Masimo's Stork Baby-Monitoring System Gets FDA Approval
18 Dezember 2023 - 7:28PM
Dow Jones News
By Ben Glickman
Masimo's baby-monitoring system Stork has received approval from
the U.S. Food and Drug Administration for medical uses.
The system is cleared by the FDA for prescription use in healthy
and sick babies up to 18 months old.
Stork allows parents to monitor babies' oxygen saturation, pulse
rate and skin temperature. Users can receive alarms regarding these
metrics and can share information with doctors remotely.
The Irvine, Calif.-based medical-device company said Monday that
the system was available at retailers nationwide as a non-medical
device and could now be used as a medical device with a
prescription.
Write to Ben Glickman at ben.glickman@wsj.com
(END) Dow Jones Newswires
December 18, 2023 13:13 ET (18:13 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
Masimo (NASDAQ:MASI)
Historical Stock Chart
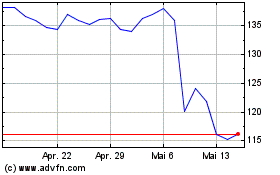
Von Apr 2024 bis Mai 2024
Masimo (NASDAQ:MASI)
Historical Stock Chart
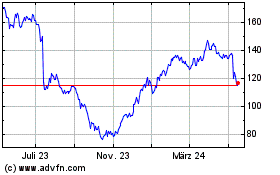
Von Mai 2023 bis Mai 2024