Innate Pharma Shares Fall Premarket on FDA Partial Study Hold
05 Oktober 2023 - 2:14PM
Dow Jones News
By Colin Kellaher
Shares of Innate Pharma dropped in premarket trading Thursday
after the biopharmaceutical company said the U.S. Food and Drug
Administration placed a partial hold on studies of its lead
proprietary program lacutamab following the death of a patient.
Innate said the partial hold, which pauses new patient
enrollment in its ongoing Phase 2 and Phase 1b studies of
lacutamab, follows a fatal case of hemophagocytic
lymphohistiocytosis, a rare hematologic disorder.
The Marseille, France, company said it is working to address
requests from the FDA, including the incorporation of risk
mitigation and management strategies for hemophagocytic
lymphohistiocytosis in the lacutamab studies.
Innate noted that patients already on study treatment who are
deriving clinical benefit can continue treatment, and that it
doesn't expect any delay in the Phase 2 study data, as all patients
had already been recruited.
Innate shares, which closed Wednesday at $2.47, were recently
down 11% to $2.19 in premarket trading.
Write to Colin Kellaher at colin.kellaher@wsj.com
(END) Dow Jones Newswires
October 05, 2023 07:59 ET (11:59 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
Innate Pharma (EU:IPH)
Historical Stock Chart
Von Apr 2024 bis Mai 2024
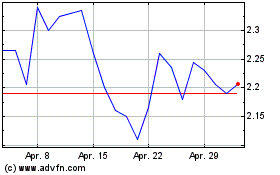
Innate Pharma (EU:IPH)
Historical Stock Chart
Von Mai 2023 bis Mai 2024