FDA Gives Marketing Authorization to Invitae Test for Cancer Genes
30 September 2023 - 2:00AM
Dow Jones News
By Ben Glickman
The Food and Drug Administration gave de novo marketing
authorization to a diagnostic test that can help detect genes
associated with elevated risk of developing cancers.
The FDA said that the test, called the Invitae Common Hereditary
Cancers Panel, was an in vitro diagnostic test designed to identify
47 genes which are linked to an elevated risk of cancer.
The de novo premarket review is a regulatory pathway through the
FDA for low- to moderate-risk new medical devices.
The FDA said the approval would allow subsequent tests of the
same type to go through a potentially quicker and cheaper
pre-market process.
Invitae tested over 9,000 samples and found an accuracy greater
than or equal to 99%.
Write to Ben Glickman at ben.glickman@wsj.com
(END) Dow Jones Newswires
September 29, 2023 19:45 ET (23:45 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
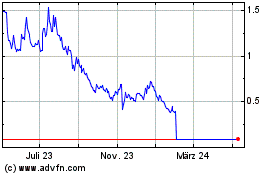
Invitae (NYSE:NVTA)
Historical Stock Chart
Von Apr 2024 bis Mai 2024
Invitae (NYSE:NVTA)
Historical Stock Chart
Von Mai 2023 bis Mai 2024