough Therapy Designation” • Phase 1 anti - CD40L monoclonal antibody to prevent organ transplant rejection
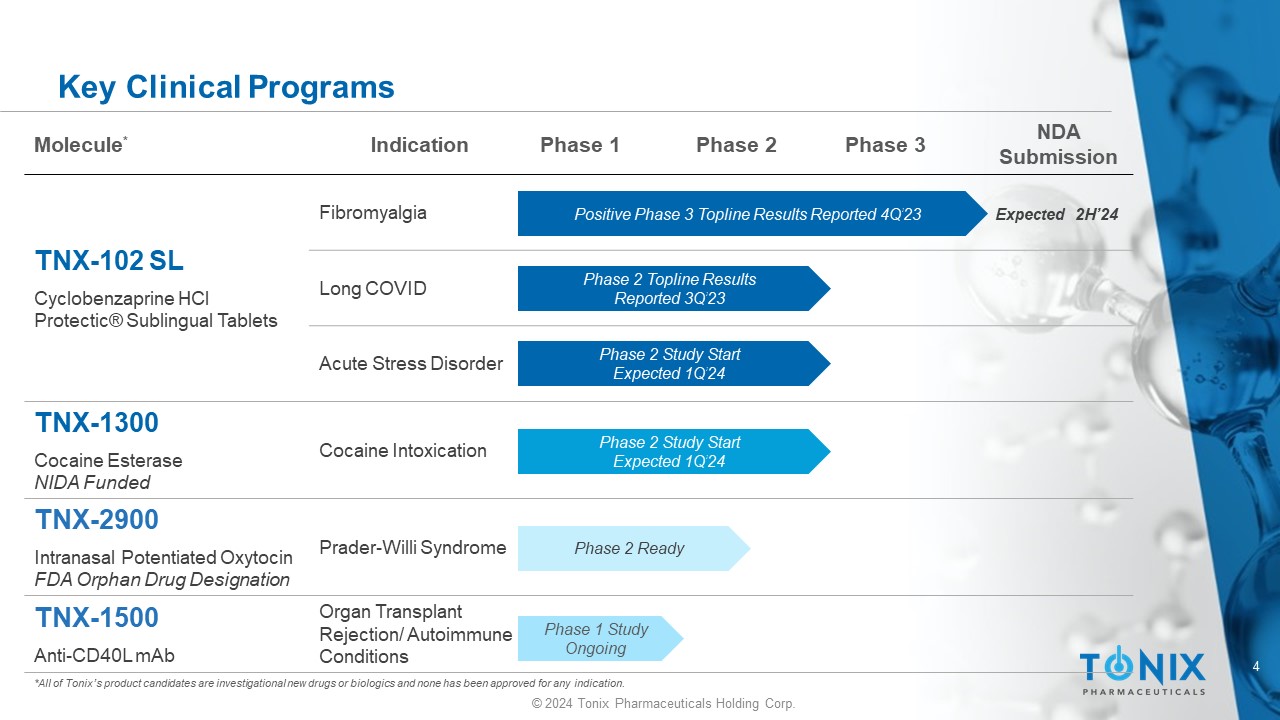
4 © 2024 Tonix Pharmaceuticals Holding Corp. Key Clinical Programs *All of Tonix’s product candidates are investigational new drugs or biologics and none has been approved for any indication. NDA Submission Phase 3 Phase 2 Phase 1 Indication Molecule * Fibromyalgia TNX - 102 SL Cyclobenzaprine HCl Protectic ® Sublingual Tablets Long COVID Acute Stress Disorder Cocaine Intoxication TNX - 1300 Cocaine Esterase NIDA Funded Prader - Willi Syndrome TNX - 2900 Intranasal Potentiated Oxytocin FDA Orphan Drug Designation Organ Transplant Rejection/ Autoimmune Conditions TNX - 1500 Anti - CD40L mAb Positive Phase 3 Topline Results Reported 4Q’23 Phase 2 Study Start Expected 1Q’24 Phase 1 Study Ongoing Expected 2H’24 Phase 2 Ready Phase 2 Topline Results Reported 3Q’23 Phase 2 Study Start Expected 1Q’24

TNX - 102 SL: Unique MOA Facilitates Restorative Sleep Centrally Acting Analgesic 5 Potent binding and antagonist activities at four key receptors facilitate restorative sleep • serotonergic - 5 - HT2A • adrenergic - α1 • histaminergic - H1 • muscarinic - M1 Relative to Oral Cyclobenzaprine o Lower daytime exposure o Avoids first - pass metabolism o Reduces risk of pharmacological interference from major metabolite Relative to Standard of Care o Potential for better tolerability while maintaining efficacy o Not scheduled nor with recognized abuse potential Key Differentiators © 2024 Tonix Pharmaceuticals Holding Corp. *TNX - 102 SL has not been approved for any indication.
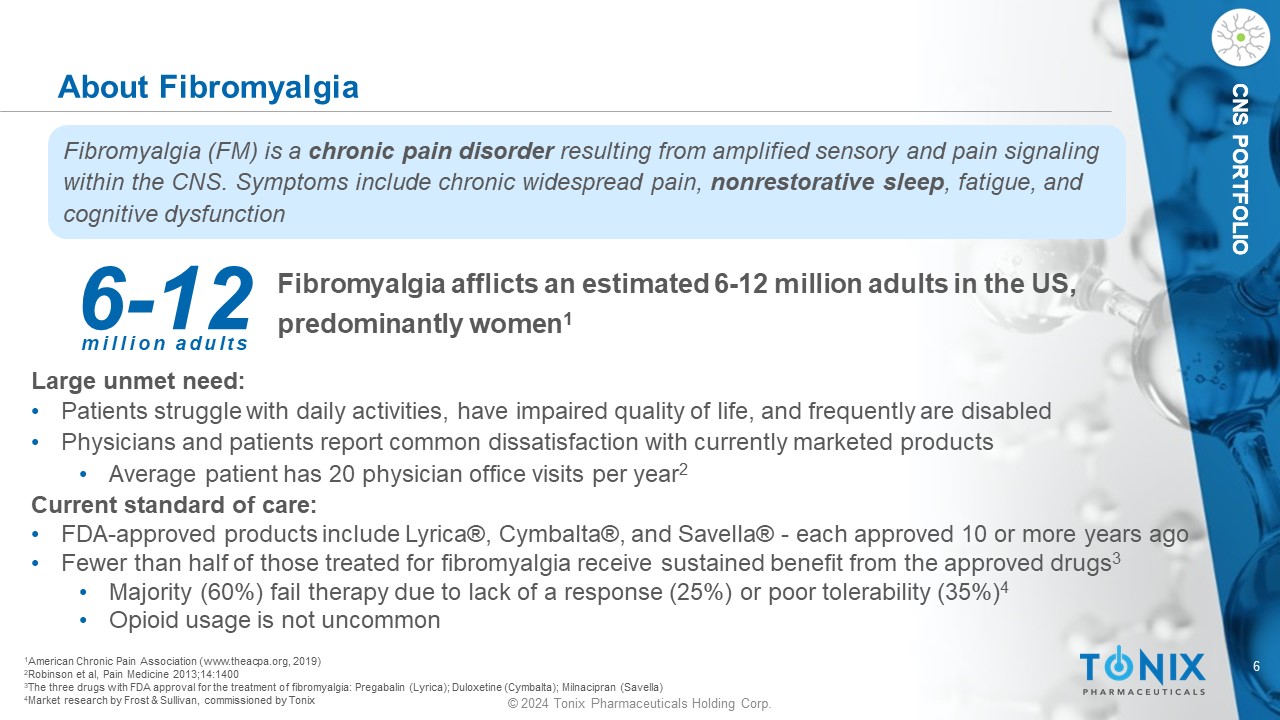
6 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO CNS PORTFOLIO Fibromyalgia a fflicts an estimated 6 - 12 million adults in the US, predominantly women 1 1 American Chronic Pain Association (www.theacpa.org, 2019) 2 Robinson et al, Pain Medicine 2013;14:1400 3 The three drugs with FDA approval for the treatment of fibromyalgia: Pregabalin (Lyrica); Duloxetine (Cymbalta); Milnacipran ( Savella ) 4 Market research by Frost & Sullivan, commissioned by Tonix About Fibromyalgia Fibromyalgia (FM) is a chronic pain disorder resulting from amplified sensory and pain signaling within the CNS. Symptoms include chronic widespread pain, nonrestorative sleep , fatigue, and cognitive dysfunction 6 - 12 million adults Large unmet need: • Patients struggle with daily activities, have impaired quality of life, and frequently are disabled • Physicians and patients report common dissatisfaction with currently marketed products • Average patient has 20 physician office visits per year 2 Current standard of care: • FDA - approved products include Lyrica®, Cymbalta®, and Savella ® - each approved 10 or more years ago • Fewer than half of those treated for fibromyalgia receive sustained benefit from the approved drugs 3 • Majority (60%) fail therapy due to lack of a response (25%) or poor tolerability (35%) 4 • Opioid usage is not uncommon
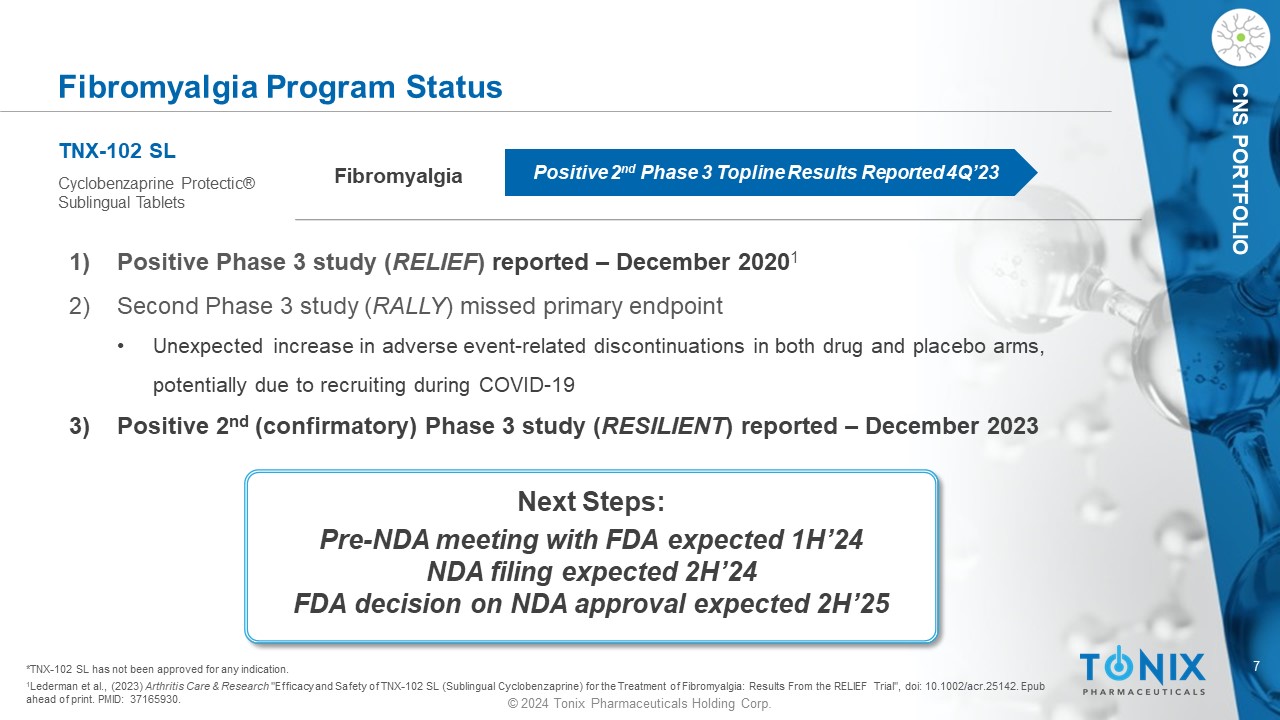
7 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Fibromyalgia Program Status Fibromyalgia TNX - 102 SL Cyclobenzaprine Protectic ® Sublingual Tablets Positive 2 nd Phase 3 Topline Results Reported 4Q’23 1) P ositive Phase 3 study ( RELIEF ) reported – December 2020 1 2) Second Phase 3 study ( RALLY ) missed primary endpoint • Unexpected increase in adverse event - related discontinuations in both drug and placebo arms, potentially due to recruiting during COVID - 19 3) Positive 2 nd (c onfirmatory ) Phase 3 study ( RESILIENT ) reported – December 2023 1 Lederman et al., (2023) Arthritis Care & Research "Efficacy and Safety of TNX - 102 SL (Sublingual Cyclobenzaprine) for the Treatment of Fibromyalgia: Results From the RELIEF Trial ", doi : 10.1002/acr.25142. Epub ahead of print. PMID: 37165930. *TNX - 102 SL has not been approved for any indication. Next Steps: Pre - NDA meeting with FDA expected 1H’24 NDA filing expected 2H’24 FDA decision on NDA approval expected 2H’25

8 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL: Phase 3 RESILIENT Study Design General s tudy c haracteristics: • Randomized, double - blind, multicenter, placebo - controlle d study in fibromyalgia • 33 U.S. sites enrolled 457 participants with fibromyalgia as defined by 2016 Revisions to the 2010/2011 FM Diagnostic C riteria 1 Primary Endpoint: • Change from baseline to Week 14 (TNX - 102 SL vs. placebo) in weekly averages of daily diary average pain severity score • Primary Endpoint, p - value = 0.00005 Placebo once - daily at bedtime TNX - 102 SL once - daily at bedtime 5.6 mg (2 x 2.8 mg tablets) * * Two - week run - in at 2.8 mg dose at bedtime followed by 12 weeks at 5.6 mg dose ClinicalTrials.gov Identifier: NCT05273749 Study Title: A Phase 3 Study to Evaluate the Efficacy and Safety of TNX - 102 SL Taken Daily in Patients With Fibromyalgia (RESILIENT) Trial ID: TNY - CY - F307 (‘RESILIENT’) 14 weeks 1 Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Häuser W, Katz RL, Mease PJ, Russell AS, Russell IJ, Walitt B. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum. 2016; 46(3):319 - 329.
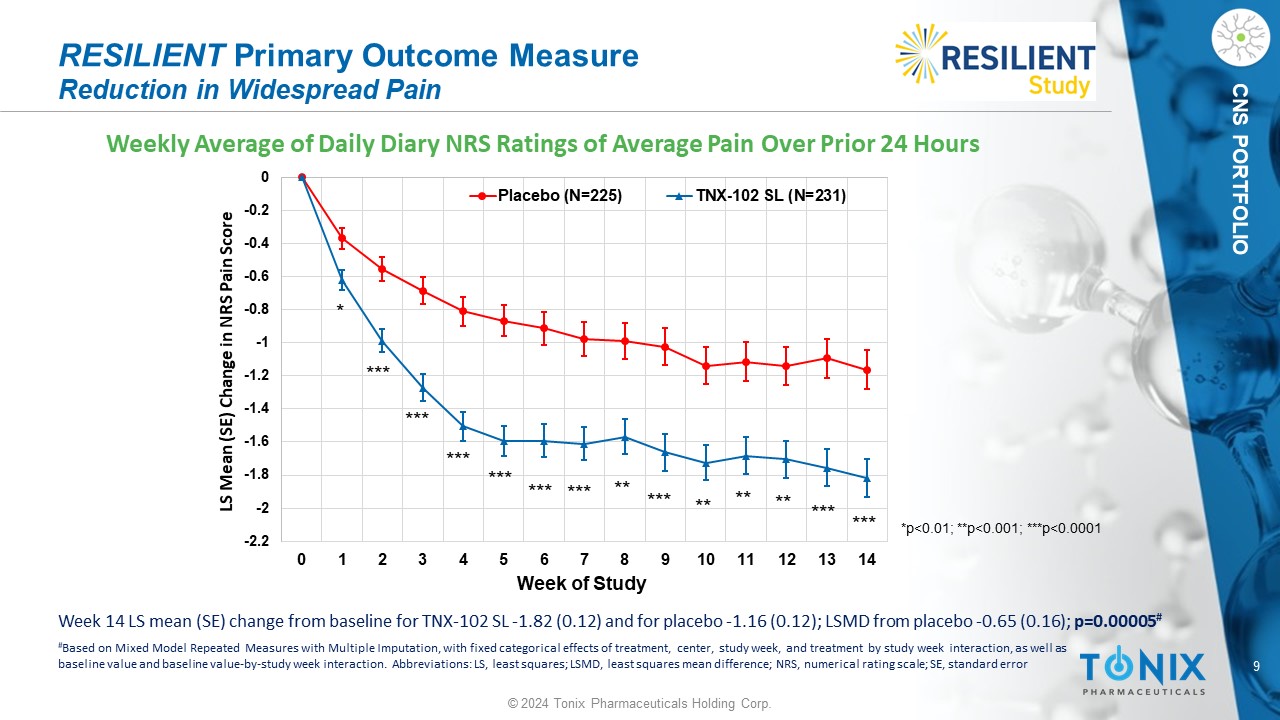
9 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Weekly Average of Daily Diary NRS Ratings of Average Pain Over Prior 24 Hours RESILIENT Primary Outcome Measure Reduction in Widespread Pain -2.2 -2 -1.8 -1.6 -1.4 -1.2 -1 -0.8 -0.6 -0.4 -0.2 0 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 LS Mean (SE) Change in NRS Pain Score Week of Study Placebo (N=225) TNX-102 SL (N=231) * *** *** ** ** ** *** *** *** *** *** *** *** ** *p<0.01; **p<0.001; ***p<0.0001 Week 14 LS mean (SE) change from baseline for TNX - 102 SL - 1.82 (0.12) and for placebo - 1.16 (0.12); LSMD from placebo - 0.65 (0.1 6); p=0.00005 # # Based on Mixed Model Repeated Measures with Multiple Imputation, with fixed categorical effects of treatment, center, study w eek , and treatment by study week interaction, as well as baseline value and baseline value - by - study week interaction. Abbreviations: LS, least squares; LSMD, least squares mean differe nce; NRS, numerical rating scale; SE, standard error
10 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO RESILIENT Pre - Specified Primary Endpoint Summary • TNX - 102 SL demonstrated statistically significant improvement in mean weekly pain scores over placebo at Week 14 • P - value of 0.00005 is highly statistically significant Additional Findings • Effect size 0.38 • All pre - specified sensitivity analyses of the primary endpoint show statistical significance (p ≤ 0.001) • Rapid onset of action: p - values <0.01 at each weekly time point, including Week 1

11 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO RESILIENT Continuous Pain Responder Graph # Analyses: Pearson’s Chi Squared test for equality of proportions Abbreviations: CI, confidence interval; DIP, difference in proportions ^pre - specified analyses but not key secondary analyses P e r c e n t o f S u b j e c t s 0 10 20 30 40 50 60 70 80 90 100 Percentage Redution in Pain ≥ 0 ≥ 10% ≥ 20% ≥ 30% ≥ 40% ≥ 50% ≥ 60% ≥ 70% ≥ 80% ≥ 90% ≥ 100% Placebo TNX-102 SL 30% Responders # ( 45.9% vs 27.1% ) p < 0.001 50% Responders # ( 22.5% vs. 13.3% ) p = 0.011

12 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Summary of Key Pre - Specified Secondary Outcome Measures *In order of statistical serial gate - keeping hierarchy (or, “waterfall”) to control overall Type 1 error **Statistical significance met Met** Week 14 Rating Scale p < 0.001 Patient Global Impression of Change (PGIC) p < 0.001 Fibromyalgia Impact Questionnaire - Symptoms p = 0.001 Fibromyalgia Impact Questionnaire - Function p < 0.001 PROMIS Sleep Disturbance p < 0.001 PROMIS Fatigue p < 0.001 Weekly average of daily Sleep Quality scores
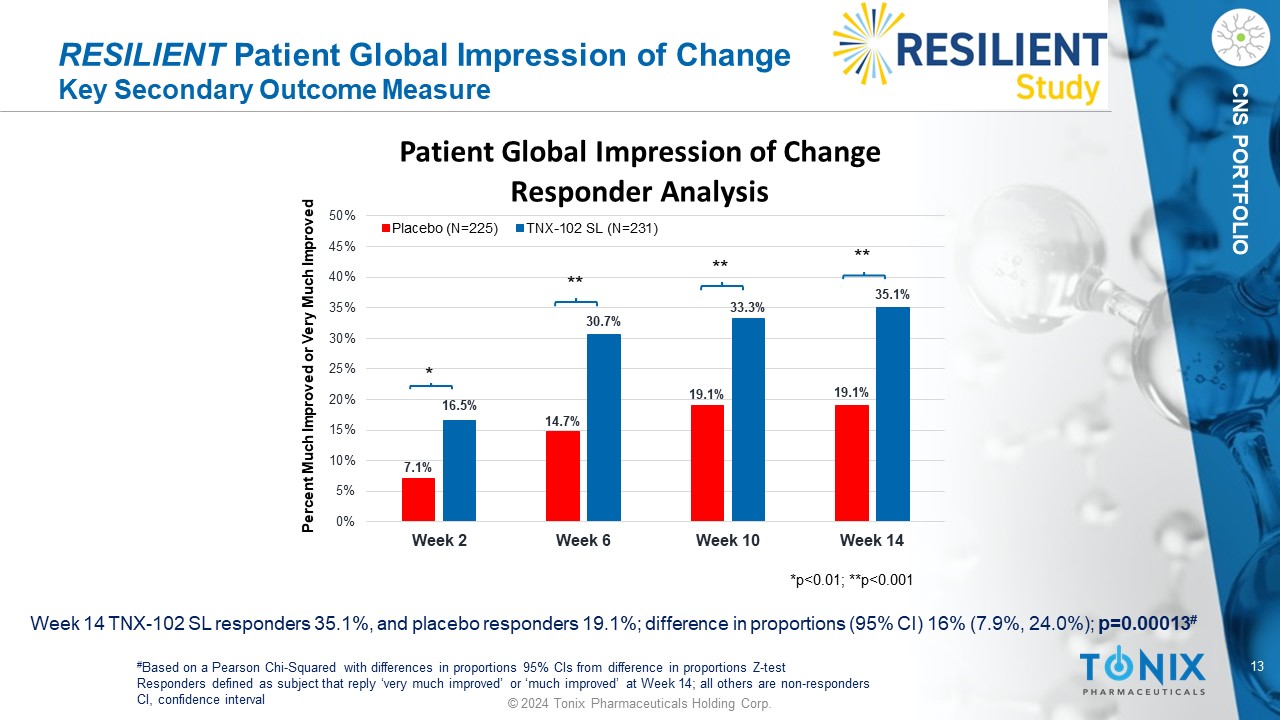
13 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO RESILIENT Patient Global Impression of Change Key Secondary Outcome Measure Week 14 TNX - 102 SL responders 35.1%, and placebo responders 19.1%; difference in proportions (95% CI) 16% (7.9%, 24.0%); p=0.00013 # # Based on a Pearson Chi - Squared with differences in proportions 95% CIs from difference in proportions Z - test Responders defined as subject that reply ‘very much improved’ or ‘much improved’ at Week 14; all others are non - responders CI, confidence interval 7.1% 14.7% 19.1% 19.1% 16.5% 30.7% 33.3% 35.1% 0% 5% 10% 15% 20% 25% 30% 35% 40% 45% 50% Week 2 Week 6 Week 10 Week 14 Patient Global Impression of Change Responder Analysis Placebo (N=225) TNX-102 SL (N=231) Percent Much Improved or Very Much Improved *p<0.01; **p<0.001 * ** ** **

14 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO RESILIENT FIQ - R Symptoms Domain Key Secondary Outcome Measure * *** *** *** Week 14 LS mean (SE) change from baseline for TNX - 102 SL - 16.0 (1.17) and for placebo - 8.4 (1.17); LSMD from placebo - 7.7 (1.62) ; p=0.000002 # # Based on Mixed Model Repeated Measures with Multiple Imputation, with fixed categorical effects of treatment, center, study w eek , and treatment by study week interaction, as well as baseline value and baseline value - by - study week interaction. -20 -18 -16 -14 -12 -10 -8 -6 -4 -2 0 Week 0 Week 2 Week 6 Week 10 Week 14 LS Mean (SE) Change in FIQ - R Symptoms Fibromyalgia Impact Questionnaire – Revised (FIQ - R) Symptoms Domain Placebo (N=225) TNX-102 SL (N=231) *p=0.003; ***p<0.0001
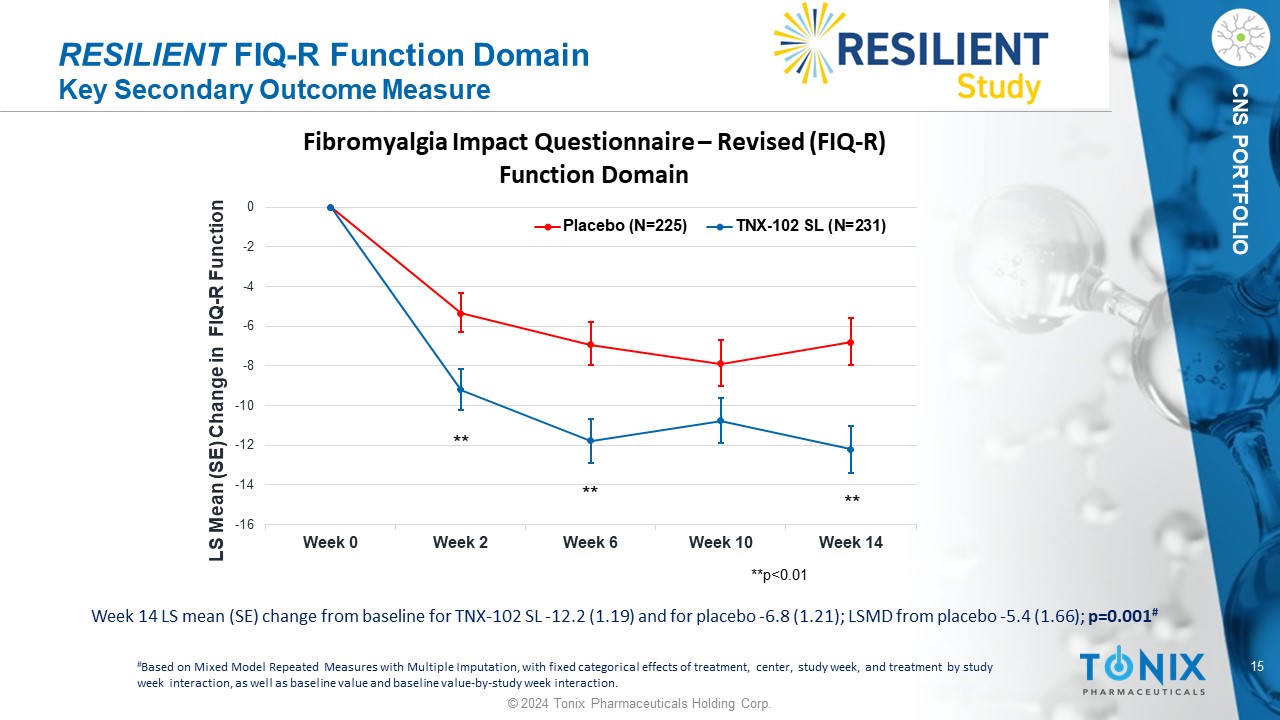
15 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO RESILIENT FIQ - R Function Domain Key Secondary Outcome Measure Week 14 LS mean (SE) change from baseline for TNX - 102 SL - 12.2 (1.19) and for placebo - 6.8 (1.21); LSMD from placebo - 5.4 (1.66) ; p=0.001 # # Based on Mixed Model Repeated Measures with Multiple Imputation, with fixed categorical effects of treatment, center, study w eek , and treatment by study week interaction, as well as baseline value and baseline value - by - study week interaction. -16 -14 -12 -10 -8 -6 -4 -2 0 Week 0 Week 2 Week 6 Week 10 Week 14 LS Mean (SE) Change in FIQ - R Function Fibromyalgia Impact Questionnaire – Revised (FIQ - R) Function Domain Placebo (N=225) TNX-102 SL (N=231) **p<0.01 ** ** **

16 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO RESILIENT PROMIS Sleep Disturbance Inventory Key Secondary Outcome Measure Week 14 LS mean (SE) change from baseline for TNX - 102 SL - 8.4 (0.57) and for placebo - 4.2 (0.56); LSMD from placebo - 4.2 (0.79); p=0.0000001 # # Based on Mixed Model Repeated Measures with Multiple Imputation, with fixed categorical effects of treatment, center, study w eek , and treatment by study week interaction, as well as baseline value and baseline value - by - study week interaction. -10 -9 -8 -7 -6 -5 -4 -3 -2 -1 0 Week 0 Week 2 Week 6 Week 10 Week 14 LS Mean (SE) Change in PROMIS SD T - score PROMIS Sleep Disturbance Placebo (N=225) TNX-102 SL (N=231) *p<0.001; ***p<0.00001 * *** *** ***
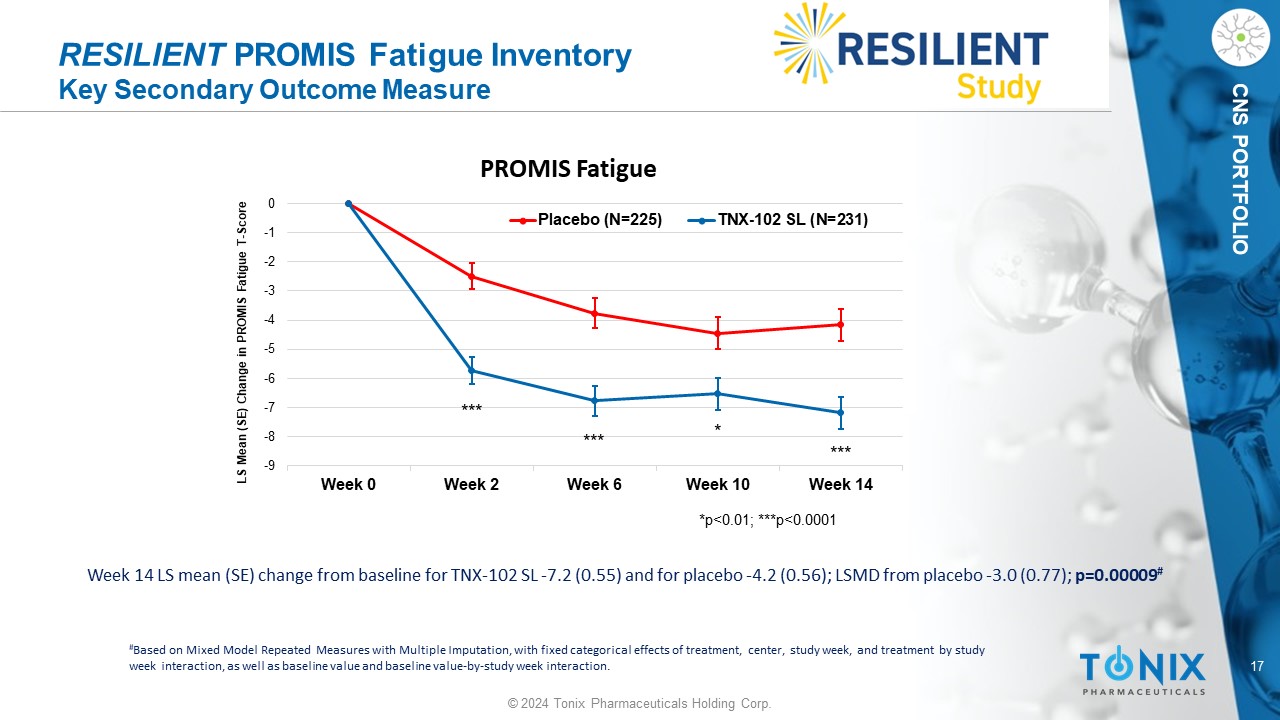
17 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO RESILIENT PROMIS Fatigue Inventory Key Secondary Outcome Measure Week 14 LS mean (SE) change from baseline for TNX - 102 SL - 7.2 (0.55) and for placebo - 4.2 (0.56); LSMD from placebo - 3.0 (0.77); p=0.00009 # # Based on Mixed Model Repeated Measures with Multiple Imputation, with fixed categorical effects of treatment, center, study w eek , and treatment by study week interaction, as well as baseline value and baseline value - by - study week interaction. -9 -8 -7 -6 -5 -4 -3 -2 -1 0 Week 0 Week 2 Week 6 Week 10 Week 14 LS Mean (SE) Change in PROMIS Fatigue T - Score PROMIS Fatigue Placebo (N=225) TNX-102 SL (N=231) *p<0.01; ***p<0.0001 * *** *** ***
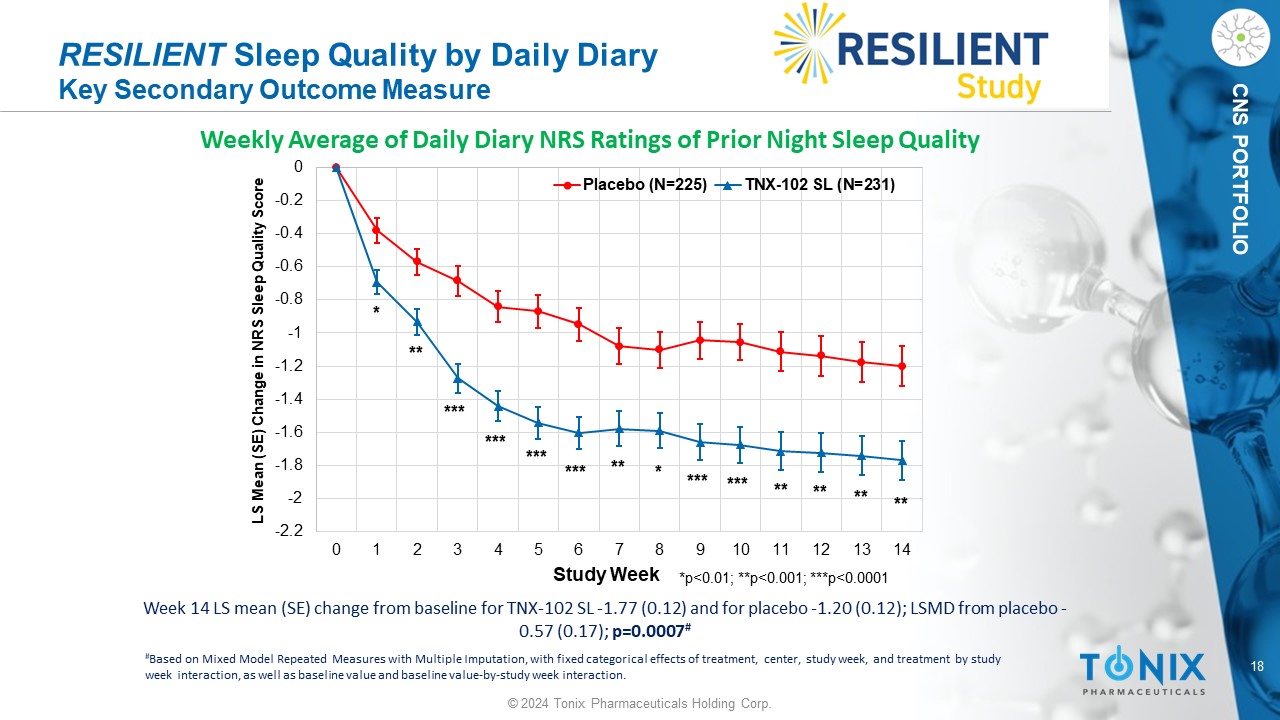
18 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Week 14 LS mean (SE) change from baseline for TNX - 102 SL - 1.77 (0.12) and for placebo - 1.20 (0.12); LSMD from placebo - 0.57 (0.17); p=0.0007 # RESILIENT Sleep Quality by Daily Diary Key Secondary Outcome Measure Weekly Average of Daily Diary NRS Ratings of Prior Night Sleep Quality -2.2 -2 -1.8 -1.6 -1.4 -1.2 -1 -0.8 -0.6 -0.4 -0.2 0 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 LS Mean (SE) Change in NRS Sleep Quality Score Study Week Placebo (N=225) TNX-102 SL (N=231) *p<0.01; **p<0.001; ***p<0.0001 *** * ** ** * ** *** ** ** ** *** *** *** *** # Based on Mixed Model Repeated Measures with Multiple Imputation, with fixed categorical effects of treatment, center, study w eek , and treatment by study week interaction, as well as baseline value and baseline value - by - study week interaction.
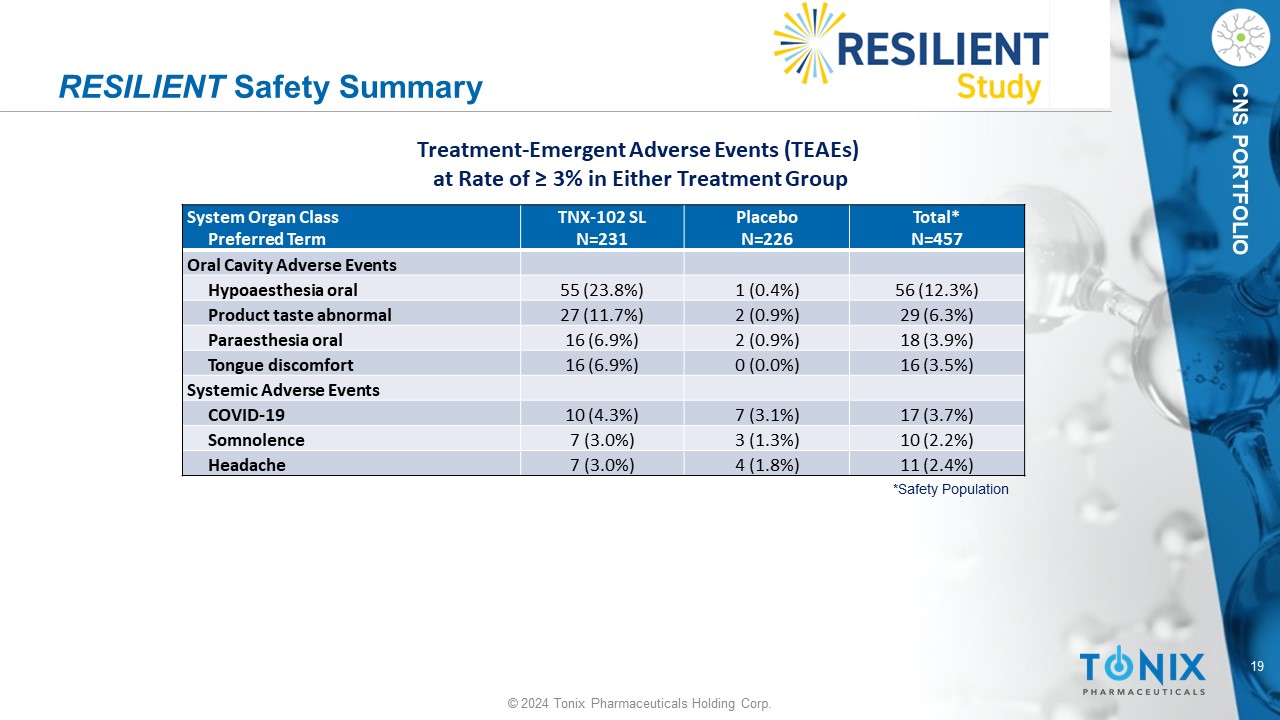
19 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO RESILIENT Safety Summary Total* N=457 Placebo N=226 TNX - 102 SL N=231 System Organ Class Preferred Term Oral Cavity Adverse Events 56 (12.3%) 1 (0.4%) 55 (23.8%) Hypoaesthesia oral 29 (6.3%) 2 (0.9%) 27 (11.7%) Product taste abnormal 18 (3.9%) 2 (0.9%) 16 (6.9%) Paraesthesia oral 16 (3.5%) 0 (0.0%) 16 (6.9%) Tongue discomfort Systemic Adverse Events 17 (3.7%) 7 (3.1%) 10 (4.3%) COVID - 19 10 (2.2%) 3 (1.3%) 7 (3.0%) Somnolence 11 (2.4%) 4 (1.8%) 7 (3.0%) Headache *Safety Population Treatment - Emergent Adverse Events (TEAEs) at Rate of ≥ 3% in Either Treatment Group
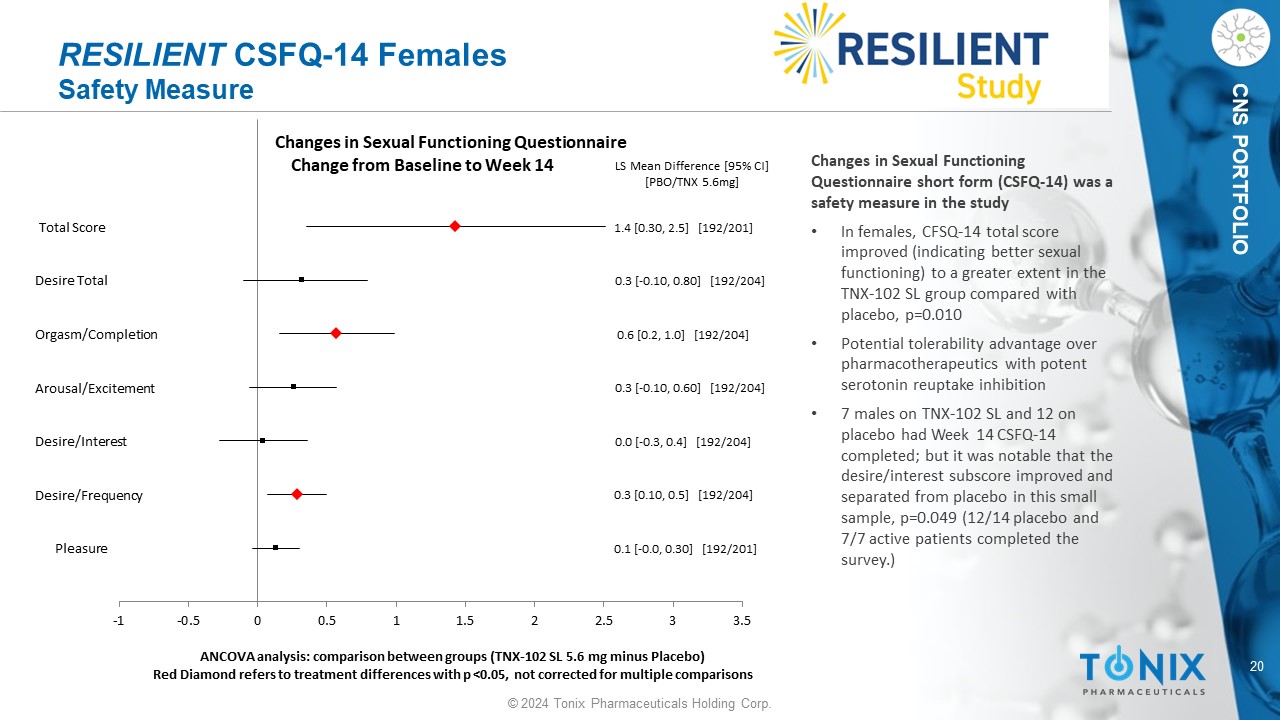
20 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO RESILIENT CSFQ - 14 Females Safety Measure Pleasure Desire/Frequency Desire/Interest Arousal/Excitement Orgasm/Completion Desire Total Total Score 0.1 [ - 0.0, 0.30] [192/201] 0.3 [0.10, 0.5] [192/204] 0.0 [ - 0.3, 0.4] [192/204] 0.3 [ - 0.10, 0.60] [192/204] 0.6 [0.2, 1.0] [192/204] 0.3 [ - 0.10, 0.80] [192/204] 1.4 [0.30, 2.5] [192/201] LS Mean Difference [95% CI] [PBO/TNX 5.6mg] -1 -0.5 0 0.5 1 1.5 2 2.5 3 3.5 ANCOVA analysis: comparison between groups (TNX - 102 SL 5.6 mg minus Placebo) Red Diamond refers to treatment differences with p <0.05, not corrected for multiple comparisons Changes in Sexual Functioning Questionnaire Change from Baseline to Week 14 Changes in Sexual Functioning Questionnaire short form (CSFQ - 14) was a safety measure in the study • In females, CFSQ - 14 total score improved (indicating better sexual functioning) to a greater extent in the TNX - 102 SL group compared with placebo, p=0.010 • Potential tolerability advantage over pharmacotherapeutics with potent serotonin reuptake inhibition • 7 males on TNX - 102 SL and 12 on placebo had Week 14 CSFQ - 14 completed; but it was notable that the desire/interest subscore improved and separated from placebo in this small sample, p=0.049 (12/14 placebo and 7/7 active patients completed the survey.)
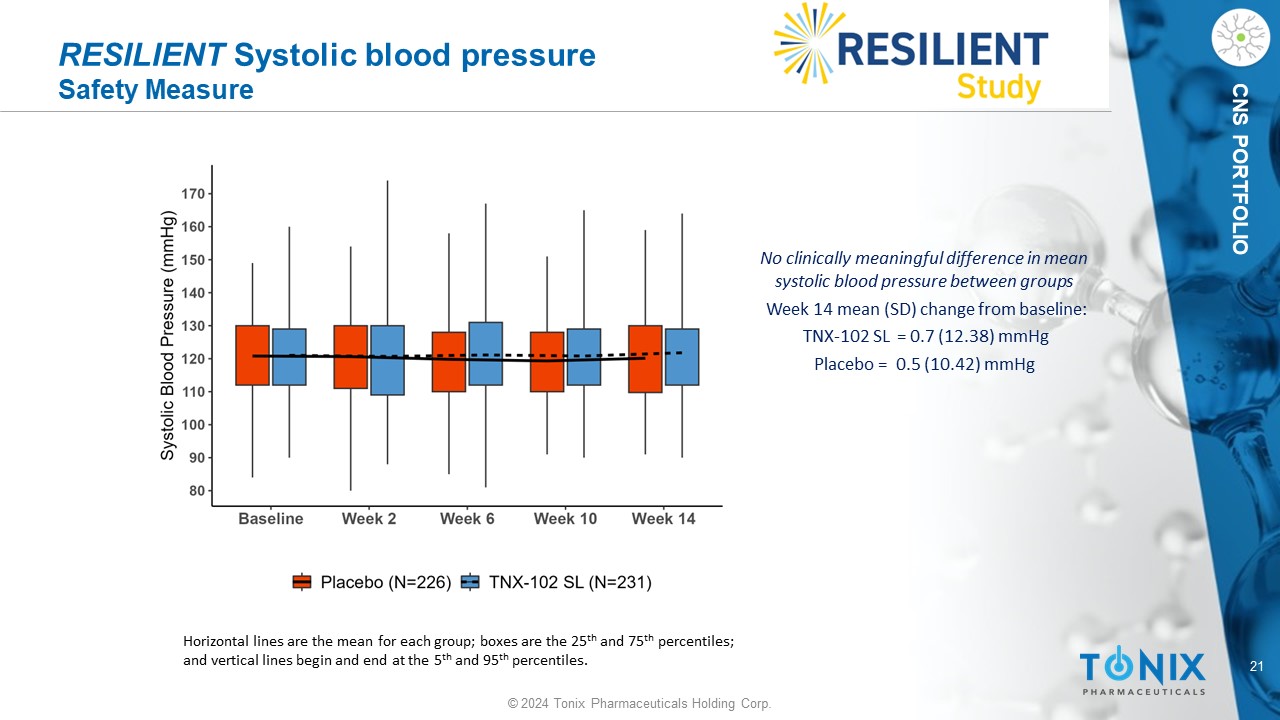
21 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO RESILIENT Systolic blood pressure Safety Measure No clinically meaningful difference in mean systolic blood pressure between groups Week 14 mean (SD) change from baseline: TNX - 102 SL = 0.7 (12.38) mmHg P lacebo = 0.5 (10.42) mmHg Horizontal lines are the mean for each group; boxes are the 25 th and 75 th percentiles; and vertical lines begin and end at the 5 th and 95 th percentiles.

22 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO RESILIENT Diastolic blood pressure Safety Measure No clinically meaningful difference in mean diastolic blood pressure between groups Week 14 mean (SD) change from baseline: TNX - 102 SL = 1.1 ( 8.60 ) mmHg P lacebo = 0.2 ( 8.22 ) mmHg Horizontal lines are the mean for each group; boxes are the 25 th and 75 th percentiles; and vertical lines begin and end at the 5 th and 95 th percentiles.
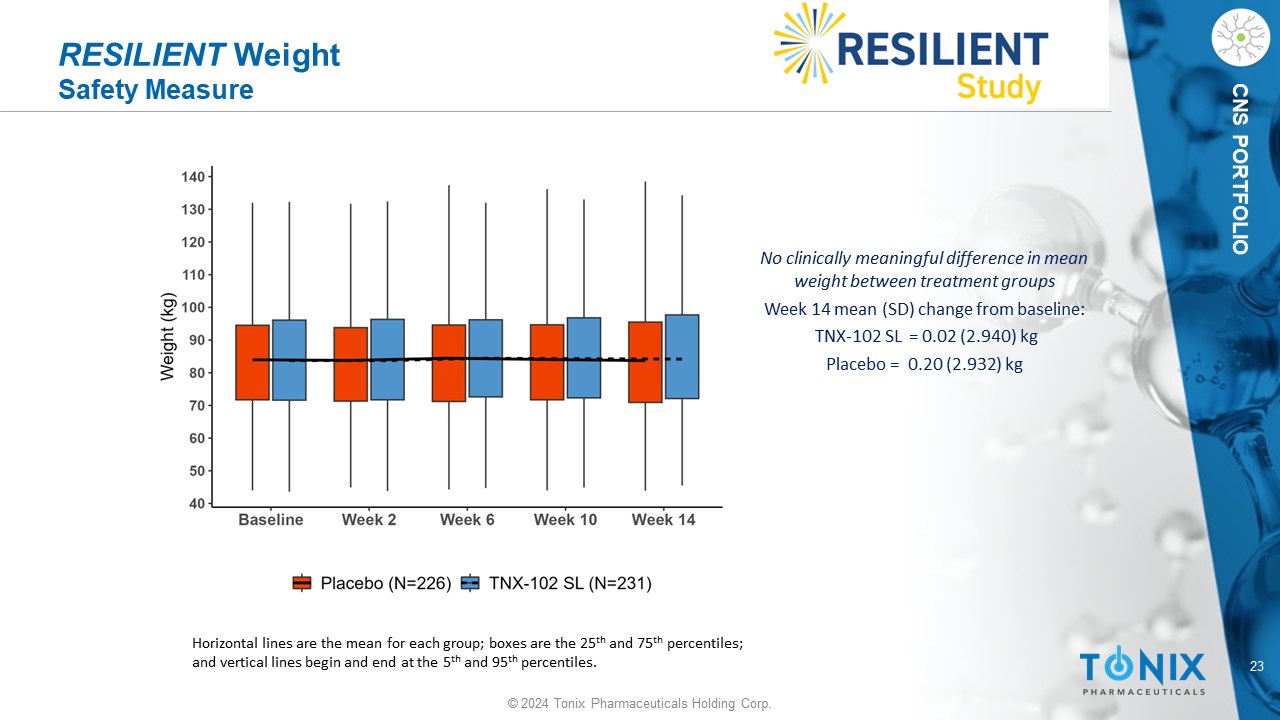
23 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO RESILIENT Weight Safety Measure No clinically meaningful difference in mean weight between treatment groups Week 14 mean (SD) change from baseline: TNX - 102 SL = 0.02 (2.94 0 ) kg P lacebo = 0.20 (2.932) kg Horizontal lines are the mean for each group; boxes are the 25 th and 75 th percentiles; and vertical lines begin and end at the 5 th and 95 th percentiles.

24 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Fibromyalgia: Market Characteristics Prevalence • One of the more common chronic pain disorders (2 - 4% of US Population) 1 Diagnosed population • Large population but underdiagnosed 2 relative to prevalence rate • Majority receive drug treatment 3 Treatment Pattern • Polypharmacy the norm - average 2.6 drugs/patient 3 • Rotation through therapy common: average ~5 drugs/year 3 • Estimated that >22 million prescriptions are issued for the treatment of fibromyalgia (on - and off - label usage) each year 4,5 Unmet Need • Majority of patients do not respond or cannot tolerate therapy 6 1 American College of Rheumatology ( www.ACRPatientInfo.org accessed May 7, 2019) – prevalence rate of 2 - 4% for U.S. adult population (~250 million) 2 Vincent et al., 2013; diagnosed prevalence rate was 1.1% of adult population or 50% of the prevalent population 3 Robinson, et al., 2012; 85% received drug treatment 4 Vincent et al, Arthritis Care Res 2013;65:786 5 Product sales derived from IMS MIDAS; IMS NDTI used to factor usage for fibromyalgia; data accessed April 2015. 6 Market research by Frost & Sullivan, commissioned by Tonix , 2011

25 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Fewer than Half of Those Treated for Fibromyalgia Receive Sustained Benefit from the Three FDA - Approved Drugs 1 Respond, but intolerant of side effects Do not respond 25% 35% 60% fa il ure rate 1 The three drugs with FDA approval for the treatment of fibromyalgia: Pregabalin (Lyrica); Duloxetine (Cymbalta); Milnacipran (Savella) 2 Market research by Frost & Sullivan, commissioned by Tonix (2011) • The treatment objective is to restore functionality and quality of life by broadly improving symptoms while avoiding significant side effects • The majority fail therapy due to lack of a response or poor tolerability 2 Treated Population

26 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Large Need for New Fibromyalgia Therapies that Provide Broad Symptom Improvement with Better Tolerability • Currently - approved medications may have side effects that limit long - term use 1 • Many patients skip doses or discontinue altogether within months of treatment initiation • Medication - related side effects may be similar to fibromyalgia symptoms • High rates of discontinuation, switching and augmentation • Attempt to treat multiple symptoms and/or avoid intolerable side effects • Average of 2 - 3 medications used simultaneously 2 • The typical patient has tried six different medications 3 • Substantial off - label use of narcotic painkillers and prescription sleep aids 3 • Among those diagnosed, more than one - third have used prescription opioids as a means of treatment 4 • TNX - 102 SL is a non - opioid, centrally - acting analgesic that could provide a new therapeutic option for fibromyalgia patients 1 Nuesch et al, Ann Rheum Dis 2013;72:955 - 62. 2 Robinson RL et al, Pain Medicine 2012;13:1366. 3 Patient Trends: Fibromyalgia”, Decision Resources, 2011. 4 Berger A, Dukes E, Martin S, Edelsberg J, Oster G, Int J Clin Pract , 2007; 61(9):1498 – 1508.

27 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Tonix Medicines: Commercial - Stage Specialty Pharma Subsidiary • Tonix Medicines is a w holly - owned subsidiary of Tonix ( NASDAQ: TNXP) • Currently marketing two products indicated for the treatment of acute migraine: Zembrace ® SymTouch ® and Tosymra ® • ~16 M in net sales 1 • Nascent commercial organization • Tonix Medicines is led by James (Jim) Hunter • Veteran pharma executive with a track record for growing early businesses • Hunter previously founded Validus with Tonix CEO, Dr. Lederman • Tonix Medicines is preparing to launch TNX - 102 SL for fibromyalgia • Fibromyalgia care is relatively concentrated to specialized providers • We believe prescribing physicians can be targeted effectively by a specialty sales force • Evolving landscape in commercial markets favors distribution channels such as specialty pharmacies 1 Tonix 10 - Q for 3Q23

28 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL: Patents and Patent Applications • U.S. Composition:* • A 75:25 cyclobenzaprine HCl - mannitol eutectic (dependent claims add a basifying agent). • 5 US Patents (Expire November 2034) • 1 Pending US Application (Would expire November 2034) • A composition of a cyclobenzaprine HCl and a basifying agent suitable for sublingual absorption. • 1 Pending US Application (Would e xpire June 2033) • U.S. Methods of Use* (Specific Indications): • Fibromyalgia • Pain, Sleep Disturbance, Fatigue • 1 Pending US Application (Would e xpire December 2041) • Early Onset Response • 1 Pending US Provisional Application (Would e xpire December 2044) • Depressive Symptoms • 1 Pending US Application (Would e xpire March 2032) • Sexual Dysfunction • 1 Pending US Application (Would expire October 2041) • PASC • 1 Pending US Application (Would e xpire June 2043) • PTSD • 1 US Patent ( Expires November 2030) • Agitation (Dementia) • 1 US Patent ( Expires December 2038) • 1 Pending US Application (Would expire December 2038) • Alcohol Use Disorder • 1 Pending US Application (Would e xpire November 2041) • Foreign Filings • Corresponding foreign patents have been filed and some have issued: • Composition (25 patents, 3 allowed applications, 16 pending applications) • Methods of Use (11 patents, 54 pending applications) *US Patents: Issued: US Patent Nos. 9,636,408; 9,956,188; 10,117,936; 10,864,175; 11,839,594; 9,918,948; 11,826,321. Pending: US Patent Application Nos. 13/918,692; 18/385,468; 13/412,571; 18/265,525; 63/612,352; 18/382,262; 18/037,815; 17/226,058; 18/212,500.

29 © 2024 Tonix Pharmaceuticals Holding Corp. PROFILE DEVELOPMENT PROGRAM Patents Issued TNX - 102 SL*: Fibromyalgia Cyclobenzaprine Protectic ® Sublingual Tablets Fibromyalgia (FM) is a chronic pain disorder resulting from amplified sensory and pain signaling within the CNS • Afflicts an estimated 6 - 12 million adults in the U.S., the majority of whom are women 1 • Symptoms include chronic widespread pain, nonrestorative sleep, fatigue, and cognitive dysfunction • Patients struggle with daily activities, have impaired quality of life, and frequently are disabled • Physicians and patients report common dissatisfaction with currently marketed products Market Entry: Fibromyalgia Additional Indications: Fibromyalgia - type Long COVID, Acute Stress Disorder (ASD), PTSD, Agitation in Alzheimer’s, Alcohol Use Disorder Status: One Positive Phase 3 study RELIEF completed, p - value = 0.01 2 Second Phase 3 study RALLY missed primary endpoint Confirmatory Phase 3 study RESILIENT positive, p - value = 0.00005 Next Steps: Pre - NDA meeting with FDA *TNX - 102 SL has not been approved for any indication. 1 American Chronic Pain Association (www.theacpa.org, 2019) 2 Lederman et al., (2023) Arthritis Care & Research "Efficacy and Safety of TNX - 102 SL (Sublingual Cyclobenzaprine) for the Treatment of Fibromyalgia: Results From the RELIEF Trial ", doi : 10.1002/acr.25142. Epub ahead of print. PMID: 37165930. When the check engine light malfunctions, the light is on even though the car is not malfunctioning

© 2024 Tonix Pharmaceuticals Holding Corp. THANK YOU

31 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Zembrace ® Important Safety Information (1 of 2) Zembrace SymTouch ( Zembrace ) can cause serious side effects, including heart attack and other heart problems, which may lead to death. Stop use and get emergency help if you have any signs of a heart attack: D iscomfort in the center of your chest that lasts for more than a few minutes or goes away and comes back; severe tightness, pain, pressure, or heaviness in your chest, throat, neck, or jaw; pain or discomfort in your arms, back, neck, jaw or stomach ; shortness of breath with or without chest discomfort ; breaking out in a cold sweat ; nausea or vomiting ; feeling lightheaded Zembrace is not for people with risk factors for heart disease (high blood pressure or cholesterol, smoking, overweight, diabetes, family history of heart disease) unless a heart exam shows no problem. Do not use Zembrace if you have: H istory of heart problems ; narrowing of blood vessels to your legs, arms, stomach, or kidney (peripheral vascular disease) ; uncontrolled high blood pressure ; hemiplegic or basilar migraines. If you are not sure if you have these, ask your provider. H ad a stroke, transient ischemic attacks (TIAs), or problems with blood circulation ; severe liver problems ; taken any of the following medicines in the last 24 hours: almotriptan , eletriptan , frovatriptan , naratriptan, rizatriptan, ergotamines , dihydroergotamine ; are taking certain antidepressants, known as monoamine oxidase (MAO) - A inhibitors or it has been 2 weeks or less since you stopped taking a MAO - A inhibitor. Ask your provider for a list of these medicines if you are not sure. A n allergy to sumatriptan or any of the components of Zembrace Tell your provider about all of your medical conditions and medicines you take, including vitamins and supplements. Zembrace can cause dizziness, weakness, or drowsiness. If so, do not drive a car, use machinery, or do anything where you need to be alert.

32 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Zembrace ® Important Safety Information (2 of 2) Zembrace may cause serious side effects including: • Changes in color or sensation in your fingers and toes; sudden or severe stomach pain, stomach pain after meals, weight loss, na usea or vomiting, constipation or diarrhea, bloody diarrhea, fever; cramping and pain in your legs or hips; feeling of heaviness or t igh tness in your leg muscles; burning or aching pain in your feet or toes while resting; numbness, tingling, or weakness in your legs; cold fe eli ng or color changes in one or both legs or feet; increased blood pressure including a sudden severe increase even if you have no history of high blood pressure; medication overuse headaches from using migraine medicine for 10 or more days each month. If your headaches g et worse, call your provider. • Serotonin syndrome, a rare but serious problem that can happen in people using Zembrace , especially when used with anti - depressant medicines called SSRIs or SNRIs. Call your provider right away if you have: mental changes such as seeing things that are not th ere (hallucinations), agitation, or coma; fast heartbeat; changes in blood pressure; high body temperature; tight muscles; or tro ubl e walking. • Hives (itchy bumps); swelling of your tongue, mouth, or throat • Seizures even in people who have never had seizures before The most common side effects of Zembrace include: pain and redness at injection site; tingling or numbness in your fingers or toes; dizziness; warm, hot, burning feeling to your face (flushing); discomfort or stiffness in your neck; feeling weak, drowsy, or ti red. Tell your provider if you have any side effect that bothers you or does not go away. These are not all the possible side effe cts of Zembrace . For more information, ask your provider. This is the most important information to know about Zembrace but is not comprehensive. For more information, talk to your provider and read the Patient Information and Instructions for Use . You can also visit www.upsher - smith.com or call 1 - 888 - 650 - 3789. For full Prescribing Information, visit: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6e5b104f - 2b9e - 416e - 92fb - ef1bdaea867d You are encouraged to report adverse effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1 - 800 - FDA - 1088. Zembrace is a prescription medicine used to treat acute migraine headaches with or without aura in adults who have been diagnosed with migraine. Zembrace is not used to prevent migraines. It is not known if it is safe and effective in children under 18 years of age.

33 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Tosymra® Important Safety Information (1 of 2) Tosymra® can cause serious side effects, including heart attack and other heart problems, which may lead to death. Stop Tosymra and get emergency medical help if you have any signs of heart attack: • D iscomfort in the center of your chest that lasts for more than a few minutes or goes away and comes back ; severe tightness, pain, pressure, or heaviness in your chest, throat, neck, or jaw ; pain or discomfort in your arms, back, neck, jaw, or stomach ; shortness of breath with or without chest discomfort; breaking out in a cold sweat ; nausea or vomiting ; feeling lightheaded Tosymra is not for people with risk factors for heart disease (high blood pressure or cholesterol, smoking, overweight, diabetes, family history of heart disease) unless a heart exam is done and shows no problem. Do not use Tosymra if you have: • H istory of heart problems ; narrowing of blood vessels to your legs, arms, stomach, or kidney (peripheral vascular disease) ; uncontrolled high blood pressure ; severe liver problems ; hemiplegic or basilar migraines. If you are not sure if you have these, ask your healthcare provider. • H ad a stroke, transient ischemic attacks (TIAs), or problems with blood circulation ; taken any of the following medicines in the last 24 hours: almotriptan , eletriptan , frovatriptan , naratriptan, rizatriptan, ergotamines , or dihydroergotamine. Ask your provider if you are not sure if your medicine is listed above • are taking certain antidepressants, known as monoamine oxidase (MAO) - A inhibitors or it has been 2 weeks or less since you stopped taking a MAO - A inhibitor . A sk your provider for a list of these medicines if you are not sure • A n allergy to sumatriptan or any ingredient in Tosymra Tell your provider about all of your medical conditions and medicines you take, including vitamins and supplements. Tosymra can cause dizziness, weakness, or drowsiness. If so, do not drive a car, use machinery, or do anything where you need to be alert.

34 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Tosymra may cause serious side effects including: • Changes in color or sensation in your fingers and toes; sudden or severe stomach pain, stomach pain after meals, weight loss, nausea or vomiting, constipation or diarrhea, bloody diarrhea, fever; cramping and pain in your legs or hips, feeling of heaviness or tightness in your leg muscles, burning or aching pain in your feet or toes while resting, numbness, tingling, or weakness in your legs, cold feeling or color changes in one or both legs or feet; increased blood pressure including a sudden severe increase even if you have no history of high blood pressure; medication overuse headaches from using migraine medicine for 10 or more days each month. If your headaches get worse, call your provider . • Serotonin syndrome, a rare but serious problem that can happen in people using Tosymra, especially when used with anti - depressant medicines called SSRIs or SNRIs. Call your provider right away if you have : mental changes such as seeing things that are not there (hallucinations), agitation, or coma; fast heartbeat; changes in blood pressure; high body temperature; tight muscles; or trouble walking. • Seizures even in people who have never had seizures before The most common side effects of Tosymra include : tingling, dizziness, feeling warm or hot, burning feeling, feeling of heaviness, feeling of pressure, flushing, feeling of tightness, numbness, application site (nasal) reactions, abnormal taste, and throat irritation. Tell your provider if you have any side effect that bothers you or does not go away. These are not all the possible side effects of Tosymra. For more information, ask your provider. This is the most important information to know about Tosymra but is not comprehensive. For more information, talk to your provider and read the Patient Information and Instructions for Use . You can also visit www.upsher - smith.com or call 1 - 888 - 650 - 3789. For full Prescribing Information, visit: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=015a5cf9 - f246 - 48bc - b91e - cd730a53d8aa You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch , or call 1 - 800 - FDA - 1088. Tosymra is a prescription medicine used to treat acute migraine headaches with or without aura in adults. Tosymra is not used to treat other types of headaches such as hemiplegic or basilar migraines or cluster headaches. Tosymra is not used to prevent migraines. It is not known if Tosymra is safe and effective in children under 18 years of age. Tosymra ® Important Safety Information (2 of 2)
v3.23.4
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Tonix Pharmaceuticals (NASDAQ:TNXP)
Historical Stock Chart
Von Mär 2024 bis Apr 2024
Tonix Pharmaceuticals (NASDAQ:TNXP)
Historical Stock Chart
Von Apr 2023 bis Apr 2024