Novavax Covid-19 Vaccine Gets Health Canada Approval for Adolescents
07 Dezember 2022 - 4:33PM
Dow Jones News
By Adriano Marchese
Novavax Inc. said Wednesday that Canada's health regulator has
approved its Covid-19 vaccine in adolescents aged 12 through
17.
The vaccine developer said that Health Canada approved its
supplement to a new drug submission for Nuvaxovid which works
towards immunization to help prevent the coronavirus in
individuals.
The approval was based on data from pediatric expansion of the
phase 3 trial in the U.S. which has showed that effectiveness was
higher in adolescents than in young adults.
Trials have found that protective efficacy was of 79.5% overall
at a time when the Delta variant was the predominant circulating
strain.
Write to Adriano Marchese at adriano.marchese@wsj.com
(END) Dow Jones Newswires
December 07, 2022 10:18 ET (15:18 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
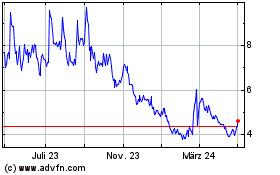
Novavax (NASDAQ:NVAX)
Historical Stock Chart
Von Mär 2024 bis Apr 2024
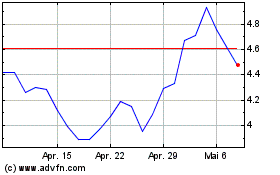
Novavax (NASDAQ:NVAX)
Historical Stock Chart
Von Apr 2023 bis Apr 2024