As
filed with the Securities and Exchange Commission on October 9, 2024
Registration
No. 333-275456
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
Post-effective
Amendment No. 1 to
FORM
S-1 ON FORM S-3
REGISTRATION
STATEMENT UNDER
THE
SECURITIES ACT OF 1933
ENSYSCE
BIOSCIENCES, INC.
(Exact
name of registrant as specified in its charter)
| Delaware |
|
2834 |
|
82-2755287 |
(State
or Other Jurisdiction of
Incorporation
or Organization) |
|
(Primary
Standard Industrial
Classification
Code Number) |
|
(I.R.S.
Employer
Identification
Number) |
7946
Ivanhoe Avenue, Suite 201
La
Jolla, California 92037
(858)
263-4196
(Address,
Including Zip Code, and Telephone Number, Including Area Code, of Registrant’s Principal Executive Offices)
Dr.
Lynn Kirkpatrick
President,
Chief Executive Officer & Director
7946
Ivanhoe Avenue, Suite 201
La
Jolla, California 92037
(858)
263-4196
(Name,
address, including zip code, and telephone number, including area code, of agent for service)
Copies
to:
Gregory
J. Rubis, Esq.
Eric
D. Kline, Esq.
Troutman
Pepper Hamilton Sanders LLP
301
Carnegie Center, Suite 400
Princeton,
New Jersey 08540
Telephone:
(609) 452-0808
Approximate
date of commencement of proposed sale to the public: As soon as practicable after this registration statement becomes effective.
If
the only securities being registered on this Form are being offered pursuant to dividend or interest reinvestment plans, please check
the following box: ☐
If
any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the
Securities Act of 1933, other than securities offered only in connection with dividend or interest reinvestment plans, check the following
box. ☒
If
this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the
following box and list the Securities Act registration statement number of the earlier effective registration statement for the same
offering. ☐
If
this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the
Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If
this Form is a post-effective amendment filed pursuant to General Instruction I.D. or a post-effective amendment thereto that shall become
effective upon filing with the Commission pursuant to Rule 462(e) under the Securities Act, check the following box. ☐
If
this Form is a post-effective amendment to a registration statement filed pursuant to General Instruction I.D. filed to register additional
securities or additional classes of securities pursuant to Rule 413(b) under the Securities Act, check the following box. ☐
Indicate
by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting
company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,”
“smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| |
Large
accelerated filer ☐ |
Accelerated
filer ☐ |
| |
Non-accelerated
filer ☒ |
Smaller
reporting company ☒ |
| |
|
Emerging
growth company ☐ |
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
PROSPECTUS

ENSYSCE
BIOSCIENCES, INC.
Up
to 1,029,387 Shares of Common Stock Underlying Notes
Up
to 3,767,091 Shares of Common Stock Underlying Warrants
This
prospectus relates to the issuance by us and the resale by the selling security holders named in this prospectus (the “Selling
Securityholders”) of up to an aggregate of 4,796,478 shares of our common stock, par value $0.0001 per share (“common
stock”), which consists of (i) up to 1,029,387 shares of common stock that (A) are issuable to the Selling Securityholders that
are party to the Securities Purchase Agreement, dated October 23, 2023 (the “October 2023 Securities Purchase Agreement”)
upon the conversion of $214,000 remaining aggregate principal amount of our secured convertible 8% original issue discount promissory
notes (the “Investor Notes”), plus accrued and unpaid interest thereon, based upon a conversion price of $1.5675 per
share and (B) were issued to Selling Securityholders party to the October 2023 Securities Purchase Agreement upon conversion of Investor
Notes; and (ii) up to 3,767,091 shares of common stock that are issuable to the Selling Securityholders upon the exercise of warrants
to purchase shares of our common stock at a per share exercise price of $1.5675 that we issued to the Selling Securityholders in the
private placement through two closings in connection with the October 2023 Securities Purchase Agreement (the “Investor Warrants”).
Investor Warrants for 2,000,000 shares of common stock were subsequently amended to provide that no exercise is permitted from August
28, 2024 until the date of stockholder approval of the issuance of securities pursuant to agreements executed on August 28, 2024 and
the exercise price for those Investor Warrants is reduced to $0.47 per share of common stock. See “Description of Capital
Stock — Warrants — Investor Warrants” beginning on page 11 of this prospectus and “Description of Capital
Stock — Convertible Promissory Notes — 2023 Secured Convertible Promissory Note” beginning on page 13 of this prospectus.
Our
registration of the securities covered by this prospectus does not mean that either we or the Selling Securityholders will issue, offer
or sell, as applicable, any of the securities being registered. The Selling Securityholders may offer, sell, or distribute all or a portion
of the securities being registered publicly or through private transactions at prevailing market prices or at negotiated prices. We will
not receive any of the proceeds from the sales of our common stock by the Selling Securityholders pursuant to this prospectus. We will,
however, receive the net proceeds of any Investor Warrants if exercised for cash. We will bear all costs, expenses and fees in connection
with the registration of these securities, including with regard to compliance with state securities or “blue sky” laws.
The Selling Securityholders will bear all commissions and discounts, if any, attributable to their sale of shares of our common stock.
See “Plan of Distribution” beginning on page 16 of this prospectus.
Our
common stock is listed on The Nasdaq Capital Market under the symbol “ENSC” and certain warrants we previously issued (the
“Public Warrants”) are quoted on the OTC Pink Open Market under the symbol “ENSCW.” On October 7, 2024,
the last reported sale price of our common stock on The Nasdaq Capital Market was $0.2199 per share and the closing bid price
for our Public Warrants as quoted on the OTC Pink Open Market was $0.028.
You
should read this prospectus and any prospectus supplement or amendment carefully before you invest in our securities.
Our
business and investing in our securities involve a high degree of risk. See “Risk Factors” beginning on page 6 of
this prospectus.
Neither
the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined
if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
Prospectus
dated [____ ], 2024.
TABLE
OF CONTENTS
GLOSSARY
| Definitions: |
|
|
| 2013
Framework |
|
Financial
reporting criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control-Integrated
Framework (2013) |
| 2021
Notes |
|
The
senior secured convertible promissory notes in the aggregate original principal amount of $15.9 million, sold in two closings on
September 24, 2021 and November 5, 2021, respectively, pursuant to the Securities Purchase Agreement entered into on September 24,
2021 |
| 2021
Omnibus Incentive Plan |
|
Ensysce
Biosciences, Inc. Amended and Restated 2021 Omnibus Incentive Plan, as amended |
| 2021
Securities Purchase Agreement |
|
Securities
Purchase Agreement, dated as of September 24, 2021, between the Company and certain institutional investors |
| 2022
Notes |
|
The
senior secured convertible promissory notes in the aggregate original principal amount of $8.48 million, sold in two closings on
June 30, 2022 and August 8, 2022, respectively, pursuant to the Securities Purchase Agreement entered into on June 30, 2022 |
| 2022
Securities Purchase Agreement |
|
Securities
Purchase Agreement, dated as of June 30, 2022, between the Company and certain institutional investors |
| 2023
Notes |
|
The
senior secured convertible promissory notes in the aggregate principal amount of $1.836 million, sold in two closings on October
25, 2023 and November 28, 2023, respectively, pursuant to the Securities Purchase Agreement entered into on October 23, 2023 |
| 2023
Securities Purchase Agreement |
|
Securities
Purchase Agreement, dated as of October 23, 2023, between the Company and certain investors |
| 2024
Warrants |
|
The
Series A Warrants and Series B Warrants, issued February 14, 2024, exercisable for an aggregate of 7,203,504 shares of common stock
and Placement Agent Warrants exercisable for an aggregate of 252,123 shares of common stock |
August 2024
Inducement Letter
August 2024 SPA ADFs |
|
Inducement Offer letter to exercise and amend
certain warrants dated August 28, 2024
Securities Purchase Agreement dated August 28, 2024
Abuse
deterrent formulations |
| ADHD |
|
Attention
deficit hyperactivity disorder |
| ANDA |
|
Abbreviated
New Drug Application |
| API |
|
Active
pharmaceutical ingredient |
| AUC |
|
Area
under the concentration time curve |
| Board |
|
Board
of Directors of Ensysce, or a committee thereof, as applicable |
| BTD |
|
Breakthrough
Therapy Designation granted by the FDA |
| Business
Combination |
|
The
definitive merger agreement among LACQ, Merger Sub and Former Ensysce, dated January 31, 2021, providing for, among other things,
and subject to terms and conditions therein, the business combination between LACQ and Former Ensysce pursuant to the merger of Merger
Sub with and into Former Ensysce, with Former Ensysce continuing as the surviving entity and as a wholly-owned subsidiary of LACQ
|
| CARA |
|
Comprehensive
Addiction and Recovery Act |
| CDC |
|
Center
for Disease Control |
| CDER |
|
Center
for Drug Evaluation and Research |
| cGMP |
|
Current
Good Manufacturing Practice |
| Cmax |
|
Maximum
plasma concentration |
| CMC |
|
Chemistry,
manufacturing, and controls |
| CMOs |
|
Contract
manufacturing organizations |
| CNS |
|
Central
nervous system |
| Company |
|
Ensysce
Biosciences, Inc. and its consolidated subsidiaries |
| COVID-19 |
|
Novel
coronavirus disease |
| Covistat |
|
A
subsidiary renamed EBIR, Inc. |
| CROs |
|
Contract
research organizations |
| CSA |
|
Controlled
Substances Act |
| CSOS |
|
Controlled
Substance Ordering System |
| DEA |
|
United
States Drug Enforcement Agency |
| DSCSA |
|
Title
II of the Federal Drug Quality and Security Act of 2013, known as the Drug Supply Chain Security Act |
| EB |
|
Ensysce
Biosciences, Inc. prior to its merger with Signature Acquisition Corp. pursuant to the EB-ST Agreement. |
| EBIR |
|
Previously
known as Covistat, Inc., EBIR, Inc. is a clinical stage pharmaceutical company that is developing a compound utilized in the Company’s
overdose protection program for the treatment of COVID-19 and 79.2%-owned subsidiary of the Company |
| EB-ST
Agreement |
|
Agreement
and Plan of Merger, dated as of December 28, 2015, by and among Signature, SAQ, and EB |
| EMA |
|
European
Medicines Agency |
| Ensysce |
|
Ensysce
Biosciences Inc. |
| EPO |
|
European
Patent Office |
| ETASU |
|
Elements
to assure a products safe use |
| Exchange
Act |
|
Securities
Exchange Act of 1934, as amended |
| FDA |
|
United
States Food and Drug Administration |
| FDC
Act |
|
Federal
Food, Drug and Cosmetic Act, as amended |
| Former
Ensysce |
|
Ensysce
Biosciences, Inc., a Delaware corporation, prior to the consummation of the merger with and into Merger Sub |
| GAAP
|
|
Generally
Accepted Accounting Principles in the United States of America |
| GCP |
|
Good
Clinical Practices |
| GMP |
|
Good
Manufacturing Practices |
| Hatch-Waxman
Act or Hatch-Waxman Amendments |
|
Drug
Price Competition and Patent Term Restoration Act of 1984 |
| HHS |
|
United
States Department of Health and Human Services |
| IMPDs |
|
Investigational
Medicinal Product Dossiers |
| IND |
|
Investigational
New Drug |
| Investor
Notes |
|
The
notes, with an aggregate principal amount of $1.836 million, issued under the October 2023 Securities Purchase Agreement |
| Investor
Warrants |
|
The
warrants issued under the October 2023 Securities Purchase Agreement |
| IRB |
|
Institutional
Review Board |
| JOBS
Act |
|
Jumpstart
Our Business Startups Act of 2012 |
| LACQ |
|
Leisure
Acquisition Corp., a Delaware Corporation |
| LACQ
Warrants |
|
Warrants
that relate to the Business Combination or were issued prior to it and are exercisable for 21,993 shares of our common stock at a
weighted average exercise price of $2,725.90 per share |
| May
2023 Securities Purchase Agreement |
|
Securities
Purchase Agreement, dated as of May 10, 2023, between the Company and certain institutional investors |
| Merger
|
|
The
merger of Merger Sub with and into Former Ensysce, with Former Ensysce continuing as the surviving entity and a wholly owned subsidiary
of LACQ, which changed its name to Ensysce Biosciences, Inc. following consummation of the Merger. |
| Merger
Agreement |
|
Agreement
and Plan of Merger, dated as of January 31, 2021, by and among LACQ, Merger Sub and Former Ensysce, providing for, among other things,
and subject to the terms and conditions therein, a business combination between Former Ensysce and LACQ pursuant to the proposed
merger of Merger Sub with and into Former Ensysce, with Former Ensysce surviving the transaction as a wholly-owned subsidiary of
LACQ, which changed its name to Ensysce Biosciences, Inc. following consummation of the Merger |
| Merger
Sub |
|
EB
Merger Sub, Inc., a Delaware corporation, a wholly-owned subsidiary of LACQ prior to the consummation of the Merger |
| MPAR® |
|
Multi-Pill
Abuse Resistance |
| MPAR®
Grant |
|
Research
and development grant related to the development of its MPAR® overdose prevention technology awarded to the Company by NIH through
NIDA in September 2018 |
| Nasdaq |
|
The
Nasdaq Stock Market LLC |
| NCE |
|
New
Chemical Entity |
| NDA |
|
New
Drug Application |
| NIDA |
|
National
Institute of Drug Abuse |
| NIH |
|
National
Institutes of Health |
| NME |
|
New
molecular entity |
| October
2023 Securities Purchase Agreement |
|
Securities
Purchase Agreement, dated as of October 23, 2023, between the Company and certain investors |
| Orange
Book |
|
FDA’s
publication Approved Drug Products with Therapeutic Equivalence Evaluations |
| OUD
Grant |
|
Research
and development grant related to the development of its TAAP/MPAR®TM abuse deterrent technology for Opioid Use Disorder
awarded to the Company by NIH/NIDA in September 2019 |
| PCT |
|
Patent
Cooperation Treaty |
| PDMA |
|
U.S.
Prescription Drug Marketing Act |
| PK |
|
Pharmacokinetics |
| Prior
Warrants |
|
Warrants
issued pursuant to the 2021 Securities Purchase Agreement and 2022 Securities Purchase Agreement, with the warrants issued in (i)
2021 exercisable for an aggregate of 4,518 shares of our common stock at an exercise price reset to $3.64 per share and (ii) 2022
exercisable for an aggregate of 38,900 shares of our common stock at an exercise price reset to $3.64 per share |
| PTA |
|
Patent
Term Adjustment |
| PTE |
|
Patent
Term Extension |
| Public
Warrants |
|
The
redeemable warrants issued by us and sold as part of the units in the LACQ IPO (whether they were purchased in the LACQ IPO or thereafter
in the open market). The Public Warrants are exercisable for an aggregate of approximately 41,666 shares of our common stock at an
exercise price of $2,760.00 per share |
| R&D |
|
Research
and Development |
| REMS |
|
Risk
evaluation and mitigation strategy |
| Reverse
Splits |
|
Reverse
splits of our common stock, one-for-twenty effected on October 28, 2022, and one-for-twelve effected on March 31, 2023 |
| SARS-CoV-2
|
|
Severe
acute respiratory syndrome coronavirus 2 |
| SAQ |
|
Signature
Acquisition Corp., a wholly-owned subsidiary of Signature |
| SEC |
|
U.S.
Securities and Exchange Commission |
| Securities
Act |
|
Securities
Act of 1933, as amended |
| Signature
|
|
Signature
Therapeutics Inc. |
| Societal |
|
Societal
CDMO |
| Societal
Agreement |
|
Manufacturing
Agreement, dated September 19, 2019, by and between Societal (formerly Recro Gaineville LLC) and the Company |
| SUPPORT
Act |
|
Substance
Use-Disorder Prevention that Promotes Opioid Recovery and Treatment for Patients and Communities Act |
| TAAP |
|
Trypsin
Activated Abuse Protection |
| TEAEs |
|
Treatment-emergent
adverse events |
| USPTO |
|
United
States Patent and Trademark Office |
ABOUT
THIS PROSPECTUS
You
should rely only on the information contained in this prospectus. We have not authorized anyone to provide you with information other
than the information that we have provided or incorporated by reference in this prospectus and your reliance on any unauthorized information
or representation is at your own risk. This prospectus may be used only in jurisdictions where offers and sales of these securities are
permitted. You should assume that the information contained in this prospectus is accurate only as of the date of this prospectus, and
that any information we have incorporated by reference is accurate only as of the date of the document incorporated by reference, regardless
of the time of delivery of this prospectus or of any sale of our securities. Our business, financial condition, results of operations
and prospects may have changed since those dates. This prospectus contains market data and industry statistics and forecasts that are
based on independent industry publications and other publicly available information. Although we believe these sources are reliable,
we do not guarantee the accuracy or completeness of this information and we have not independently verified this information. In addition,
the market and industry data and forecasts that may be included in this prospectus may involve estimates, assumptions and other risks
and uncertainties and are subject to change based on various factors, including those discussed under the heading “Risk Factors”
contained in this prospectus. Accordingly, investors should not place undue reliance on this information.
The
representations, warranties and covenants made by us in any agreement that is filed as an exhibit to any document that is incorporated
by reference herein were made solely for the benefit of the parties to such agreement, including, in some cases, for the purpose of allocating
risk among the parties to such agreements, and should not be deemed to be a representation, warranty or covenant to you. Moreover, such
representations, warranties or covenants were accurate only as of the date when made. Accordingly, such representations, warranties and
covenants should not be relied on as accurately representing the current state of our affairs.
To
the extent there is a conflict between the information contained in this prospectus, on the one hand, and the information contained in
any document incorporated by reference filed with the U.S. Securities and Exchange Commission (the “SEC”) before the date
of this prospectus, on the other hand, you should rely on the information in this prospectus. If any statement in a document incorporated
by reference is inconsistent with a statement in another document incorporated by reference having a later date, the statement in the
document having the later date modifies or supersedes the earlier statement.
Neither
we nor the Selling Securityholders has authorized anyone to provide you with any information or to make any representations other than
those contained in this prospectus, any post-effective amendment, or any applicable prospectus supplement prepared by or on behalf of
us or to which we have referred you. We and the Selling Securityholders take no responsibility for and can provide no assurance as to
the reliability of, any other information that others may give you. Neither we, nor the Selling Securityholders, will make an offer to
sell these securities in any jurisdiction where the offer or sale is not permitted. You should assume that the information appearing
in this prospectus, any post-effective amendment and any applicable prospectus supplement to this prospectus is accurate only as of the
date on its respective cover.
This
prospectus contains summaries of certain provisions contained in some of the documents described herein, but reference is made to the
actual documents for complete information. All the summaries are qualified in their entirety by the actual documents. Copies of certain
documents have been filed as exhibits to the registration statement of which this prospectus is a part, and you may obtain copies of
those documents as described below under “Where You Can Find More Information.”
We
own or have rights to trademarks, trade names and service marks that we use in connection with the operation of our business. In addition,
our name, logos and website name and address are our trademarks or service marks. Solely for convenience, in some cases, the trademarks,
trade names and service marks referred to in this prospectus are listed without the applicable ®, ™ and SM symbols, but we
will assert, to the fullest extent under applicable law, our rights to these trademarks, trade names and service marks. Other trademarks,
trade names and service marks appearing in this prospectus are the property of their respective owners.
On
June 30, 2021, we consummated the transactions contemplated by the Merger Agreement, with the Company surviving in the Merger. In connection
with the consummation of the Business Combination, LACQ changed its name to “Ensysce Biosciences, Inc.”
On
October 28, 2022, we effected a one-for-twenty reverse split of our common stock (the “2022 Reverse Split”). On March
31, 2023, we effected a one-for-twelve reverse split of our common stock (the “2023 Reverse Split” and together with
the 2022 Reverse Split the “Reverse Splits”). All share and per share information has been restated retroactively,
giving effect to the Reverse Splits for all periods presented.
Unless
the context indicates otherwise, references in this prospectus to the “Company,” “Ensysce,” “we,”
“us,” “our,” and similar terms refer to Ensysce Biosciences, Inc. (f/k/a Leisure Acquisition Corp.)
and its consolidated subsidiaries.
CAUTIONARY
NOTE REGARDING FORWARD-LOOKING STATEMENTS
This
prospectus includes statements that express our opinions, expectations, beliefs, plans, objectives, assumptions, or projections regarding
future events or future results and therefore are, or may be deemed to be, “forward-looking statements.” These forward-looking
statements can generally be identified by the use of forward-looking terminology, including the terms “believes,”
“estimates,” “anticipates,” “expects,” “seeks,” “projects,”
“intends,” “plans,” “may,” “will,” or “should”
or, in each case, their negative or other variations or comparable terminology. These forward-looking statements include all matters
that are not historical facts. They appear in several places throughout this registration statement/prospectus and include statements
regarding our intentions, beliefs or current expectations concerning, among other things, results of operations, financial condition,
liquidity, prospects, growth, strategies and the markets in which we operate. Such forward-looking statements are based on available
current market material and management’s expectations, beliefs and forecasts concerning future events impacting our company. Factors
that may impact such forward-looking statements include:
| |
● |
the
risk that our common stock will be delisted from Nasdaq; |
| |
|
|
| |
● |
our
ability to continue as a going concern for the next twelve months; |
| |
|
|
| |
● |
our
estimates regarding expenses, revenue, capital requirements and timing and availability of and the need for additional financing
will almost certainly not match actual amounts and timing; |
| |
|
|
| |
● |
the
risk that our lead product candidate PF614 and PF614-MPAR may not be successful in limiting or impeding abuse, overdose, or misuse
or providing additional safety upon commercialization; |
| |
|
|
| |
● |
reliance
by us on third-party contract research organizations, or CROs, for our research and development activities and clinical trials; |
| |
|
|
| |
● |
the
need for substantial additional funding to complete the development and commercialization of our product candidates; |
| |
|
|
| |
● |
the
risk that our clinical trials may fail to replicate positive results from earlier preclinical studies or clinical trials conducted
by us or third parties; |
| |
|
|
| |
● |
the
risk that the potential product candidates that we develop may not progress through clinical development or receive required regulatory
approvals within expected timelines or at all; |
| |
|
|
| |
● |
the
risk that clinical trials may not confirm any safety, potency, or other product characteristics described or assumed in this prospectus; |
| |
|
|
| |
● |
the
risk that we will be unable to successfully market or gain market acceptance of our product candidates; |
| |
|
|
| |
● |
the
risk that our product candidates may not be beneficial to patients or successfully commercialized; |
| |
|
|
| |
● |
the
risk that we have overestimated the size of the target market, patients’ willingness to try new therapies, and the willingness
of physicians to prescribe these therapies; |
| |
|
|
| |
● |
effects
of competition;
|
| |
● |
the
risk that third parties on which we depend for laboratory, clinical development, manufacturing, and other critical services will
fail to perform satisfactorily; |
| |
|
|
| |
● |
the
risk that we will be unable to obtain and maintain sufficient intellectual property protection for our investigational products or
will infringe the intellectual property protection of others; |
| |
|
|
| |
● |
the
loss of key members of our management team; |
| |
|
|
| |
● |
changes
in our regulatory environment; |
| |
|
|
| |
● |
the
ability to attract and retain key scientific, medical, commercial, or management personnel; |
| |
|
|
| |
● |
changes
in our industry; |
| |
|
|
| |
● |
our
ability to remediate any material weaknesses or establish and maintain effective internal controls over financial reporting; |
| |
|
|
| |
● |
the
risk that we may not be able to regain and maintain compliance with applicable listing standards of Nasdaq; |
| |
|
|
| |
● |
other
factors disclosed in this prospectus; and |
| |
|
|
| |
● |
other
factors beyond our control. |
The
forward-looking statements contained in this prospectus are based on our current expectations and beliefs concerning future developments
and their potential effects on our company. There can be no assurance that future developments affecting us will be those that we have
anticipated. These forward-looking statements involve risks, uncertainties or other assumptions that may cause actual results or performance
to be materially different from those expressed or implied by these forward-looking statements. These risks and uncertainties include,
but are not limited to, those factors described under the heading “Risk Factors” in this prospectus. Should one or
more of these risks or uncertainties materialize, or should any of the assumptions prove incorrect, actual results may vary in material
respects from those projected in these forward-looking statements. We will not undertake any obligation to update or revise any forward-looking
statements, whether because of new information, future events or otherwise, except as may be required under applicable securities laws.
RISK
FACTORS
An
investment in our securities involves a high degree of risk. You should carefully consider the risks and uncertainties described under
“Risk Factors” in our Annual Report on Form 10-K for the fiscal year ended December 31, 2023, and any subsequent Quarterly
Report on Form 10-Q and our other filings with the SEC, all of which are incorporated by reference herein, together with the Risk Factors
set forth below, before making an investment decision. The occurrence of one or more of the events or circumstances described in these
risk factors, alone or in combination with other events or circumstances, may have an adverse effect on our business, cash flows, financial
condition and results of operations. You should also carefully consider the following risk factors in addition to the other information
included in this registration statement/prospectus, including matters addressed in the section entitled “Cautionary Note Regarding
Forward-Looking Statements.” We may face additional risks and uncertainties that are not presently known to us or that we currently
deem immaterial, which may also impair our business or financial condition.
Risks
Related to this Offering and Our Securities
We
need to raise additional capital after this offering to support our operations.
We
will not receive any of the proceeds from the sales of our common stock by the Selling Securityholders pursuant to this prospectus. We
will, however, receive the net proceeds of any Investor Warrants if exercised for cash.
We
have incurred substantial losses since our inception. Net losses and negative cash flows have had, and will continue to have, an adverse
effect on our stockholders’ equity and working capital. We expect to continue to incur significant losses for the foreseeable future
as we continue our research and development of, and seek regulatory approvals for, our product candidates.
Our
current cash on hand is sufficient to fund operations into the third quarter of 2024. The report of our independent registered public
accounting firm on our financial statements for the years ended December 31, 2023, and 2022 contains explanatory language that substantial
doubt exists about our ability to continue as a going concern. We have reduced expenses because we do not have access to sufficient cash
and liquidity to finance our business operations as currently contemplated and may be compelled to reduce further general and administrative
expenses and delay research and development projects until we are able to obtain sufficient financing. We may find it difficult to raise
money on terms favorable to us or at all. The failure to obtain sufficient capital to support our operations would have a material adverse
effect on our business, financial condition and results of operations. If sufficient financing is not received timely, we would then
need to pursue a plan to license or sell assets, seek to be acquired by another entity, cease operations and/or seek bankruptcy protection.
If
we are unable to regain and maintain compliance with the listing standards of Nasdaq, our common stock may become delisted, which could
have a material adverse effect on our ability to raise funding, which could negatively impact our business, capital and financial condition.
On
August 20, 2024, we received notice from the Listing Qualifications Department of The Nasdaq Stock Market LLC (“Nasdaq”)
stating that due to the Company’s non-compliance with the $2.5 million stockholders’ equity requirement set forth in Nasdaq
Listing Rule 5550(b)(1) as of June 30, 2024, the Company is subject to delisting unless it timely requests a hearing before the Nasdaq
Hearings Panel (the “Panel”). The Company timely requested a hearing before the Panel, which request stays
any further action by Nasdaq pending the conclusion of the hearing process.
On
March 27, 2024, the Company received a notice (the “Deficiency Letter”) from Nasdaq stating that the Company was not in compliance
with Nasdaq Listing Rule 5550(a)(2) because the bid price for the Company’s common stock had closed below $1.00 per share for the
previous 30 consecutive business days. Nasdaq requires that Ensysce common stock have a minimum bid price of at least $1 per share (the
“Minimum Bid Price”). In accordance with Nasdaq listing rule 5810(c)(3)(A), the Company has 180 calendar days, or until September
23, 2024, to regain compliance. The Deficiency Letter states that to regain compliance, the bid price for the Company’s common
stock must close at the Minimum Bid Price for a minimum of ten consecutive business days during the compliance period ending September
23, 2024. The Deficiency Letter does not explicitly address that the Nasdaq staff may require a longer period for compliance with the
Minimum Bid Price in some circumstances, but generally not more than 20 consecutive business days.
The
Company did not regain compliance with
the Minimum Bid Price by September 23, 2024, and Nasdaq staff provided written notice to the Company that this non-compliance
would also be addressed at the upcoming hearing before the Panel.
There
can be no assurance that the Company will be granted more time to attempt to comply with the Equity Rule or be able to regain
or maintain compliance with the Equity Rule, the Minimum Bid Price requirement or other Nasdaq listing standards.
If
you purchase our securities in this offering you may experience future dilution as a result of future equity offerings or other equity
issuances.
To
raise additional capital, we will need to offer and issue additional shares of our common stock or other securities convertible into
or exchangeable for our common stock in the future. We cannot assure you that we will be able to sell shares or other securities in any
other offering at a price per share that is equal to or greater than the price per share paid by investors in this offering, and investors
purchasing other securities in the future could have rights superior to existing stockholders. The price per share at which we sell additional
shares of our common stock or other securities convertible into or exchangeable for our common stock in future transactions may be higher
or lower than the price per share in this offering.
In
addition, we have a significant number of warrants and stock options outstanding. Further, we may choose to raise additional capital
due to market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating
plans.
The
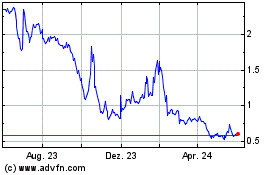
market price of our common stock and the trading volume of our common stock has been and may continue to be, highly volatile, and such
volatility could cause the market price of our common stock to decrease.
Between
January 1, 2024, and October 7, 2024, the market
price of our common stock fluctuated from a low of $0.22 per share to a high of $2.06 per share, and our stock price continues
to fluctuate. The market price and trading volume of our common stock may continue to fluctuate significantly in response to numerous
factors, some of which are beyond our control, such as:
| |
● |
our
ability to grow our revenue and customer base; |
| |
|
|
| |
● |
The
announcement or the market introduction of new products or product enhancements by us or our competitors; |
| |
|
|
| |
● |
the
trading volume of our common stock; |
| |
|
|
| |
● |
developments
concerning regulatory oversight and approvals; |
| |
|
|
| |
● |
variations
in our and our competitors’ results of operations; |
| |
|
|
| |
● |
changes
in earnings estimates or recommendations by securities analysts, if our common stock
is
covered by analysts; |
| |
|
|
| |
● |
successes
or challenges in our collaborative arrangements or alternative funding sources; |
| |
|
|
| |
● |
developments
in the health care and life science industries; |
| |
|
|
| |
● |
the
results of product liability or intellectual property lawsuits; |
| |
|
|
| |
● |
adverse
effects on our business condition and results of operations from general economic and market conditions and overall fluctuations
in the United States and international markets, including deteriorating market conditions due to investor concerns regarding inflation
and Russia’s war on Ukraine; |
| |
|
|
| |
● |
future
issuances of common stock or other securities; |
| |
|
|
| |
● |
the
addition or departure of key personnel; |
| |
|
|
| |
● |
announcements
by us or our competitors of acquisitions, investments or strategic alliances; and |
| |
|
|
| |
● |
general
market conditions and other factors, including factors unrelated to our operating performance. |
Further,
the stock market in general and micro-cap clinical trial pharmaceutical issuers in particular, have recently experienced extreme price
and volume fluctuations. Continued market fluctuations could result in extreme volatility in the price of our common stock, which could
cause a decline in the value of our common stock and the loss of some or all your investment.
We
have never paid dividends on our capital stock, and we do not anticipate paying dividends in the foreseeable future.
We
have never paid dividends on any of our capital stock and currently intend to retain any future earnings to fund the growth of our business.
We may also enter into credit agreements or other borrowing arrangements in the future that will restrict our ability to declare or pay
cash dividends on our common stock.
USE
OF PROCEEDS
The
Selling Securityholders will receive all the proceeds from this offering. We will, however, receive the net proceeds of any Investor
Warrants if exercised for cash. Proceeds, if any, received from the exercise of such Investor Warrants will be used for working capital
for general corporate purposes. No assurances can be given that any of such Investor Warrants will be exercised. The Selling Securityholders
will pay any underwriting discounts and commissions and expenses incurred by the Selling Securityholders for brokerage, accounting, tax
or legal services or any other expenses incurred by the Selling Securityholders in disposing of the shares. We will bear all other costs,
fees and expenses incurred in effecting the registration of the shares covered by this prospectus, including all registration and filing
fees, and fees and expenses for our counsel and our independent registered public accountants.
DESCRIPTION
OF CAPITAL STOCK
The
following summary of the material terms of our common stock and description of certain of our other securities is not intended to be
a complete summary of the rights and preferences of our common stock or such other securities. We urge you to read the third amended
and restated certificate of incorporation in its entirety for a complete description of the rights and preferences of our common stock.
See “Where You Can Find More Information.”
Pursuant
to the third amended and restated certificate of incorporation, our authorized capital stock consists of 250,000,000 shares of common
stock, $0.0001 par value, and 1,500,000 shares of undesignated preferred stock, $0.0001 par value.
Common
Stock
As
of October 7, 2024, 13,870,510 shares of our common stock were issued and outstanding.
Common
stockholders of record are entitled to one vote for each share held on all matters to be voted on by stockholders. Unless specified in
our amended and restated certificate of incorporation or bylaws, or as required by applicable provisions of the DGCL or applicable stock
exchange rules, the affirmative vote of a majority of our shares of common stock that are voted is required to approve any such matter
voted on by our stockholders. Our Board is divided into three classes, each of which will generally serve for a term of three years with
only one class of directors being elected in each year. There is no cumulative voting with respect to the election of directors or any
other matter, with the result that the holders of more than 50% of the shares voted for the election of directors can elect all the directors.
Our stockholders are entitled to receive ratable dividends when, as and if declared by the Board out of funds legally available therefor.
In
the event of a liquidation, dissolution or winding up, our stockholders are entitled to share ratably in all assets remaining available
for distribution to them after payment of liabilities and after provision is made for each class of stock, if any, having preference
over the common stock. Our stockholders have no preemptive or other subscription rights. There are no sinking fund provisions applicable
to the common stock.
Certain
Anti-Takeover Provisions of our Third Amended and Restated Certificate of Incorporation and our Bylaws
Our
third amended and restated certificate of incorporation and bylaws contain provisions that may delay, defer or discourage another party
from acquiring control of us. These provisions, which are summarized below, discourage coercive takeover practices or inadequate takeover
bids. These provisions are also designed to encourage persons seeking to acquire control of us to first negotiate with our Board, which
may result in an improvement of the terms of any such acquisition in favor of our stockholders. However, they also give the Board the
power to discourage acquisitions that some stockholders may favor.
Classified
Board
Our
third amended and restated certificate of incorporation provides that our Board is classified into three classes of directors. As a result,
in most circumstances, a person can gain control of our Board only by successfully engaging in a proxy contest at two or more annual
meetings.
Authorized
but Unissued Shares
Our
authorized but unissued shares of common and preferred stock are available for future issuances without stockholder approval and could
be utilized for a variety of corporate purposes, including future offerings to raise additional capital, acquisitions and employee benefit
plans. However, the listing requirements of the Nasdaq, which apply if and so long as our common stock remains listed on the Nasdaq,
require stockholder approval of certain issuances equal to or exceeding 20% of the then outstanding voting power or then outstanding
number of shares of common stock. Additional shares that may be used in the future may be issued for a variety of corporate purposes,
including future public offerings, to raise additional capital, or to facilitate acquisitions. The existence of authorized but unissued
and unreserved common stock and preferred stock could render it more difficult or discourage an attempt to obtain control of us by means
of a proxy contest, tender offer, merger or otherwise.
Special
Meetings of Stockholders
Our
bylaws provide that special meetings of our stockholders may be called only by a majority vote of our Board.
Advance
Notice Requirements for Stockholder Proposals and Director Nominations
Our
bylaws provide that stockholders seeking to bring business before our annual meeting of stockholders, or to nominate candidates for election
as directors at our annual meeting of stockholders, must provide timely notice of their intent in writing. To be timely, a stockholder’s
notice will need to be received by the company secretary at our principal executive offices not later than the close of business on the
90th day nor earlier than the close of business on the 120th day prior to the anniversary date of the immediately
preceding annual meeting of stockholders. Pursuant to Rule 14a-8 of the Exchange Act, proposals seeking inclusion in our annual proxy
statement must comply with the notice periods contained therein. Our bylaws also specify certain requirements as to the form and content
of a stockholders meeting. These provisions may preclude our stockholders from bringing matters before our annual meeting of stockholders
or from making nominations for directors at our annual meeting of stockholders.
Amendment
of Charter or Bylaws
The
amendment, alteration or repeal of the provisions of the third amended and restated certificate of incorporation governing limitation
of director liability, indemnification and advancement of expenses or the adoption of any provision or bylaw inconsistent with those
provisions may only be effected by the affirmative vote of the stockholders holding at least sixty five percent (65%) of the voting power
of our outstanding shares entitled to vote generally in the election of directors, voting together as a single class, at a meeting of
the stockholders called for that purpose. The affirmative vote of the stockholders holding at least 65% of the voting power of all outstanding
shares of our capital stock is required for any amendment of the indemnification provisions in the bylaws or adoption of a provision
inconsistent with them.
Exclusive
Forum
Under
our charter, unless we consent in writing to the selection of an alternative forum, subject to certain limitations, the sole and exclusive
forum will be the Court of Chancery of the State of Delaware (or, if such court does not have jurisdiction, the Superior Court of the
State of Delaware, or, if the Superior Court of the State of Delaware also does not have jurisdiction, the United States District Court
for the District of Delaware) for:
| |
● |
any
derivative action or proceeding brought on our behalf; |
| |
|
|
| |
● |
any
action asserting a claim of breach of a fiduciary duty owed by any of our directors, officers or other employees to us or our stockholders; |
| |
|
|
| |
● |
any
action asserting a claim against us arising pursuant to any provision of the DGCL, our charter or our bylaws (as either may be amended,
restated, modified, supplemented or waived from time to time); |
| |
|
|
| |
● |
any
action to interpret, apply, enforce or determine the validity of our charter or our bylaws; and |
| |
|
|
| |
● |
any
action asserting a claim against us governed by the internal affairs doctrine. |
For
the avoidance of doubt, the foregoing provisions of our charter will not apply to any action or proceeding asserting a claim under the
Securities Act or the Exchange Act. These provisions of our charter could limit the ability of our stockholders to obtain a favorable
judicial forum for certain disputes with us or with our current or former directors, officers or other employees, which may discourage
such lawsuits against us and our current or former directors, officers and employees. Alternatively, if a court were to find these provisions
of our charter inapplicable to, or unenforceable in respect of, one or more of the types of actions or proceedings listed above, we may
incur additional costs associated with resolving such matters in other jurisdictions, which could adversely affect our business, financial
condition and results of operations.
Delaware
Anti-Takeover Statute
We
are subject to the provisions of Section 203 of the Delaware General Corporation Law (sometimes referred to as Section 203) regulating
corporate takeovers. In general, Section 203 prohibits a publicly held Delaware corporation from engaging, under specified circumstances,
in a business combination with an interested stockholder for a period of three years following the date the person became an interested
stockholder unless:
| |
● |
prior
to the date of the transaction, the board of directors of the corporation approved either the business combination or the transaction
which resulted in the stockholder becoming an interested stockholder; |
| |
|
|
| |
● |
upon
consummation of the transaction that resulted in the stockholder becoming an interested stockholder, the stockholder owned at least
85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining
the number of shares of voting stock outstanding (but not the outstanding voting stock owned by the stockholder)(1) shares owned
by persons who are directors and also officers and (2) shares owned by employee stock plans in which employee participants do not
have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer;
or |
| |
|
|
| |
● |
on
or subsequent to the date of the transaction, the business combination is approved by the board of directors and authorized at an
annual or special meeting of stockholders, and not by written consent, by the affirmative vote of at least 66-2/3% of the outstanding
voting stock that is not owned by the interested stockholder. |
Generally,
a business combination includes a merger, asset or stock sale, or other transaction resulting in a financial benefit to the interested
stockholder. An interested stockholder is a person who, together with affiliates and associates, owns or, within three years prior to
the determination of interested stockholder status, did own 15% or more of a corporation’s outstanding voting securities. We expect
the existence of this provision to have an anti-takeover effect with respect to transactions our board of directors do not approve in
advance. We also anticipate that Section 203 may also discourage attempts that might result in a premium over the market price for the
shares of our common stock held by stockholders.
The
provisions of Delaware law, our certificate of incorporation and our bylaws could have the effect of discouraging others from attempting
hostile takeovers and, consequently, they may also inhibit temporary fluctuations in the market price of our common stock that often
result from actual or rumored hostile takeover attempts. These provisions may also have the effect of preventing changes in our management.
It is possible that these provisions could make it more difficult to accomplish transactions that stockholders may otherwise deem to
be in their best interests.
Warrants
Investor
Warrants
On
October 25, 2023, we completed the first of two closings under the October 2023 Securities Purchase Agreement, whereby we issued to the
selling securityholders signatory thereto warrants (the “Investor Warrants”) to purchase 1,255,697 shares of common
stock in the aggregate. On November 28, 2023, we completed the second closing and issued Investor Warrants to purchase 2,511,394 shares
of common stock. The Investor Warrants are exercisable for five years from the date of issuance at a per share common stock exercise
price of $1.5675. Investor Warrants to purchase 2,443,187 shares of common stock were outstanding as of October 3, 2024. On August
28, 2024, we executed an Inducement Offer to Exercise Common Stock Purchase Warrants (the “August 2024 Inducement Letter”)
that, among other things, reduced the exercise price of 2,000,000 of the Investor Warrants issued November 28, 2023, that are held by
one investor to $0.47 per share of common stock. These warrants were amended such that they are not exercisable from August 28, 2024,
until the date approval is received from our stockholders for the securities to be issued under the August 2024 Inducement Letter and
a related securities purchase agreement among the signatories to the August 2024 Inducement Letter. Additional information about the
August 2024 Inducement Letter and related securities purchase agreement is set forth below under “Description of Capital Stock
– Warrants – 2024 Warrants.” Stockholder approval for the issuance of the 2,000,000 Investor Warrants is required
to be sought until approval is obtained. The results of our stockholder meetings are disclosed in Current Reports on Form 8-K filed with
the SEC. Our obligations under the Investor Warrants are secured by all our and our subsidiaries’ assets and are guaranteed
jointly and severally by our subsidiaries.
Prior
Warrants
On
September 24, 2021, we entered into the 2021 Securities Purchase Agreement, whereby we issued to the purchasers signatory thereto warrants
to purchase 4,518 shares of the common stock in the aggregate (the “2021 Prior Warrants”) in closings on September
24, 2021 and November 5, 2021. The 2021 Prior Warrants have a reset per share exercise price of $3.637 and are exercisable for five years
following issuance. Our obligations under the 2021 Prior Warrants are secured by all our and our subsidiaries’ assets and are guaranteed
jointly and severally by our subsidiaries.
On
July 1, 2022, and August 8, 2022, we completed closings under the 2022 Securities Purchase Agreement, whereby we issued to the
purchasers signatory thereto warrants (the “2022 Prior Warrants”) to purchase 38,900 shares of the common stock in
the aggregate. The 2022 Prior Warrants have a reset per share exercise price of $3.637 and are exercisable for five years following issuance.
Our obligations under the Prior Warrants are secured by all of our and our subsidiaries’ assets and are guaranteed jointly and
severally by our subsidiaries.
Other
Warrants
On
December 9, 2022, we issued warrants, pursuant to a registered offering, to purchase 549,993 shares of our common stock in the aggregate
with a per share exercise price of $12.81. The warrants are exercisable for five years from the date of issuance.
On
February 2, 2023, we issued warrants to purchase 297,619 shares of our common stock with an exercise price of $8.58 per share. These
warrants expire five and one-half years after the original issue date. On February 2, 2023, we also issued warrants to purchase 20,832
shares of our common stock with exercisable price of $12.60 per share. These warrants expire five years after the original issue date.
On
May 12, 2023, we issued: (i) warrants of five-year duration to purchase 1,800,876 shares of our common stock in the aggregate with an
exercise price of $3.637 per share; (ii) warrants of eighteen-month duration to purchase 1,800,876 shares of our common stock with an
exercise price of $3.637 per share and (iii) warrants of five-year duration to purchase 126,061 shares of our common stock with an exercise
price of $4.8588 per share. On May 12, 2023, we also issued pre-funded warrants to purchase 222,072 shares of our common stock with an
exercise price of $0.0001 per share.
As
described below under “Description of Capital Stock–Warrants–2024 Warrants”, the warrants to purchase
3,601,752 shares of our common stock with an exercise price of $3.637 were subsequently repriced and exercised.
Over
time, we have issued, to a number of third parties, other warrants that may be exercised for common stock. Some of these warrants are
traded on the OTC Pink Open Market under the symbol “ENSCW”.
2024
Warrants
On
February 12, 2024, we entered into an inducement offer letter agreement (the “February Inducement Letter”)
with certain holders (the “Holders”) of certain of our existing warrants to purchase up to an aggregate of
3,601,752 shares of the common stock issued to the Holders on May 12, 2023, and having an original exercise price of $3.637 per
share (the “Existing Warrants”). Pursuant to the Inducement Letter, the Holders agreed to exercise for cash their
Existing Warrants to purchase an aggregate of 3,601,752 shares of common stock at a reduced exercise price of $1.31 per share in consideration
of our agreement to issue new unregistered Series A Warrants to purchase up to 3,601,752 shares of common stock and new unregistered
Series B Warrants to purchase up to 3,601,752 shares of common stock. The Series A Warrants have an exercise price of $1.06 per share,
are exercisable immediately upon issuance and have a term equal to eighteen months from the date of issuance. The Series B Warrants have
an exercise price of $1.06 per share, are exercisable immediately upon issuance and will expire on May 12, 2028. In connection with the
execution of the Inducement Letter, we issued to a placement agent unregistered warrants to purchase up to 252,123 shares of common stock
(the “Placement Agent Warrants”). The Placement Agent Warrants expire on May 12, 2028, and have an exercise price
of $1.6375 per share of common stock (equal to 125% of the reduced exercise price of $1.31 per Existing Warrant).
The
August 2024 Inducement Letter also included an agreement with certain holders (the “Induced Holders”) of our existing
warrants to purchase up to an aggregate of 7,203,504 shares of the common stock issued to the Induced Holders on February 14, 2024 and
having an original exercise price of $1.06 per share (the “February Warrants”). Pursuant to the August 2024 Inducement
Letter, the Induced Holders agreed to exercise for cash their February Warrants to purchase an aggregate of 7,203,504 shares of common
stock at a reduced exercise price of $0.47 per share in consideration of our agreement to issue new unregistered Series A-3 Warrants
to purchase up to 10,805,256 shares of common stock and new unregistered Series A-4 Warrants to purchase up to 10,805,256 shares of common
stock. The Series A-3 Warrants have an exercise price of $0.47 per share, are exercisable at any time on or after stockholder approval
is received and have a term of exercise equal to eighteen months from the date of stockholder approval. The Series B Warrants have an
exercise price of $0.47 per share, are exercisable at any time on or after stockholder approval is received and have a term of exercise
equal to five years from the date of stockholder approval. We also reduced the exercise price per share on Investor Warrants issued in
November 2023 for 2,000,000 shares of common stock from $1.06 to $0.47, as described above under “Description of Capital Stock
– Warrants – Investor Warrants.” On August 28, 2024, we also entered into Securities Purchase Agreement (the “August
2024 SPA”) pursuant to which we issued to the Induced Holders new unregistered Series A-3 Warrants, described above, to purchase
up to 3,553,194 shares of common stock and new unregistered Series A-4 Warrants, described above, to purchase up to 3,553,194 shares
of common stock. In connection with the execution of the August 2024 Inducement Letter the August 2024 SPA and a registered direct offering
that closed on August 29, 2024, we issued to a placement agent unregistered warrants to purchase up to 752,969 shares of common stock
(the “August Placement Agent Warrants”). The August Placement Agent Warrants expire on August 28, 2029, and have an
exercise price of $0.5875 per share of common stock (equal to 125% of the reduced exercise price of $0.47 per February Warrant).
Convertible
Promissory Notes
2021
Secured Convertible Promissory Notes
On
September 24, 2021, we entered into a securities purchase agreement (the “2021 Securities Purchase Agreement”) whereby
we issued to the purchasers signatory thereto the 2021 Notes in the aggregate principal amount of $15.9 million for an aggregate purchase
price of $15 million. Our obligations under the 2021 Securities Purchase Agreement are secured by all of our and our subsidiaries’
assets and are guaranteed jointly and severally by our subsidiaries. Principal and interest under the 2021 Notes have been satisfied
in full.
We
previously registered with the SEC the resale of the shares of common stock issuable upon conversion of the 2021 Notes, as well as the
shares of common stock issuable upon the exercise of the 2021 Prior Warrants (as defined above).
2022
Secured Convertible Promissory Notes
On
June 30, 2022, we entered into a securities purchase agreement (the “2022 Securities Purchase Agreement”) whereby
we issued to the purchasers signatory thereto the 2022 Notes in the aggregate principal amount of $8.48 million for an aggregate purchase
price of $8.0 million in two closings under the 2022 Securities Purchase Agreement, the first closing occurring on July 1, 2022 and the
second occurring on August 8, 2022. Our obligations under the 2022 Securities Purchase Agreement are secured by all of our and our subsidiaries’
assets and are guaranteed jointly and severally by our subsidiaries. Principal and interest under the 2022 Notes have been satisfied
in full.
We
previously registered with the SEC the resale of the shares of common stock issuable upon conversion of the 2022 Notes, as well as the
shares of common stock issuable upon the exercise of the 2022 Prior Warrants (as defined above).
2023
Secured Convertible Promissory Note
On
October 23, 2023, we entered into a securities purchase agreement (the “October 2023 Securities Purchase Agreement”)
whereby at the first of two closings, on October 25, 2023, we issued to certain investors, including our Chairman, Investor Notes in
the principal amount of $612,000 for a purchase price of $566,667. At the second closing, on November 28, 2023, we issued additional
Investor Notes in the principal amount of $1,224,000 for a purchase price of $1,133,333, which increased the aggregate principal amount
raised under the 2023 Securities Purchase Agreement to $1,836,000 for an aggregate purchase price of $1,700,000. The Investor Notes,
subject to an original issue discount of eight percent (8%), had a term of six months and accrued interest at the rate of 6.0% per annum.
The Investor Notes are convertible into the common stock, at a per share conversion price equal to $1.5675. Under the Investor Notes,
commencing 90 days after issuance (January 2024), we were obligated to redeem one third (1/3rd) of the original principal
amount under the applicable Investor Note, plus accrued but unpaid interest, liquidated damages and any other amounts then owing to the
holder of such Investor Note. All Investor Notes, other than those issued to our Chairman, were repaid in full, at a premium, in February
2024. On April 25, 2024, the Company and our Chairman entered into a forbearance agreement that will expire on April 25, 2025 relating
to $216,000 of the Notes held by him. Upon termination of the forbearance period, the Company will owe the remaining outstanding principal
balance together with unpaid interest. The Company may pay the Investor Notes held by the Chairman in full at any time prior to the conclusion
of the forbearance period.
The
Investor Notes may not be converted to the extent that, after giving effect to such conversion, the Purchaser, together with its affiliates
and any other person acting as a group as defined pursuant to Section 13(d) of the Securities Exchange Act of 1934, as amended (the affiliates
and such persons, the “Attribution Parties”), would beneficially own in excess of 4.99% of the number of shares of the common
stock outstanding immediately prior to, and immediately after giving effect to, the conversion of all or any portion of the Investor
Notes (the “Beneficial Ownership Limitation”). The Purchasers may adjust the Beneficial Ownership limitation at any time,
upon 61 days’ notice, provided that the Beneficial Ownership Limitation may not be adjusted above 9.99% of the number of shares
of common stock outstanding immediately prior to, and immediately after giving effect to, the conversion of all or any portion of the
Investor Notes.
Through
the registration statement of which this prospectus is a part we registered with the SEC the resale of the shares of common stock issuable
upon conversion of the Investor Notes, as well as the shares of common stock issuable upon the exercise of the Investor Warrants. The
Investor Notes contain certain covenants, and events of default and triggering events, respectively, which would require repayment of
the obligations outstanding pursuant to such instruments. Our obligations under the Investor Notes are secured by all of our and our
subsidiaries’ assets and guaranteed jointly and severally by our subsidiaries.
SELLING
SECURITYHOLDERS
This
prospectus relates to the resale by the Selling Securityholders from time to time of up to 4,796,478 shares of our common stock and shares
of our common stock underlying the Investor Warrants. The Selling Securityholders may offer and sell some, all or none of the common
stock set forth below pursuant to this prospectus and any accompanying prospectus supplement. As used in this prospectus, the term “Selling
Securityholders” includes the persons listed in the table below, together with any additional selling securityholders listed in
a subsequent amendment to this prospectus, and their pledgees, donees, transferees, assignees, successors, designees and others who later
come to hold any of the Selling Securityholders’ interests in the common stock, other than through a public sale.
The
table below lists the Selling Securityholders and provides information about the beneficial ownership of the shares of common stock held
by each Selling Securityholder. The second column lists the number of shares of common stock owned by each Selling Securityholder, based
on exercise of the Investor Warrants and other warrants held by each selling securityholder, without regard to any limitations on exercises
contained therein. The third column lists the shares of common stock being offered by this prospectus by the Selling Securityholders.
This prospectus generally covers the resale of the maximum number of shares of common stock issuable upon exercise of the Investor Warrants,
determined as if the Investor Warrants had been exercised in full, in each case without regard to any limitations on the exercise of
the Investor Warrants. The fourth column assumes the sale of all shares offered by the Selling Securityholders by this prospectus. In
the fifth column, the applicable percentage ownership of common stock beneficially owned is based on approximately 13,870,510
shares of common stock outstanding as of October 7, 2024, accounting for any limitations on the exercise of warrants contained
therein.
We
have determined beneficial ownership in accordance with the rules of the SEC and beneficial ownership includes voting or investment power
over securities. We have prepared the table based on information given to us by, or on behalf of, the Selling Securityholders.
Please
see the section titled “Plan of Distribution” in this prospectus for further information regarding the Selling Securityholders’
methods of distributing these shares.
| Name | |
Shares Owned Prior to Offering | | |
Shares Underlying Notes
and
Investor Warrants Registered for Sale Hereby | | |
Shares Owned After Offering | | |
Percent Beneficially Owned After Offering | |
| 3i, LP (1) | |
| 8,614,524 | | |
| 2,000,000 | | |
| 6,614,524 | | |
| 4.99 | % |
| Bob Gower (2) | |
| 766,706 | | |
| 585,120 | | |
| 181,586 | | |
| 1.31 | % |
| * |
Less
than 1% |
| |
|
| (1) |
Includes
(i) 6,153,622 shares of common stock
issuable upon the exercise of Series A-3 Warrants and Series A-4 Warrants to purchase shares of common stock that were
issued to the Selling Securityholder pursuant to an Inducement Agreement entered into in August 2024, which warrants are not exercisable
until the date on which stockholder approval is received with respect to the issuance of the shares of common stock issuable upon
exercise of such warrants, and are also subject to, as applicable, certain beneficial ownership limitations, which provide that
a holder of such warrants will not have the right to exercise any portion thereof if such holder, together with its affiliates, would
beneficially own in excess of 4.99%, of the number of shares of common stock outstanding immediately after giving effect to such
exercise, provided that such holder may decrease such limitation and further provided that upon at least 61 days’ prior notice
to us, such holder may increase such limitation up to a maximum of 9.99% of the number of shares of common stock outstanding;
provided that the limitation for Series A-3 Warrants and Series A-4 Warrants aggregating 1,522,798 of those shares of common stock
is set at 9.99%, (ii) 2,000,000 shares of common stock issuable upon the exercise of warrants to purchase shares of common
stock that were issued to the Selling Securityholder pursuant to the October 2023 Securities Purchase Agreement, which warrants are
subject to, as applicable, certain beneficial ownership limitations, which provide that a holder of such warrants will not have the
right to exercise any portion thereof if such holder, together with its affiliates, would beneficially own in excess of 4.99%, of
the number of shares of common stock outstanding immediately after giving effect to such exercise, provided that such holder may
decrease such limitation and further provided that upon at least 61 days’ prior notice to us, such holder may increase such
limitation up to a maximum of 9.99% of the number of shares of common stock outstanding, and further provided that warrants exercisable
for those shares of common stock have been amended and are not exercisable until the date on which stockholder approval is received
with respect to the issuance of the shares of common stock issuable upon exercise of such warrants, (iii) 19,450 shares of common
stock, issuable upon the exercise of warrants to purchase shares of common stock that were issued to the Selling Securityholder pursuant
to the 2022 Securities Purchase Agreement, which warrants are subject to, as applicable, certain beneficial ownership limitations,
which provide that a holder of such warrants will not have the right to exercise any portion thereof if such holder, together with
its affiliates, would beneficially own in excess of 4.99%, of the number of shares of common stock outstanding immediately after
giving effect to such exercise, provided that such holder may decrease such limitation and further provided that upon at least 61
days’ prior notice to us, such holder may increase such limitation up to a maximum of 9.99% of the number of shares of common
stock outstanding, and (iv) 2,258 shares of common stock, issuable upon the exercise of warrants to purchase shares of common stock
that were issued to the Selling Securityholder pursuant to the 2021 Securities Purchase Agreement, which warrants are subject to,
as applicable, certain beneficial ownership limitations, which provide that a holder of such warrants will not have the right to
exercise any portion thereof if such holder, together with its affiliates, would beneficially own in excess of 4.99%, of the number
of shares of common stock outstanding immediately after giving effect to such exercise, provided that such holder may decrease such
limitation and further provided that upon at least 61 days’ prior notice to us, such holder may increase such limitation up
to a maximum of 9.99% of the number of shares of common stock outstanding. The maximum number of shares to be sold includes all shares
of common stock issuable upon exercise of warrants without giving effect to such beneficial ownership limitation. The business address
of 3i, LP is 2 Wooster Street, 2nd Floor, New York, NY 10013. 3i, LP’s principal business is that of a private investor.
Maier Joshua Tarlow is the manager of 3i Management, LLC, the general partner of 3i, LP, and has sole voting control and investment
discretion over securities beneficially owned directly or indirectly by 3i Management, LLC and 3i, LP. Mr. Tarlow disclaims any beneficial
ownership of the securities beneficially owned directly by 3i, LP and indirectly by 3i Management, LLC. |
| (2) |
Dr.
Gower is the Chairman of our Board of Directors, a position he has held since 2008. His ownership consists of (i) 141,933
shares of common stock issuable upon the conversion of up to $216,000 principal amount of secured convertible promissory notes that
were or are expected to be issued to the Selling Securityholder pursuant to the October 2023 Securities Purchase Agreement, plus
accrued and unpaid interest thereon, which shares underlying the secured convertible promissory notes are subject to, as applicable,
certain beneficial ownership limitations, which provide that a holder of such secured convertible promissory notes will not have
the right to convert any portion thereof if such holder, together with its affiliates, would beneficially own in excess of 4.99%,
of the number of shares of common stock outstanding immediately after giving effect to such conversion, provided that such holder
may decrease such limitation and further provided that upon at least 61 days’ prior notice to us, such holder may increase
such limitation up to a maximum of 9.99% of the number of shares of common stock outstanding, (ii) 443,187 shares of common stock
issuable upon the exercise of warrants to purchase shares of common stock that were issued to the Selling Securityholder
pursuant to the October 2023 Securities Purchase Agreement, which warrants are subject to, as applicable, certain beneficial ownership
limitations, which provide that a holder of such warrants will not have the right to exercise any portion thereof if such holder,
together with its affiliates, would beneficially own in excess of 4.99%, of the number of shares of common stock outstanding immediately
after giving effect to such exercise, provided that such holder may decrease such limitation and further provided that upon at least
61 days’ prior notice to us, such holder may increase such limitation up to a maximum of 9.99% of the number of shares of common
stock outstanding, (iii) 172 shares subject to options, (iv) 59,524 shares that may be acquired through the exercise of warrants
purchased in a December 2022 registered offering, and (v) 121,890 shares of common stock. The business address of Bob Gower is 101
Westcott, Unit 303, Houston, Texas 77007. Mr. Gower has sole voting control and investment discretion over securities beneficially
owned by him. |
PLAN
OF DISTRIBUTION
Each
Selling Securityholder, and any of their pledgees, assignees and successors-in-interest may, from time to time, sell any or all of their
Company securities covered hereby on the Nasdaq Capital Market or any other stock exchange, market or trading facility on which such
securities are traded or in private transactions. These sales may be at fixed or negotiated prices.
A
Selling Securityholder may use any one or more of the following methods when selling securities:
| |
● |
ordinary
brokerage transactions and transactions in which the broker dealer solicits purchasers; |
| |
|
|
| |
● |
block
trades in which the broker dealer will attempt to sell the securities as agent but may position and resell a portion of the block
as principal to facilitate the transaction; |
| |
|
|
| |
● |
purchases
by a broker dealer as principal and resale by the broker dealer for its account; |
| |
|
|
| |
● |
an
exchange distribution in accordance with the rules of the applicable exchange; |
| |
|
|
| |
● |
privately
negotiated transactions; |
| |
|
|
| |
● |
settlement
of short sales entered after the effective date of the registration statement of which this prospectus is a part; |
| |
|
|
| |
● |
in
transactions through broker dealers that agree with the Selling Securityholders to sell a specified number of such securities at
a stipulated price per security; |
| |
|
|
| |
● |
through
the writing or settlement of options or other hedging transactions, whether through an options exchange or otherwise; |
| |
|
|
| |
● |
a
combination of any such methods of sale; or |
| |
|
|
| |
● |
any
other method permitted pursuant to applicable law. |
The
Selling Securityholders may also sell securities under Rule 144 under the Securities Act, if available, rather than under this prospectus.
Broker
dealers engaged by the Selling Securityholders may arrange for other brokers dealers to participate in sales. Broker dealers may receive
commissions or discounts from the Selling Securityholders (or, if any broker dealer acts as agent for the purchaser of securities, from
the purchaser) in amounts to be negotiated, but, except as set forth in a supplement to this prospectus, in the case of an agency transaction
not in excess of a customary brokerage commission in compliance with FINRA Rule 2440; and in the case of a principal transaction a markup
or markdown in compliance with FINRA IM-2440.
In
connection with the sale of the securities or interests therein, the Selling Securityholders may enter hedging transactions with broker-dealers
or other financial institutions, which may in turn engage in short sales of the securities while hedging the positions
they assume. The Selling Securityholders may also sell securities short and deliver these securities to close out their short positions,
or loan or pledge the securities to broker-dealers that in turn may sell these securities. The Selling Securityholders may also enter
into option or other transactions with broker-dealers or other financial institutions or create one or more derivative securities which
require the delivery to such broker-dealer or other financial institution of securities offered by this prospectus, which securities
such broker-dealer or other financial institution may resell pursuant to this prospectus (as supplemented or amended to reflect such
transaction).
The
Selling Securityholders and any broker-dealers or agents that participate in selling the securities may be deemed to be “underwriters”
within the meaning of the Securities Act in connection with such sales. In such event, any commissions received by such broker-dealers
or agents and any profit on the resale of the securities purchased by them may be deemed to be underwriting commissions or discounts
under the Securities Act. Each Selling Securityholder has informed the Company that it does not have any written or oral agreement or
understanding, directly or indirectly, with any person to distribute the securities. In no event shall any broker-dealer receive fees,
commissions and markups which, in the aggregate, would exceed eight percent (8%).
The
Company is required to pay certain fees and expenses incurred by the Company incident to the registration of the securities.
Because
Selling Securityholders may be deemed to be “underwriters” within the meaning of the Securities Act, they will be subject
to the prospectus delivery requirements of the Securities Act including Rule 172 thereunder. In addition, any securities covered by this
prospectus which qualify for sale pursuant to Rule 144 under the Securities Act may be sold under Rule 144 rather than under this prospectus.
The Selling Securityholders have advised us that there is no underwriter or coordinating broker acting in connection with the proposed
sale of the resale securities by the Selling Securityholders.
We
agreed to keep this prospectus effective for the Selling Securityholders for common stock issued upon conversion of Investor notes and
for common stock resold following exercise of the Investor Warrants (the “resale securities”) at all times until no
holder of the Investor Warrants holds any Investor Warrants or resale securities or certain other requirements are met. The resale securities
will be sold only through registered or licensed brokers or dealers if required under applicable state securities laws. In addition,
in certain states, the resale securities covered hereby may not be sold unless they have been registered or qualified for sale in the
applicable state or an exemption from the registration or qualification requirement is available and is complied with.
Under
applicable rules and regulations under the Exchange Act, any person engaged in the distribution of the resale securities may not simultaneously
engage in market making activities with respect to the common stock for the applicable restricted period, as defined in Regulation M,
prior to the commencement of the distribution. In addition, the Selling Securityholders will be subject to applicable provisions of the
Exchange Act and the rules and regulations thereunder, including Regulation M, which may limit the timing of purchases and sales of securities
of the common stock by the Selling Securityholders or any other person. We will make copies of this prospectus available to the Selling
Securityholders and have informed them of the need to deliver a copy of this prospectus to each purchaser at or prior to the time of
the sale (including by compliance with Rule 172 under the Securities Act).
LEGAL
MATTERS
The
validity of the securities offered hereby will be passed upon for us by Troutman Pepper Hamilton Sanders LLP. Any underwriters or agents
will be advised about other issues relating to the offering by counsel to be named in the applicable prospectus supplement.
EXPERTS
Our consolidated
financial statements as of December 31, 2023 and 2022, and for the years ended, appearing in our Annual Report on Form
10-K for the year ended December 31, 2023, incorporated in this prospectus by reference, have been audited by
Moss Adams LLP, an independent registered public accounting firm, as stated in their report (which report expresses an unqualified
opinion and includes an explanatory paragraph regarding the existence of substantial doubt about the Company’s ability
to continue as a going concern). Such consolidated financial statements are incorporated by reference in
reliance upon the report of such firm given their authority as experts in accounting and auditing.
WHERE
YOU CAN FIND MORE INFORMATION
This
prospectus is part of the registration statement on Form S-3 filed with the SEC under the Securities Act and does not contain all the
information set forth in the registration statement. Whenever a reference is made in this prospectus to any of our contracts, agreements
or other documents, the reference may not be complete and you should refer to the exhibits that are a part of the registration statement
or the exhibits to the reports or other documents incorporated herein by reference for a copy of such contract, agreement or other document.
We
are currently subject to the reporting requirements of the Exchange Act, and in accordance therewith file periodic reports, proxy statements
and other information with the SEC. Our SEC filings are available to you on the SEC’s website at http://www.sec.gov and in the
“Investors” section of our website at www.ensysce.com. Our website and the information contained on that site, or connected
to that site, are not incorporated into and are not a part of this prospectus.
DOCUMENTS
INCORPORATED BY REFERENCE
The
SEC allows us to “incorporate by reference” information from other documents that we file with it, which means that we can
disclose important information to you by referring you to those documents. The information incorporated by reference is considered to
be part of this prospectus. Information in this prospectus supersedes information incorporated by reference that we filed with the SEC
prior to the date of this prospectus, while information that we file later with the SEC will automatically update and supersede the information
in this prospectus. We incorporate by reference into this prospectus and the registration statement of which this prospectus is a part
the information or documents listed below that we have filed with the SEC:
| |
● |
Our
Annual Report on Form 10-K for the year ended December 31, 2023 filed with the SEC on March 15, 2024; |
| |
|
|
| |
● |
Our
Quarterly Report on Form 10-Q for the quarter ended March
31, 2024, filed with the SEC on May 13, 2024, and for the quarter ended June
30, 2024, filed with the SEC on August 14, 2024; |
| |
|
|
| |
● |
Our
Current Reports on Form 8-K filed with the SEC on February
14, 2024, March
15, 2024, March
29, 2024, May
9, 2024, May
13, 2024, May
28, 2024, August
14, 2024, August
23, 2024 and August 30, 2024 (in each case other than any portions thereof deemed furnished and not filed); |
| |
|
|
| |
● |
All
other reports filed with the SEC pursuant to Section 13(a) or 15(d) of the Exchange Act, since the end of the fiscal year covered
by the Annual Report on Form 10-K referenced above; and |
| |
|
|
| |
● |
The
Form 8-A filed with the SEC on November 28, 2017 by our predecessor corporation, Leisure Acquisition Corp., included a description
of common stock that was updated with the filing of our: (i) Third Amended and Restated Certificate of Incorporation as Exhibit 3.1
to our Current Report on Form 8-K, which was filed with the SEC on July 7, 2021 and (ii) Certificate of Amendment to Third Amended
and Restated Certificate of Incorporation that was filed as Exhibit 3.1(b) to our Registration Statement on Form S-1 (File No. 333-268038),
which was filed with the SEC on October 28, 2022. |
We
also incorporate by reference any future filings (other than any filings or portions of such reports that are not deemed “filed”
under the Exchange Act in accordance with the Exchange Act and applicable SEC rules, including current reports furnished under Item 2.02
or Item 7.01 of Form 8-K and exhibits furnished on such form that are related to such items unless such Form 8-K expressly provides to
the contrary) made with the SEC pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act, including those made after the date
of the initial filing of the registration statement of which this prospectus is a part and prior to the effectiveness of the registration
statement, until we file a post-effective amendment that indicates the termination of the offering of the securities made by this prospectus
and will become a part of this prospectus from the date that such documents are filed with the SEC. Information in such future filings
updates and supplements the information provided in this prospectus. Any statements in any such future filings will automatically be
deemed to modify and supersede any information in any document we previously filed with the SEC that is incorporated or deemed to be
incorporated herein by reference to the extent that statements in the later filed document modify or replace such earlier statements.
We
will furnish without charge to you, upon written or oral request, a copy of any or all of the documents incorporated by reference, including
exhibits to these documents by writing or telephoning us at the following address or phone number. Ensysce Biosciences, Inc., 7946 Ivanhoe
Avenue, Suite 201, La Jolla, California 92037, telephone number (858) 263-4196. You may also access this information on our website at
www.ensysce.com by viewing the “SEC Filings” subsection of the “Investors” menu. No additional information is
deemed to be part of or incorporated by reference into this prospectus.
Up
to 1,029,387 Shares of Common Stock Underlying Notes
Up
to 3,767,091 Shares of Common Stock Underlying Warrants

ENSYSCE
BIOSCIENCES, INC.
PROSPECTUS
[_______
__], 2024
PART
II
INFORMATION
NOT REQUIRED IN PROSPECTUS
Item
13. Other Expenses of Issuance and Distribution.
The
following is a statement of estimated expenses payable by the registrant, other than placement agent fees and expenses, in connection
with the offering described in this registration statement. All amounts are estimates except the SEC registration fee.
| SEC registration fee | |
$ | 835 | |
| Accounting fees and expenses | |
| 15,000 | |
| Legal fees and expenses | |
| 15,000 | |
| Miscellaneous | |
| 5,000 | |
| Total | |
$ | 35,835 | |
Item
14. Indemnification of Directors and Officers.
Section
102(b)(7) of the Delaware General Corporation Law (the “DGCL”) allows a corporation to provide in its certificate
of incorporation that a director of the corporation will not be personally liable to the corporation or its stockholders for monetary
damages for breach of fiduciary duty as a director, except where the director breached the duty of loyalty, failed to act in good faith,
engaged in intentional misconduct or knowingly violated a law, authorized the payment of a dividend or approved a stock repurchase in
violation of Delaware corporate law or obtained an improper personal benefit. The registrant’s third amended and restated certificate
of incorporation provides for this limitation of liability.
Section
145 of the DGCL, provides, among other things, that a Delaware corporation may indemnify any person who was, is or is threatened to be
made, party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative
(other than an action by or in the right of such corporation), by reason of the fact that such person is or was an officer, director,
employee or agent of such corporation or is or was serving at the request of such corporation as a director, officer, employee or agent
of another corporation or enterprise. The indemnity may include expenses (including attorneys’ fees), judgments, fines and amounts
paid in settlement actually and reasonably incurred by such person in connection with such action, suit or proceeding, provided such
person acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the corporation’s best interests
and, with respect to any criminal action or proceeding, had no reasonable cause to believe that his or her conduct was unlawful. A Delaware
corporation may indemnify any persons who were or are a party to any threatened, pending or completed action or suit by or in the right
of the corporation by reason of the fact that such person is or was a director, officer, employee or agent of another corporation or
enterprise. The indemnity may include expenses (including attorneys’ fees) actually and reasonably incurred by such person in connection
with the defense or settlement of such action or suit, provided such person acted in good faith and in a manner he or she reasonably
believed to be in or not opposed to the corporation’s best interests, provided further that no indemnification is permitted without
judicial approval if the officer, director, employee or agent is adjudged to be liable to the corporation. Where an officer or director
is successful on the merits or otherwise in the defense of any action referred to above, the corporation must indemnify him or her against
the expenses (including attorneys’ fees) which such officer or director has actually and reasonably incurred.
Section
145 further authorizes a corporation to purchase and maintain insurance on behalf of any person who is or was a director, officer, employee
or agent of the corporation or is or was serving at the request of the corporation as a director, officer, employee or agent of another
corporation or enterprise, against any liability asserted against such person and incurred by such person in any such capacity, or arising
out of his or her status as such, whether or not the corporation would otherwise have the power to indemnify such person under Section
145.
The
registrant’s amended and restated bylaws provide that it must indemnify and advance expenses to its directors and officers to the
full extent authorized by the DGCL.
The
registrant has entered into indemnification agreements with each of its directors and executive officers. Such agreements may require
the registrant, among other things, to advance expenses and otherwise indemnify its executive officers and directors against certain
liabilities that may arise by reason of their status or service as executive officers or directors, to the fullest extent permitted by
law.
The
indemnification rights set forth above shall not be exclusive of any other right which an indemnified person may have or hereafter acquire
under any statute, any provision of the registrant’s third amended and restated certificate of incorporation, the registrant’s
amended and restated bylaws, agreement, vote of stockholders or disinterested directors or otherwise. Notwithstanding the foregoing,
the registrant shall not be obligated to indemnify a director or officer in respect of a proceeding (or part thereof) instituted by such
director or officer, unless such proceeding (or part thereof) has been authorized by the board of directors pursuant to the applicable
procedure outlined in the registrant’s amended and restated bylaws.
Section
174 of the DGCL provides, among other things, that a director, who willfully or negligently approves of an unlawful payment of dividends
or an unlawful stock purchase or redemption, may be held jointly and severally liable for such actions. A director who was either absent
when the unlawful actions were approved or dissented at the time may avoid liability by causing his or her dissent to such actions to
be entered in the books containing the minutes of the meetings of the board of directors at the time such action occurred or immediately
after such absent director receives notice of the unlawful acts.
The
registrant maintains and expects to maintain standard policies of insurance that provide coverage (1) to its directors and officers against
loss rising from claims made by reason of breach of duty or other wrongful act and (2) to the registrant with respect to indemnification
payments that the registrant may make to such directors and officers.
These
provisions may discourage stockholders from bringing a lawsuit against the registrant’s directors for breach of their fiduciary
duty. These provisions also may have the effect of reducing the likelihood of derivative litigation against directors and officers, even
though such an action, if successful, might otherwise benefit the registrant and its stockholders. Furthermore, a stockholder’s
investment may be adversely affected to the extent the registrant pays the costs of settlement and damage awards against officers and
directors pursuant to these indemnification provisions.
The
registrant believes that these provisions, the insurance and the indemnity agreements are necessary to attract and retain talented and
experienced officers and directors.
Item
15. Recent Sales of Unregistered Securities.
Set
forth below is information regarding shares of securities sold by us within the past three years. Also included is the consideration
received by us for such shares and information relating to the section of the Securities Act, or rule of the Securities and Exchange
Commission, under which exemption from registration was claimed. Share and per share amounts reported in this Item 15 have been retroactively
restated to reflect the Reverse Splits.
| |
● |
On
February 18, 2022, we issued 208 shares of common stock to MZHCI, LLC in connection with our execution of an investor relations consulting
agreement with MZHCI, LLC on December 20, 2021, through which we receive ongoing stock market support services and other consulting
services. The issuance of our shares was a private transaction between MZHCI, LLC and us. We received no proceeds in connection with
our issuance of those 208 shares. |
| |
|
|
| |
● |
On
June 30, 2022, we entered into a Securities Purchase Agreement for an aggregate financing of $8.0 million with institutional investors.
The Company issued to the investors (i) 2022 Notes in the aggregate principal amount of $8.48 million for an aggregate purchase price
of $8.0 million and (ii) warrants to purchase 38,900 shares of the Company’s common stock in the aggregate at an exercise price
of $170.04 per share, which was subsequently reduced to $24.07. The first funding of $4.0 million occurred on July 1, 2022 and the
second funding of $4.0 million occurred on August 9, 2022. Pursuant to the 2022 Notes, shares of Company common stock were issued
to these investors in satisfaction of principal and interest payments. The conversion price of the 2022 Notes (and exercise price
of the related warrants) was subsequently reset lower such that a greater amount of principal on the 2022 Notes could be extinguished
for shares. The proceeds were used for working capital purposes subject to certain customary restrictions. Effective May 12, 2023,
we reduced the per share exercise price of the warrants to $3.637 by amending the warrants in return for an additional offering price
of $0.125 per amended warrant, such reduction disclosed in a prospectus supplement filed with the SEC on May 12, 2023. |
| |
● |
On
October 19, 2022, we issued 1,187 shares of common stock to Dr. Lynn Kirkpatrick and 2,651 shares of Company common stock to Dr.
Bob Gower (collectively, the “K&G Shares”). The K&G Shares were issued in satisfaction (reimbursement) of an
obligation to a third-party vendor previously incurred by the Company that was paid by Drs. Kirkpatrick and Gower. The reimbursement
replaced registered but restricted shares on a one-for-one basis with unregistered and restricted shares. The aggregate market value
of the K&G Shares on the transfer date was $191,618. The transaction involved the receipt by two insiders of unregistered and
restricted shares of common stock. |
| |
|
|
| |
● |
On
January 4, 2023, we issued 44,444 shares of common stock to Gem Yield Bahamas Limited as final payment of a commitment fee due in
the form of cash or freely tradeable shares under a share purchase agreement, dated December 29, 2020. The final payment of the commitment
fee was due in the form of freely tradeable shares 18 months after the public listing date of the common stock. The final payment
of $400,000 of the commitment fee was converted into shares of common stock at a price per share of approximately $9.00. |
| |
|
|
| |
● |
On
February 2, 2023, we issued warrants to purchase up to 297,619 shares of our common stock in a private placement that occurred concurrently
with a registered direct offering of an identical number of shares of our common stock. The warrants were issued in the same amounts
and to the same purchasers in the registered direct offering. Each warrant is exercisable for five and one-half years from the date
of issuance for one share of our common stock at an exercise price of $8.58 per share. We engaged H.C. Wainwright & Co., LLC
(the “placement agent”), as our exclusive placement agent in connection with the offering of our shares pursuant to the
registered direct offering and for the offering of the warrants pursuant to the private placement. In connection with that February
2, 2023 public and private offerings, the placement agent received: (i) a placement agent fee of $210,000, (ii) reimbursement of
accountable expenses of $50,000, (iii) reimbursement of non-accountable expenses of $35,000, (iv) reimbursement of $15,950 for clearing
expenses and (v) warrants exercisable for five years to purchase 20,832 shares of common stock with a per share exercise price of
$12.60. |
| |
|
|
| |
● |
On
October 23, 2023, we entered into a Securities Purchase Agreement for an aggregate financing of $1.7 million with investors. At the
first closing, which occurred on October 25, 2023, the Company issued to the investors (i) senior secured convertible promissory
notes in the aggregate principal amount of $612,000 for an aggregate purchase price of $566,667 and (ii) warrants to purchase 1,255,697
shares of the Company’s common stock in the aggregate. At the second closing, which occurred on November 28, 2023, the Company
issued to the investors referenced above, (i) additional notes in the aggregate principal amount of $1,224,000 for an aggregate purchase
price of $1,133,333 and (i) additional warrants to purchase 2,511,394 shares of common stock in the aggregate. The combined notes
are subject to an original issue discount of 8%, have a term of six months from their respective date of issuance and accrue interest
at the rate of 6.0% per annum. The notes are convertible into common Stock, at a per share conversion price equal to $1.5675. Beginning
ninety days following issuance of the respective notes, the Company was obligated to redeem one third of the original principal amount
under the applicable note, plus accrued but unpaid interest, liquidated damages and any other amounts then owing to the holder of
such note. The balance of accrued but unpaid interest, liquidated damages and any other amounts then owing to the holder of such
note was due over the remainder of the note term. The Company was required to pay the redemption amount in cash with a premium of
10% or, at the election of the purchaser at any time, some or all of the principal amount and interest may be paid by conversion
of shares under the note into common stock based on a conversion price equal to $1.5675. The warrants have an exercise price of $1.5675,
the same as the conversion price, and are exercisable for five years following issuance, issuance to occur on each of the first and
second closing dates. The resale of shares of common issuable upon conversion of the notes and exercise of the warrants were registered
by means of this registration statement. |
| |
|
|
| |
● |
On
February 12, 2024, we entered into an inducement offer letter agreement (the “February Inducement Letter”)
with certain holders (the “Holders”) of certain of our existing warrants to purchase up to an aggregate of 3,601,752
shares of common stock issued to the Holders on May 12, 2023 and having an exercise price of $3.637 per share (the “Existing
Warrants”). The shares of common stock issuable upon exercise of the Existing Warrants are registered pursuant to an effective
registration statement on Form S-1 (File No. 333-271480). Pursuant to the February Inducement Letter, the Holders agreed to
exercise for cash their Existing Warrants to purchase an aggregate of 3,601,752 shares of common stock at a reduced exercise price
of $1.31 per share in consideration of our agreement to issue new unregistered Series A Warrants (the “Series A Warrants”)
to purchase up to 3,601,752 shares of common stock and new unregistered Series B Warrants (the “Series B Warrants”)
to purchase up to 3,601,752 shares of common stock (collectively, the “New Warrant Shares”). The Series A Warrants
have an exercise price of $1.06 per share, are exercisable immediately upon issuance and have a term equal to eighteen months from
the date of issuance. The Series B Warrants have an exercise price of $1.06 per share, are exercisable immediately upon issuance
and will expire on May 12, 2028. We were assisted by a placement agent on a best-efforts basis, and compensated the placement with,
among other things, unregistered warrants (the “February Placement Agent Warrants”) to purchase up to 252,123
shares of common stock. The February Placement Agent Warrants expire on May 12, 2028, and have an exercise price of $1.6375
per share of common stock (equal to 125% of the reduced exercise price of $1.31 per Existing Warrant). The resale of the
shares of common stock issuable upon the exercise of the Series A Warrants, Series B Warrants and February Placement Agent Warrants
are registered pursuant to an effective registration statement on Form S-3 (File No. 333-276537). |
| |
|
|
| |
● |
On August 28, 2024, we entered into an inducement offer
letter agreement (the “August 2024 Inducement Letter”) with certain holders (the “Induced Holders”)
of certain of our existing warrants to purchase up to an aggregate of 7,203,504 shares of the common stock issued to the Induced
Holders on February 14, 2024 and having an original exercise price of $1.06 per share (the “February Warrants”).
Pursuant to the August 2024 Inducement Letter, the Induced Holders agreed to exercise for cash their February Warrants to purchase
an aggregate of 7,203,504 shares of common stock at a reduced exercise price of $0.47 per share in consideration of our agreement
to issue new unregistered Series A-3 Warrants (“Series A-3 Warrants”) to purchase up to 10,805,256 shares of common
stock and new unregistered Series A-4 Warrants (“Series A-4 Warrants”) to purchase up to 10,805,256 shares of
common stock. The Series A-3 Warrants have an exercise price of $0.47 per share, are exercisable at
any time on or after stockholder approval is received and have a term of exercise equal to eighteen months from the date of stockholder
approval. The Series B Warrants have an exercise price of $0.47 per share, are exercisable at any time on or after stockholder approval
is received and have a term of exercise equal to five years from the date of stockholder approval. We also reduced the exercise price
per share on warrants issued in November 2023 for 2,000,000 shares of common stock from $1.06 to $0.47. On August 28, 2024, we also
entered into Securities Purchase Agreement (the “August 2024 SPA”) pursuant to which we issued to the Induced
Holders new unregistered Series A-3 Warrants, described above, to purchase up to 3,553,194 shares of common stock and new unregistered
Series A-4 Warrants, described above, to purchase up to 3,553,194 shares of common stock. In connection with the execution of the
August 2024 Inducement Letter the August 2024 SPA and a registered direct offering that closed on August 29, 2024, we issued to a
placement agent unregistered warrants to purchase up to 752,969 shares of common stock (the “August Placement Agent Warrants”).
The August Placement Agent Warrants expire on August 28, 2029, and have an exercise price of $0.5875 per share of common stock (equal
to 125% of the reduced exercise price of $0.47 per February Warrant). We have filed a registration statement providing for the resale
of the shares of common stock issuable upon exercise of the Series A-3 Warrants, Series A-4 Warrants and August Placement Agent Warrants. |
Except
as otherwise stated, none of the foregoing transactions involved any underwriters, underwriting discounts or commissions, or any public
offering. Unless otherwise set forth above, we believe each of these transactions was exempt from registration under the Securities Act
in reliance on Section 4(a)(2) of the Securities Act (and Regulation D promulgated thereunder) as transactions by an issuer not involving
any public offering or Rule 701 promulgated under Section 3(b) of the Securities Act as transactions by an issuer under benefit plans
and contracts relating to compensation as provided under Rule 701. The recipients of the securities in each of these transactions represented
their intentions to acquire the securities for investment only and not with a view to or for sale in connection with any distribution
thereof, and appropriate legends were placed on the share certificates issued in these transactions. All recipients had adequate access,
through their relationships with us, to information about us. The sales of these securities were made without any general solicitation
or advertising.
Item16.
Exhibits and Financial Statement Schedules.
(a)
Exhibits.
Exhibit
Index
| No. |
|
Description
of Exhibit |
| 2.1† |
|
Agreement and Plan of Merger, dated January 31, 2021, by and among Leisure Acquisition Corp., Ensysce Biosciences, Inc. and EB Merger Sub, Inc. (incorporated by reference to Exhibit 2.1 filed with the registrant’s Registration Statement on Form S-4 (File No.333-254279) initially filed on March 15, 2021). |
| 3.1(a) |
|
Third Amended and Restated Certificate of Incorporation of Ensysce Biosciences, Inc. (incorporated by reference to Exhibit 3.1 filed with the registrant’s Current Report on Form 8-K (File No. 001-38306) on July 7, 2021). |
| 3.1(b) |
|
Certificate of amendment to Third Amended and Restated Certificate of Incorporation (incorporated by reference to Exhibit 3.1(b) filed with the registrant’s Registration Statement on Form S-1 (File No. 333-268038) on October 28, 2022). |
| 3.1(c) |
|
Certificate of Second Amendment to Third Amended and Restated Certificate of Incorporation (incorporated by reference to Exhibit 3.1 filed with the registrant’s Current Report on Form 8-K (File No. 001-38306) on October 27, 2022). |
| 3.1(d) |
|
Certificate of Third Amendment to Third Amended and Restated Certificate of Incorporation (incorporated by reference to Exhibit 3.1 filed with the registrant’s Current Report on Form 8-K (File No. 001-38306) on March 30, 2023). |
| 3.1(e) |
|
Certificate of Designation of the Series A Preferred Stock of Ensysce Biosciences, Inc., dated February 1, 2023 (incorporated by reference to Exhibit 3.1 to the registrant’s Registration Statement on Form 8-A, filed on February 1, 2023, File No. 000-56516). |
| 3.1(f) |
|
Certificate of Amendment to Certificate of Designation of the Series A Preferred Stock of Ensysce Biosciences, Inc., dated February 7, 2023 (incorporated by reference to Exhibit 3.2 to the Company’s Registration Statement on Form 8-A/A (Amendment No. 1), filed on February 7, 2023, File No. 000-56516). |
| 3.2(a) |
|
Amended and Restated Bylaws of Ensysce Biosciences, Inc. (incorporated by reference to Exhibit 3.2 filed with the registrant’s Current Report on Form 8-K (File No. 001-38306) on July 7, 2021). |
| 3.2(b) |
|
Amendment, dated October 5, 2023, to Amended and Restated Bylaws of Ensysce Biosciences, Inc. (incorporated by reference to Exhibit 3.2(b) filed with the registrant’s Registration Statement on Form S-1 (File No. 333-275456) on November 9, 2023). |
| 4.1 |
|
Warrant Agreement, dated December 1, 2017, between the Leisure Acquisition Corp. and Continental Stock Transfer & Trust Company (incorporated by reference to Exhibit 4.1 filed with the registrant’s Current Report on Form 8-K (File No. 001-38306) on December 5, 2017). |
| 4.2 |
|
Investor Rights Agreement between Ensysce Biosciences, Inc. and the Investors listed on the signature pages thereto dated as of May 11, 2018 (incorporated by reference to Exhibit 4.6 filed with the registrant’s Registration Statement on Form S-4 (File No.333-254279) initially filed on March 15, 2021). |
| 4.3 |
|
Form of Warrant Certificate issued to previous holders of Private Placement Warrants and other private warrants (incorporated by reference to Exhibit 4.8 filed with the registrant’s Registration Statement on Form S-4 (File No.333-254279) initially filed on March 15, 2021). |
| 4.4 |
|
Form of Senior Secured Convertible Promissory Note issued by the Company pursuant to and in accordance with the Securities Purchase Agreement (incorporated by reference to Exhibit 4.6 filed with the registrant’s Current Report on Form 8-K (File No. 001-38306) initially filed on September 27, 2021). |
| 4.5 |
|
Form of Common Stock Purchase Warrant to be issued by the Company pursuant to and in accordance with the Securities Purchase Agreement (incorporated by reference to Exhibit 4.7 filed with the registrant’s Current Report on Form 8-K (File No. 001-38306) initially filed on September 27, 2021). |
| 4.6 |
|
Form of Senior Secured Convertible Promissory Note issued by Ensysce Biosciences, Inc. pursuant to and in accordance with a 2022 Securities Purchase Agreement (incorporated by reference to Exhibit 4.6 filed with the registrant’s Current Report on Form 8-K (File No. 001-38306) on August 9, 2022). |
| 4.7 |
|
Form of Common Stock Purchase Warrant issued by Ensysce Biosciences, Inc. pursuant to and in accordance with a 2022 Securities Purchase Agreement (incorporated by reference to Exhibit 4.7 filed with the registrant’s Current Report on Form 8-K (File No. 001-38306) on August 9, 2022). |
| 4.8 |
|
Form of warrant delivered by Ensysce Biosciences, Inc. in December 2022 in connection with an underwritten offering (incorporated by reference to Exhibit 4.10 filed with the registrant’s Post-Effective Amendment No. 1 to the registrant’s Registration Statement on Form S-1 (File No. 333-268038) filed December 8, 2022). |
| 4.9 |
|
Form of pre-funded warrant delivered by Ensysce Biosciences, Inc. in December 2022 in connection with an underwritten offering (incorporated by reference to Exhibit 4.11 filed with the registrant’s Post-Effective Amendment No. 1 to the registrant’s Registration Statement on Form S-1 (File No. 333-268038) filed December 8, 2022). |
| 4.10 |
|
Form of warrant issued in connection with a private placement conducted concurrently with a public offering (incorporated by reference to Exhibit 4.1 filed with the registrant’s Current Report on Form 8-K (File No. 001-38306) on February 7, 2023). |
| 4.11 |
|
Form of warrant issued to a placement agent or its designees in connection with a private placement conducted concurrently with a public offering (incorporated by reference to Exhibit 4.2 filed with the registrant’s Current Report on Form 8-K (File No. 001-38306) on February 7, 2023). |
| 4.12 |
|
Form of common warrant (incorporated by reference to Exhibit 4.12 filed with the registrant’s Post-Effective Amendment No. 1 to the Registration Statement on Form S-1 (File No. 333-271480) on May 17, 2023). |
| 4.13 |
|
Form of pre-funded warrant (incorporated by reference to Exhibit 4.13 filed with the registrant’s Post-Effective Amendment No. 1 to the Registration Statement on Form S-1 (File No. 333-271480) on May 17, 2023). |
| 4.14 |
|
Form of placement agent warrant (incorporated by reference to Exhibit 4.14 filed with the registrant’s Post-Effective Amendment on Form S-1 (File No. 333-271480) on May 17, 2023). |
| 4.15 |
|
Form of warrants amended in connection with the execution of a Securities Purchase Agreement on May 10, 2023 (incorporated by reference to Exhibit 4.15 filed with the registrant’s Post-Effective Amendment No. 1 to the Registration Statement on Form S-1 (File No. 333-271480) on May 17, 2023). |
| 4.16 |
|
Form of common warrant issued in October 2023 and November 2023 (incorporated by reference to Exhibit 4.16 filed with the registrant’s Registration Statement on Form S-1 (File No. 333-275456) on November 9, 2023). |
| 4.17 |
|
Form of October 2023 Secured Convertible Promissory Note (incorporated by reference to Exhibit 4.6 filed with the registrant’s Current Report on Form 8-K (File No. 001-38306) on October 24, 2023). |
| 4.18 |
|
Form of Series A/B common stock purchase warrant issued February 14, 2024 (incorporated by reference to Exhibit 4.1 filed with the registrant’s Current Report on Form 8-K (File No. 001-38306) on February 14, 2024). |
| 4.19 |
|
Form of placement agent warrant issued February 14, 2024 (incorporated by reference to Exhibit 4.2 filed with the registrant’s Current Report on Form 8-K (File No. 001-38306) on February 14, 2024). |
| 4.20 |
|
Form of Series A-3/A-4 common stock purchase warrant issued August 29, 2024 (incorporated by reference to Exhibit 4.2 filed with the registrant’s Current Report on Form 8-K (File No. 001-38306) on August 30, 2024). |
| 5*** |
|
Opinion of Troutman Pepper Hamilton Sanders LLP. |
| 10.1 |
|
Registration Rights Agreement, dated December 1, 2017, among Leisure Acquisition Corp. and certain securityholders (incorporated by reference to Exhibit 10.2 filed with the registrant’s Current Report on Form 8-K (File No. 001-38306) on December 5, 2017). |
| 10.2 |
|
Warrant Purchase Agreement, dated December 1, 2017, between Leisure Acquisition Corp. and certain security holders (incorporated by reference to Exhibit 10.3 filed with the registrant’s Current Report on Form 8-K (File No. 001-38306) on December 5, 2017). |
| 10.3(a)
|
|
Form
of Director and Officer Indemnity Agreement (incorporated by reference to Exhibit 10.8 filed with the registrant’s Registration
Statement on Form S-1 (File No.333-221330) initially filed on November 3, 2017). |
| 10.3(b) |
|
Form
of Indemnification Agreement executed by each of the Ensysce directors and executive officers (incorporated by reference to Exhibit
10.6 filed with the registrant’s Form 10-Q (File No. 001-38306) initially filed on November 15, 2021). |
| 10.4+ |
|
Executive
Employment Agreement, by and between the Company and Dr. Lynn Kirkpatrick, dated September 14, 2021 (incorporated by reference to
Exhibit 10.44 filed with the registrant’s Amendment Number 1 to its Registration Statement on Form S-1 (File No.333-260478)
filed on October 29, 2021). |
| 10.5 |
|
Agreement
and Plan of Merger by and among the Signature Therapeutics, Inc., Signature Acquisition Corp. and the Company dated December 28,
2015 (incorporated by reference to Exhibit 10.21 filed with the registrant’s Registration Statement on Form S-4 (File No.333-254279)
initially filed on March 15, 2021). |
| 10.6+ |
|
Executive
Employment Agreement, by and between the Company and Geoffrey Birkett, dated August 21, 2021 (incorporated by reference to Exhibit
10.45 filed with the registrant’s Amendment Number 1 to its Registration Statement on Form S-1 (File No.333-260478) filed on
October 29, 2021) |
| 10.7+ |
|
Employment
Agreement between the Company and David Humphrey dated February 11, 2021 (incorporated by reference to Exhibit 10.26 filed with the
registrant’s Registration Statement on Form S-4 (File No.333-254279) initially filed on March 15, 2021). |
| 10.8+ |
|
Amendment
to Offer Letter between the Company and David Humphrey dated February 23, 2021 (incorporated by reference to Exhibit 10.27 filed
with the registrant’s Registration Statement on Form S-4 (File No.333-254279) initially filed on March 15, 2021). |
| 10.9(a)+ |
|
Amended and Restated 2021 Omnibus Incentive Plan, as further amended (incorporated by reference to Annex A to the registrant’s Definitive Proxy Statement on Schedule DEF 14A (File No. 001-38306) filed on July 14, 2023). |
| 10.9(b)+ |
|
Amended
and Restated 2021 Omnibus Incentive Plan Form of Stock Option Grant Notice and Award Agreement (incorporated by reference to Exhibit
10.22(a) filed with the registrant’s Annual Report on Form 10-K (File No. 001-38306) filed on March 31, 2022). |
| 10.9(c)+ |
|
Form
of Restricted Stock Unit Agreement under the Amended and Restated 2021 Omnibus Incentive Plan (incorporated by reference to Exhibit
10.9(c) filed with the registrant’s Registration Statement on Form S-1 (File No. 333-275456) on November 9, 2023). |
| 10.11† |
|
Technology
Transfer Agreement by and among the Company, Covistat, Inc., Mucokinetica, Ltd., Roderick Hall and Peter Cole dated August 5, 2020
(incorporated by reference to Exhibit 10.30 filed with the registrant’s Registration Statement on Form S-4 (File No.333-254279)
initially filed on March 15, 2021). |
| 10.12 |
|
Manufacturing
Agreement between Societal CDMO (formerly Recro Gaineville LLC) and the Company dated September 11, 2019 (incorporated by reference
to Exhibit 10.35 filed with the registrant’s Registration Statement on Form S-4 (File No.333-254279) initially filed on March
15, 2021). |
| 10.13(a) |
|
Form
of Exchange Agreement between Leisure Acquisition Corp. and the holders of Private Placement Warrants (incorporated by reference
to Exhibit 10.36(a) filed with the registrant’s Registration Statement on Form S-4 (File No.333-254279) initially filed on
March 15, 2021). |
| 10.13(b) |
|
Form
of Exchange Agreement to be entered into by the Company with each of the Sponsors and the Strategic Investor (incorporated by reference
to Exhibit 10.36(b) filed with the registrant’s Registration Statement on Form S-4 (File No.333-254279) initially filed on
March 15, 2021). |
| 10.14(a)† |
|
Securities
Purchase Agreement, dated September 24, 2021 by and among the Company and the purchasers signatory thereto (incorporated by reference
to Exhibit 10.1 filed with the registrant’s Current Report on Form 8-K (File No. 001-38306) initially filed on September 27,
2021). |
| 10.14(b) |
|
Registration
Rights Agreement, dated September 24, 2021, by and among the Company and the parties signatory thereto (incorporated by reference
to Exhibit 10.2 filed with the registrant’s Current Report on Form 8-K (File No. 001-38306) initially filed on September 27,
2021). |
| 10.14(c) |
|
Subsidiary
Guarantee, dated September 24, 2021, by and among the Company and the purchasers signatory thereto (incorporated by reference to
Exhibit 10.3 filed with the registrant’s Current Report on Form 8-K (File No. 001-38306) initially filed on September 27, 2021). |
| 10.14(d)† |
|
Security
Agreement, dated September 24, 2021, by and among the Company, EBI OpCo, Inc., Covistat, Inc. and the other parties signatory thereto
(incorporated by reference to Exhibit 10.4 filed with the registrant’s Current Report on Form 8-K (File No. 001-38306) initially
filed on September 27, 2021). |
| 10.14(e) |
|
Patent
Security Agreement, dated September 24, 2021, by and among the Company, EBI OpCo, Inc., Covistat, Inc. and the other parties signatory
thereto (incorporated by reference to Exhibit 10.5 filed with the registrant’s Current Report on Form 8-K (File No. 001-38306)
initially filed on September 27, 2021). |
| 10.14(f) |
|
Letter
Agreement, dated December 27, 2021, by and among the Company and the parties signatory thereto (incorporated by reference to Exhibit
10.6 filed with the registrant’s Current Report on Form 8-K (File No. 001-38306) initially filed on December 27, 2021). |
| 10.14(g) |
|
Second
Letter Agreement, dated January 16, 2022, by and among the Company and the parties signatory thereto (incorporated by reference to
Exhibit 10.7 filed with the registrant’s Current Report on Form 8-K (File No. 001-38306) initially filed on January 18, 2022). |
| 10.15(a) |
|
Securities
Purchase Agreement, dated June 30, 2022, by and among the Company and the purchasers signatory thereto (incorporated by reference
to Exhibit 10.1 filed with the registrant’s Current Report on Form 8-K (File No. 001-38306) on July 6, 2022). |
| 10.15(b) |
|
Registration
Rights Agreement, dated June 30, 2022, by and among the Company and the parties signatory thereto (incorporated by reference to Exhibit
10.2 filed with the registrant’s Current Report on Form 8-K (File No. 001-38306) on July 6, 2022). |
| 10.15(c) |
|
Subsidiary
Guarantee, dated June 30, 2022, by and among the Company and the purchasers signatory thereto (incorporated by reference to Exhibit
10.3 filed with the registrant’s Current Report on Form 8-K (File No. 001-38306) on July 6, 2022). |
| 10.15(d) |
|
Security
Agreement, dated June 30, 2022, by and among the Company, EBI OpCo, Inc., Covistat, Inc. and the other parties signatory thereto
(incorporated by reference to Exhibit 10.4 filed with the registrant’s Current Report on Form 8-K (File No. 001-38306) on July
6, 2022). |
| 10.15(e) |
|
Patent
Security Agreement, dated June 30, 2022, by and among the Company, EBI OpCo, Inc., Covistat, Inc. and the other parties signatory
thereto (incorporated by reference to Exhibit 10.5 filed with the registrant’s Current Report on Form 8-K (File No. 001-38306)
on July 6, 2022). |
| 10.15(f) |
|
Letter
Agreement, dated January 12, 2023, by and among the Company and the parties signatory thereto (incorporated by reference to Exhibit
10.6 filed with the registrant’s Current Report on Form 8-K (File No. 001-38306) on January 13, 2023). |
| 10.16 |
|
October
2023 Securities Purchase Agreement (incorporated by reference to Exhibit 10.1 filed with the registrant’s Current Report on
Form 8-K (File No. 001-38306) on October 24, 2023). |
| 10.17 |
|
Form
of Registration Rights Agreement (incorporated by reference to Exhibit 10.2 filed with the registrant’s Current Report on Form
8-K (File No. 001-38306) on October 24, 2023). |
| 10.18 |
|
Form
of Subsidiary Guaranty (incorporated by reference to Exhibit 10.3 filed with the registrant’s Current Report on Form 8-K (File
No. 001-38306) on October 24, 2023). |
| 10.19 |
|
Form
of Security Agreement (incorporated by reference to Exhibit 10.4 filed with the registrant’s Current Report on Form 8-K (File
No. 001-38306) on October 24, 2023). |
| 10.20 |
|
Form
of Patent Security Agreement (incorporated by reference to Exhibit 10.5 filed with the registrant’s Current Report on Form
8-K (File No. 001-38306) on October 24, 2023). |
| 10.21 |
|
Form
of Inducement Letter Agreement, dated as of February 12, 2024 (incorporated by reference to Exhibit 10.1 filed with the registrant’s
Current Report on Form 8-K (File No. 001-38306) on February 14, 2024). |
| 10.22 |
|
Form
of Waiver, dated February 12, 2024, under the Securities Purchase Agreement dated October 23, 2023 (incorporated by reference to
Exhibit 10.2 filed with the registrant’s Current Report on Form 8-K (File No. 001-38306) on February 14, 2024). |
| 10.23 |
|
Inducement Letter Agreement, dated August 28, 2024 (incorporated by reference to Exhibit 10.2 filed with the registrant’s Current Report on Form 8-K (File No. 001-38306) on August 30, 2024). |
| 14 |
|
Company’s
Code of Business Conduct (incorporated by reference to Exhibit 14 filed with the registrant’s Annual Report on Form 10-K (File
No. 001-38306) on March 30, 2023). |
| 21.1 |
|
List
of Subsidiaries (incorporated by reference to Exhibit 21 filed with the Registration Statement on Form S-1 (333-268038) filed on
October 28, 2022). |
| 23.1*** |
|
Consent of Troutman Pepper Hamilton Sanders LLP (included in Exhibit 5). |
| 23.2* |
|
Consent of Moss Adams LLP. |
| 24.1*** |
|
Power of Attorney (included on signature page to this registration statement). |
| 107*** |
|
Filing Fee Table. |
| (101) |
|
Interactive
Data File |
| |
++ |
Inline
XBRL Instance Document (the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within
the Inline XBRL document) |
| 101.SCH |
++ |
Inline
XBRL Taxonomy Extension Schema Document |
| 101.CAL |
++ |
Inline
XBRL Taxonomy Extension Calculation Document |
| 101.LAB |
++ |
Inline
XBRL Taxonomy Extension Label Linkbase Document |
| 101.PRE |
++ |
Inline
XBRL Taxonomy Extension Presentation Linkbase Document |
| 101.DEF |
++ |
Inline
XBRL Taxonomy Extension Definition Linkbase Document |
| * |
Filed
herewith. |
| ** |
To
be filed by amendment. |
| *** |
Previously
filed. |
| † |
Certain
schedules (or similar attachments) to this Exhibit have been omitted in accordance with Regulation S-K Item 601(a)(5) or 601(b)(2),
as applicable. The registrant agrees to furnish supplementally a copy of all omitted schedules to the Securities and Exchange Commission
upon its request. |
| + |
Denotes
compensatory plans or arrangements or management contracts. |
| ++ |
Pursuant
to Rule 406T of Regulation S-T, this interactive data file is deemed not “filed” or part of a registration statement
or prospectus for purposes of Section 11 or 12 of the Securities Act, is deemed not “filed” for purposes of Section
18 of the Exchange Act, and otherwise is not subject to liability under these sections. |
Item
17. Undertakings.
The
undersigned registrant hereby undertakes:
| (1) |
To
file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement: (i) to
include any prospectus required by Section 10(a)(3) of the Securities Act; (ii) to reflect in the prospectus any facts or events
arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually
or in the aggregate, represent a fundamental change in the information set forth in the registration statement (notwithstanding the
foregoing, any increase or decrease in the volume of securities offered (if the total dollar value of securities offered would not
exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected
in the form of prospectus filed with the SEC pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent
no more than a 20% change in the maximum aggregate offering price set forth in the “Calculation of Filing Fee Tables”
or “Calculation of Registration Fee” table, as applicable, table in the effective registration statement); and (iii)
to include any material information with respect to the plan of distribution not previously disclosed in the registration statement
or any material change to such information in the registration statement. |
| |
|
| (2) |
That,
for the purpose of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be a
new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be
deemed to be the initial bona fide offering thereof. |
| |
|
| (3) |
To
remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the
termination of the offering. |
| |
|
| (4) |
The
undersigned registrant hereby undertakes that, for purposes of determining any liability under the Securities Act of 1933, each filing
of the registrant’s annual report pursuant to section 13(a) or section 15(d) of the Securities Exchange Act of 1934 (and, where
applicable, each filing of an employee benefit plan’s annual report pursuant to section 15(d) of the Securities Exchange Act
of 1934) that is incorporated by reference in the registration statement shall be deemed to be a new registration statement relating
to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering
thereof. |
| |
|
| (5) |
That,
for the purpose of determining liability under the Securities Act to any purchaser, each prospectus filed pursuant to Rule 424(b)
as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than
prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date
it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is
part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement
or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first
use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration
statement or made in any such document immediately prior to such date of first use. |
| |
|
| (6) |
For
purposes of determining any liability under the Securities Act of 1933, the information omitted
from the form of prospectus filed as part of this registration statement in reliance upon
Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule
424(b) (1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration
statement as of the time it was declared effective.
For
the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of
prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such
securities at that time shall be deemed to be the initial bona fide offering thereof. |
SIGNATURES
Pursuant
to the requirements of the Securities Act of 1933, the registrant certifies that it has reasonable grounds to believe that
it meets all of the requirements for filing on Form S-3 and has duly caused this registration statement to be signed on its behalf
by the undersigned, thereunto duly authorized, in City of San Diego, State of California, on October 9, 2024.
| |
ENSYSCE
BIOSCIENCES, INC. |
| |
|
|
| |
By: |
/s/
Dr. Lynn Kirkpatrick |
| |
Name: |
Dr.
Lynn Kirkpatrick |
| |
Title: |
President,
Chief Executive Officer and Director |
Pursuant
to the requirements of the Securities Act, this registration statement has been signed by the following persons in the capacities indicated
on October 9, 2024
| Name |
|
Title |
| |
|
|
| By: |
/s/
Dr. Lynn Kirkpatrick |
|
President,
Chief Executive Officer and Director |
| |
Dr.
Lynn Kirkpatrick |
|
(Principal
Executive Officer) |
| |
|
|
|
| By: |
/s/
David Humphrey |
|
Chief
Financial Officer, Secretary and Treasurer |
| |
David
Humphrey |
|
(Principal
Financial and Accounting Officer) |
| |
|
|
|
| By: |
*/s/
Andrew Benton |
|
Director |
| |
Andrew
Benton |
|
|
| |
|
|
|
| By: |
*/s/
William Chang |
|
Director |
| |
William
Chang |
|
|
| |
|
|
|
| By: |
*/s/
Bob Gower |
|
Director
and Chairman of the Board |
| |
Bob
Gower |
|
|
| |
|
|
|
| By: |
*/s/
Adam Levin |
|
Director |
| |
Adam
Levin |
|
|
| |
|
|
|
| By: |
*/s/
Steve Martin |
|
Director |
| |
Steve
Martin |
|
|
| |
|
|
|
| By: |
*/s/
Lee Rauch |
|
Director |
| |
Lee
Rauch |
|
|
| |
|
|
|
| By: |
*/s/
Curtis Rosebraugh |
|
Director |
| |
Curtis
Rosebraugh |
|
|
| |
|
|
|
| *By: |
/s/
Dr. Lynn Kirkpatrick |
|
As
Attorney-In-Fact for the persons noted above with
an asterisk |
| |
Dr.
Lynn Kirkpatrick |
|
|
Exhibit
23.2
CONSENT
OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
We
consent to the incorporation by reference in this Post-effective Amendment No. 1 to Registration Statement on Form S-1
on Form S-3 (No. 333-275456) of Ensysce Biosciences, Inc. (the “Company”) of our report dated March 14, 2024, related to
the consolidated financial statements of the Company (which report expresses an unqualified opinion and includes an explanatory paragraph
regarding the existence of substantial doubt about the Company’s ability to continue as a going concern). We also consent
to the reference to us under the heading “Experts” in such Registration Statement.
/s/
Moss Adams LLP
San
Diego, California
October
9, 2024
Ensysce Biosciences (NASDAQ:ENSC)
Historical Stock Chart
Von Mär 2025 bis Apr 2025
Ensysce Biosciences (NASDAQ:ENSC)
Historical Stock Chart
Von Apr 2024 bis Apr 2025