false
0001610820
A1
0001610820
2024-07-18
2024-07-18
0001610820
BCTX:CommonSharesNoParValueMember
2024-07-18
2024-07-18
0001610820
BCTX:WarrantsToPurchaseCommonSharesNoParValueMember
2024-07-18
2024-07-18
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
WASHINGTON,
D.C. 20549
FORM
8-K
CURRENT
REPORT
PURSUANT
TO SECTION 13 OR 15(d) OF THE
SECURITIES
EXCHANGE ACT OF 1934
Date
of Report (Date of earliest event reported): July 18, 2024
BRIACELL
THERAPEUTICS CORP.
(Exact
name of registrant as specified in its charter)
| British
Columbia |
|
47-1099599 |
(State
or other jurisdiction
of
incorporation or organization) |
|
(I.R.S.
Employer
Identification
No.) |
| |
|
|
Suite
300 - 235 15th Street
West
Vancouver, BC V7T 2X1 |
|
V7T
2X1 |
| (Address
of principal executive offices) |
|
(Zip
Code) |
(604)
921-1810
(Registrant’s
telephone number, including area code)
Commission
File No. 001-40101
Check
the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under
any of the following provisions (see General Instruction A.2. below):
| ☐ |
Written
communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| |
|
| ☐ |
Soliciting
material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| |
|
| ☐ |
Pre-commencement
communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| |
|
| ☐ |
Pre-commencement
communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities
registered under Section 12(b) of the Act:
| Title
of each class |
|
Trading
Symbol(s) |
|
Name
of each exchange on which registered |
| Common
Shares, no par value |
|
BCTX |
|
The
Nasdaq Stock Market LLC |
| Warrants
to purchase common shares, no par value |
|
BCTXW |
|
The
Nasdaq Stock Market LLC |
Indicate
by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (17 CFR §230.405)
or Rule 12b-2 of the Securities Exchange Act of 1934 (17 CFR §240.12b-2).
Emerging
growth company ☒
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
Item
7.01 Regulation FD Disclosure.
On
July 18, 2024, BriaCell Therapeutics Corp. (the “Company”) issued a press release
announcing significantly higher progression free survival for its top responder patient in the Phase 2 study of the Company’s Bria-IMT™
regimen in combination with an immune checkpoint inhibitor in metastatic breast cancer. A copy of the press release is attached to this
Current Report on Form 8-K as Exhibit 99.1.
The
information in this Current Report on Form 8-K under Item 7.01, including the information contained in Exhibit 99.1, is being furnished
to the Securities and Exchange Commission, and shall not be deemed to be “filed” for the purposes of Section 18 of the Securities
Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, and shall
not be deemed to be incorporated by reference into any filing under the Securities Act of 1933, as amended, or the Exchange Act, except
as shall be expressly set forth by a specific reference in such filing.
Item
9.01 Condensed interim financial statements and Exhibits
EXHIBIT
INDEX
SIGNATURES
Pursuant
to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by
the undersigned hereunto duly authorized.
| |
BRIACELL
THERAPEUTICS CORP. |
| |
|
| |
/s/
William V. Williams |
| July
18, 2024 |
William
V. Williams |
| |
President
and Chief Executive Officer |
Exhibit
99.1

BriaCell
Quadruples Progression Free Survival (PFS) in Patient with “Eye-Bulging” Metastatic Breast Cancer
| ● |
Progression
free survival (PFS) extended to 9.1 months in ADC resistant patient - quadruple the PFS of patients in similar studies 1,2,
3 |
| ● |
Significant
reduction of “Eye-Bulging” metastatic breast cancer tumor was previously reported |
| ● |
Heavily
pre-treated patient had failed 8 prior regimens including antibody-drug conjugate (ADC) therapy and continues to receive BriaCell
treatment |
PHILADELPHIA,
PA and VANCOUVER, British Columbia, July 18, 2024— BriaCell Therapeutics Corp. (Nasdaq: BCTX, BCTXW) (TSX: BCT) (“BriaCell”
or the “Company”), a clinical-stage biotechnology company that develops novel immunotherapies to transform cancer care, is
pleased to report significantly higher PFS for its top responder patient in the Phase 2 study of BriaCell’s Bria-IMT™ regimen
in combination with an immune checkpoint inhibitor in metastatic breast cancer. The patient remains alive and she continues to receive
BriaCell’s treatment regimen.
“We
are extremely pleased with the unprecedented survival benefit in this very-difficult-to-treat patient,” stated Dr. William V. Williams,
BriaCell’s President and CEO. “This data represents a step forward in our efforts to build on our knowledge and successes
to transform cancer care for patients. We expect to replicate this positive data in our ongoing Phase 3 study and bring relief to cancer
patients whose medical needs remain unmet.”
“Despite
recent advances in cancer therapy, metastatic breast cancer remains an unmet medical need, as current treatments are limited by poor
survival and harsh side effects,” commented Dr. Giuseppe Del Priore, BriaCell’s Chief Medical Officer. “The Bria-IMT™
regimen produced a much longer than expected survival benefit in addition to its favorable safety and tolerability in this patient suggesting
its potential as a therapeutic option for these cancer patients.”
The
patient had a large right orbital lesion (behind the right eye) and a right temporal lobe lesion (in the right side of the brain). The
temporal lobe lesion is no longer detectable, while the orbital lesion has continued to shrink markedly (see figure showing resolution
of proptosis post treatment (small arrows) with reduction in tumor indicated by the large arrows). In addition, her tumor markers (blood
tests that correlate with the amount of tumor in the body) have markedly decreased from her pre-treatment levels.
Figure
1: Responder Images - Bria-IMT™ Regimen
| Pre-Treatment |
3 Months on Treatment |
9 Months on Treatment |
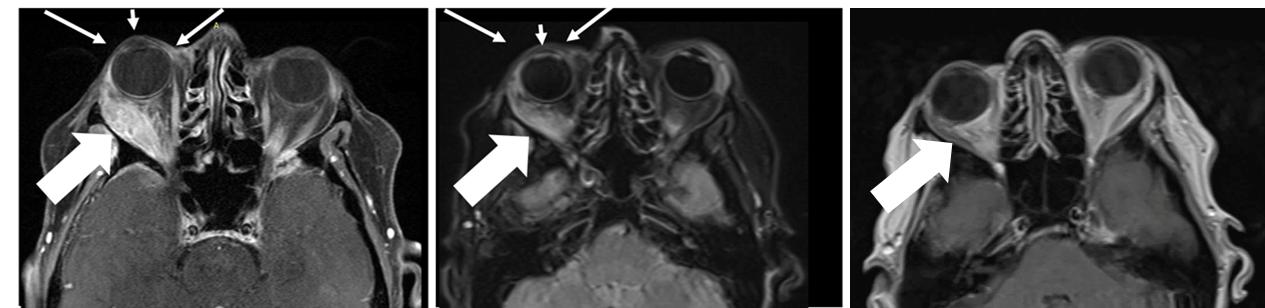 |
Table
1: Bria-IMT™ and historical clinical data in MBC patients who failed multiple prior treatments
| Study | |
PFS
(months) | |
| Top
Responder Patient | |
| 9.1 | + |
| Bardia,
A. et. al. 1 | |
| 1.7 | |
| Tripathy
D. et. al. 2 | |
| 1.9 | |
| O’Shaughnessy
J. et. al. non-TNBC 3 | |
| 2.3 | |
| O’Shaughnessy
J. et. al. TNBC 3 | |
| 1.6 | |
1,2,3
Data is shown for the intent to treat population for the control group treated with treatment of physician’s choice, which
is the comparator in the BriaCell’s ongoing pivotal Phase 3 study.
2
This paper describes patients with brain metastases, which were also present in the patient described.
+
Indicates the patient is ongoing in the study.
References
| 1.
|
Bardia
A, et al. Final Results From the Randomized Phase III ASCENT Clinical Trial in Metastatic Triple-Negative Breast Cancer and Association
of Outcomes by Human Epidermal Growth Factor Receptor 2 and Trophoblast Cell Surface Antigen 2 Expression. J Clin Oncol. 2024 May
20;42(15):1738-1744. doi: 10.1200/JCO.23.01409. Epub 2024 Feb 29. PMID: 38422473. |
| 2.
|
Tripathy
D, et al. Treatment with etirinotecan pegol for patients with metastatic breast cancer and brain metastases: final results from the
phase 3 ATTAIN randomized clinical trial. JAMA Oncol. 2022;8(7):1047-1052. doi:10.1001/jamaoncol.2022.0514. |
| 3.
|
O’Shaughnessy
J et al. Analysis of patients without and with an initial triple-negative breast cancer diagnosis in the phase 3 randomized ASCENT
study of sacituzumab govitecan in metastatic triple-negative breast cancer. Breast Cancer Res Treat. 2022 Sep;195(2):127-139. doi:
10.1007/s10549-022-06602-7. Epub 2022 May 11. PMID: 35545724; PMCID: PMC9374646. |
About
BriaCell Therapeutics Corp.
BriaCell
is a clinical-stage biotechnology company that develops novel immunotherapies to transform cancer care. More information is available
at https://briacell.com/.
Safe
Harbor
This
press release contains “forward-looking statements” that are subject to substantial risks and uncertainties. All statements,
other than statements of historical fact, contained in this press release are forward-looking statements. Forward-looking statements
contained in this press release may be identified by the use of words such as “anticipate,” “believe,” “contemplate,”
“could,” “estimate,” “expect,” “intend,” “seek,” “may,” “might,”
“plan,” “potential,” “predict,” “project,” “target,” “aim,” “should,”
“will,” “would,” or the negative of these words or other similar expressions, although not all forward-looking
statements contain these words. Forward-looking statements, including those about BriaCell replicating positive data in its ongoing Phase
3 study; BriaCell’s Bria-IMT™ regimen bringing relief to cancer patients whose medical needs remain unmet; and the Bria-IMT™
regimen becoming a therapeutic option for metastatic breast cancer patients, are based on BriaCell’s current expectations and are
subject to inherent uncertainties, risks, and assumptions that are difficult to predict. Further, certain forward-looking statements
are based on assumptions as to future events that may not prove to be accurate. These and other risks and uncertainties are described
more fully under the heading “Risks and Uncertainties” in the Company’s most recent Management’s Discussion and
Analysis, under the heading “Risk Factors” in the Company’s most recent Annual Information Form, and under “Risks
and Uncertainties” in the Company’s other filings with the Canadian securities regulatory authorities and the U.S. Securities
and Exchange Commission, all of which are available under the Company’s profiles on SEDAR+ at www.sedarplus.ca and
on EDGAR at www.sec.gov. Forward-looking statements contained in this announcement are made as of this date, and BriaCell
Therapeutics Corp. undertakes no duty to update such information except as required under applicable law.
Neither
the Toronto Stock Exchange nor its Regulation Services Provider (as that term is defined in the policies of the Toronto Stock Exchange)
accepts responsibility for the adequacy or accuracy of this release.
Contact
Information
Company
Contact:
William
V. Williams, MD
President
& CEO
1-888-485-6340
info@briacell.com
Media
Relations:
Jules
Abraham
CORE
IR
julesa@coreir.com
Investor
Relations Contact:
CORE
IR
investors@briacell.com
v3.24.2
Cover
|
Jul. 18, 2024 |
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Jul. 18, 2024
|
| Entity File Number |
001-40101
|
| Entity Registrant Name |
BRIACELL
THERAPEUTICS CORP.
|
| Entity Central Index Key |
0001610820
|
| Entity Tax Identification Number |
47-1099599
|
| Entity Incorporation, State or Country Code |
A1
|
| Entity Address, Address Line One |
Suite
300
|
| Entity Address, Address Line Two |
235 15th Street
|
| Entity Address, City or Town |
West
Vancouver
|
| Entity Address, State or Province |
BC
|
| Entity Address, Postal Zip Code |
V7T
2X1
|
| City Area Code |
(604)
|
| Local Phone Number |
921-1810
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Entity Emerging Growth Company |
true
|
| Elected Not To Use the Extended Transition Period |
false
|
| Common Shares, no par value |
|
| Title of 12(b) Security |
Common
Shares, no par value
|
| Trading Symbol |
BCTX
|
| Security Exchange Name |
NASDAQ
|
| Warrants to purchase common shares, no par value |
|
| Title of 12(b) Security |
Warrants
to purchase common shares, no par value
|
| Trading Symbol |
BCTXW
|
| Security Exchange Name |
NASDAQ
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14a
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=BCTX_CommonSharesNoParValueMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=BCTX_WarrantsToPurchaseCommonSharesNoParValueMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
BriaCell Therapeutics (NASDAQ:BCTX)
Historical Stock Chart
Von Nov 2024 bis Dez 2024

BriaCell Therapeutics (NASDAQ:BCTX)
Historical Stock Chart
Von Dez 2023 bis Dez 2024
