0001377121
false
0001377121
2023-07-04
2023-07-04
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section
13 or 15(d)
of the Securities
Exchange Act of 1934
Date of Report (Date of earliest event reported): July 4, 2023
PROTAGONIST THERAPEUTICS, INC.
(Exact name of registrant as specified
in its charter)
| Delaware |
|
001-37852 |
|
98-0505495 |
(State or other jurisdiction
of incorporation) |
|
(Commission
File Number) |
|
(IRS Employer
Identification No.) |
7707 Gateway Blvd., Suite 140
Newark, California 94560-1160
(Address of principal executive offices,
including zip code)
(510) 474-0170
(Registrant’s telephone number, including
area code)
Not Applicable
(Former name or former address, if changed
since last report.)
Check the appropriate box below if the Form 8-K filing is intended
to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ¨ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ¨ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ¨ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ¨ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which
registered |
| Common Stock, par value $0.00001 |
|
PTGX |
|
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging
growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities
Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ¨
If an emerging
growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any
new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
Item 8.01. Other Events
On July 4, 2023, Protagonist Therapeutics, Inc. (the
“Company”) made available an updated corporate presentation, which includes additional information regarding the Phase
2b FRONTIER 1 trial of JNJ-2113 in patients with moderate-to-severe plaque psoriasis and updated timing for expected enrollment
completion for the Phase 3 VERIFY trial of rusfertide in patients with polycythemia vera. A copy of the corporate presentation is
filed as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated by reference herein.
Item 9.01 Financial Statements and Exhibits
(d) Exhibits.
SIGNATURES
Pursuant to the requirements of the Securities
Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| |
Protagonist Therapeutics, Inc. |
| |
|
| Dated: July 5, 2023 |
|
| |
|
| |
By: |
/s/ Asif Ali |
| |
|
Asif Ali |
| |
|
Chief Financial Officer |
Exhibit 99.1

1 COMPANY OVERVIEW Dinesh V. Patel, Ph.D. President & CEO July 04, 2023

Forward - looking Statements 2 This presentation and the accompanying oral presentation contain forward - looking statements made pursuant to the safe harbor pro visions of the Private Securities Litigation Reform Act of 1995. All statements other than statements of historical facts contained in this pre sentation, including statements regarding our future results of operations and financial position, business strategy, product candidates, capital res ources, potential markets for our product candidates, our plans related to potential future collaboration arrangements, the impact on our business or p rod uct candidates of actions or determinations of the U.S. Food and Drug Administration (“FDA”), enrollment in our clinical trials, any potential impact o n o ur business related to COVID - 19, our potential receipt of milestone payments and royalties under our Collaboration Agreement with Janssen Biotech, Inc. , are forward - looking statements. In some cases, you can identify forward - looking statements by terminology such as “anticipate,” “believe,” “continue ,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potentially” “predict,” “should,” “will” or the negative of these terms or other similar exp ressions. The forward - looking statements made in this presentation involve known and unknown risks, uncertainties and other important fact ors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievemen ts expressed or implied by the forward - looking statements. These forward - looking statements are subject to risks and uncertainties, including those discuss ed in Protagonist’s filings with the Securities and Exchange Commission, including in the “Risk Factors” and “Management’s Discussion and Analysi s o f Financial Condition and Results of Operations” sections of most recently filed periodic reports on Form 10 - K and Form 10 - Q and subsequent filings an d in the documents incorporated by reference therein. Because forward - looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified and some of which are beyond our control, you should not rely on these forward - looking statements as pre dictions of future events. The events and circumstances reflected in our forward - looking statements may not be achieved or occur and actual results could differ materially from those projected in the forward - looking statements. Except as required by applicable law, we do not plan to publicly update or revise any forward - looking statements contained herein, whether as a result of any new information, future events, changed circumstances or othe rwi se. This presentation concerns products that are under clinical investigation and which have not yet been approved for marketing by the U.S. Food and Drug Administration. They are currently limited by Federal law to investigational use, and no representation is made as to their s afe ty or effectiveness for the purposes for which they are being investigated. The trademarks included herein are the property of the owners thereof and are us ed for reference purposes only. Such use should not be construed as an endorsement of such products. Nothing contained in this presentation i s, or should be construed as, a recommendation, promise or representation by the presenter or Protagonist or any director, employee, agent or ad visor of Protagonist. This presentation does not purport to be all inclusive or to contain all the information you may desire.
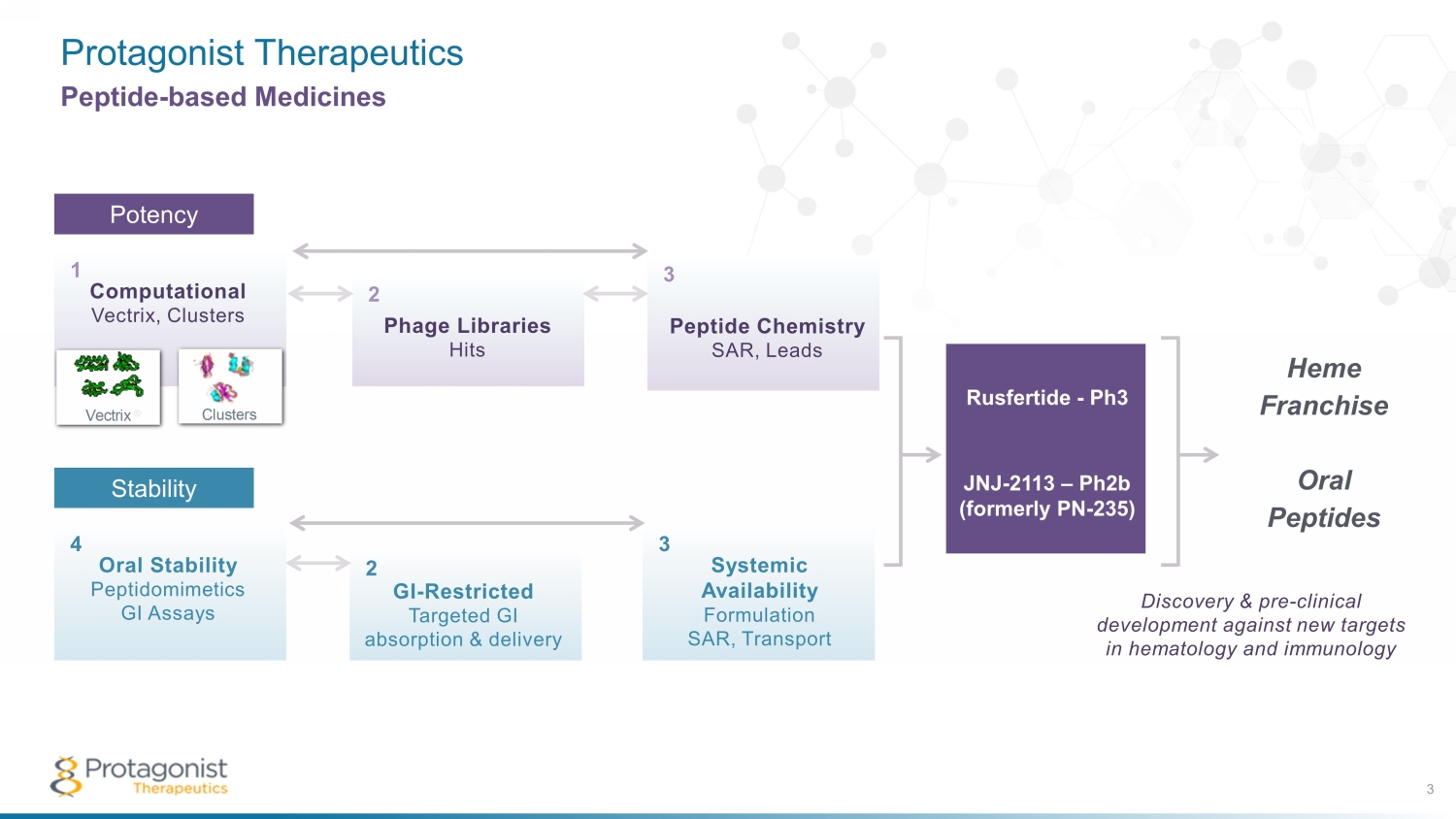
Protagonist Therapeutics 3 Peptide - based Medicines Potency Peptide Chemistry SAR, Leads Phage Libraries Hits Computational Vectrix, Clusters Vectrix ® Clusters 1 2 3 POTENCY Oral Stability Peptidomimetics GI Assays GI-Restricted Targeted GI absorption & delivery Systemic Availability Formulation SAR, Transport 4 5 6 STABILITY Computational Vectrix , Clusters 1 Peptide Chemistry SAR, Leads Phage Libraries Hits Computational Vectrix, Clusters Vectrix ® Clusters 1 2 3 POTENCY Oral Stability Peptidomimetics GI Assays GI-Restricted Targeted GI absorption & delivery Systemic Availability Formulation SAR, Transport 4 5 6 STABILITY Peptide Chemistry SAR, Leads 3 Phage Libraries Hits 2 Stability Oral Stability Peptidomimetics GI Assays 4 Systemic Availability Formulation SAR, Transport 3 GI - Restricted Targeted GI absorption & delivery 2 Rusfertide - Ph3 JNJ - 2113 – P h2b (formerly PN - 235) Heme Franchise Oral Peptides Discovery & pre - clinical development against new targets in hematology and immunology
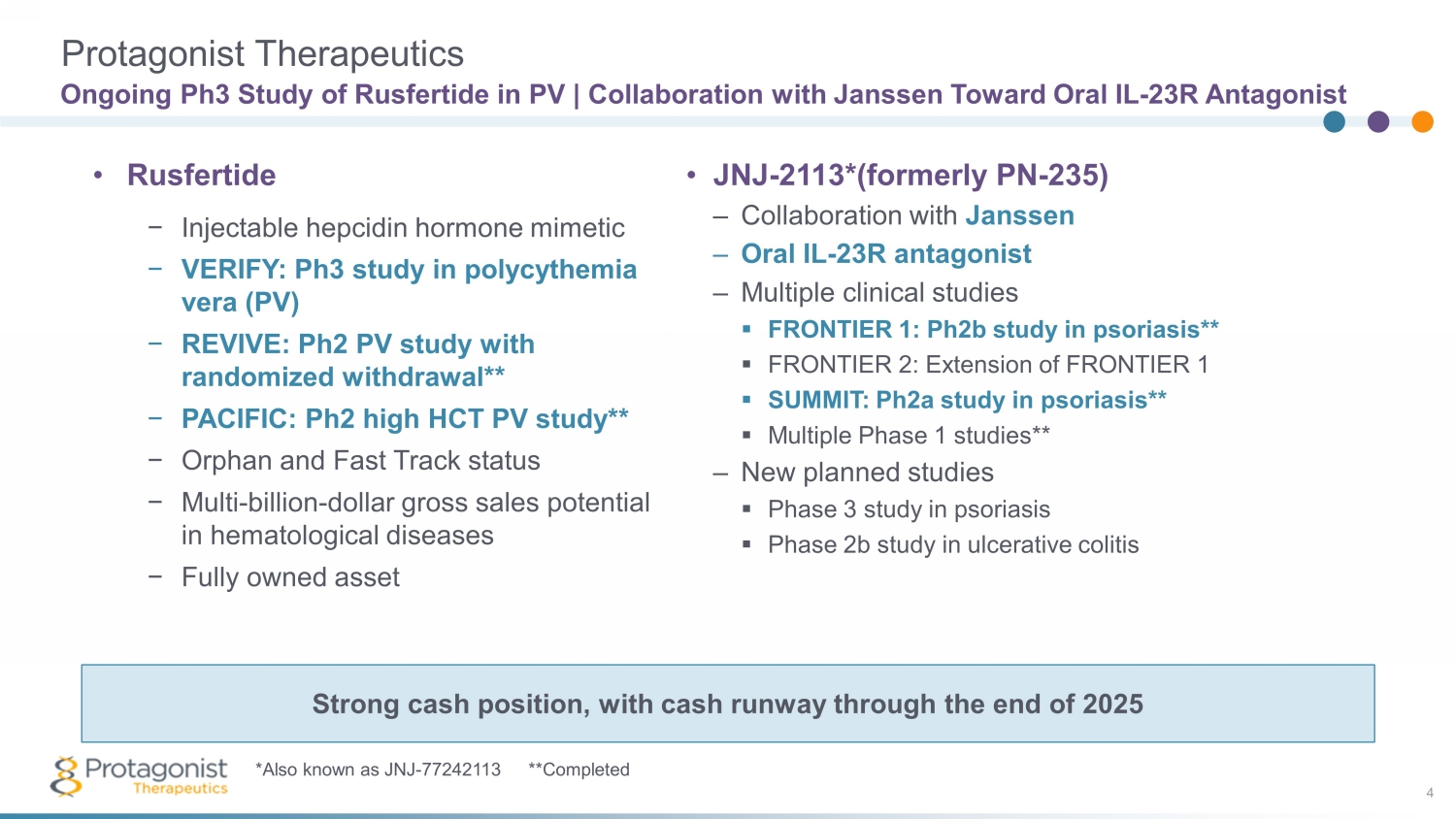
Ongoing Ph3 Study of Rusfertide in PV | Collaboration with Janssen Toward Oral IL - 23R Antagonist 4 • JNJ - 2113*(formerly PN - 235) – Collaboration with Janssen – Oral IL - 23R antagonist – Multiple clinical studies ▪ FRONTIER 1: Ph2b study in psoriasis** ▪ FRONTIER 2: Extension of FRONTIER 1 ▪ SUMMIT: Ph2a study in psoriasis** ▪ Multiple Phase 1 studies** – New planned studies ▪ Phase 3 study in psoriasis ▪ Phase 2b study in ulcerative colitis • Rusfertide − Injectable hepcidin hormone mimetic − VERIFY: Ph3 study in polycythemia vera (PV) − REVIVE: Ph2 PV study with randomized withdrawal** − PACIFIC: Ph2 high HCT PV study** − Orphan and Fast Track status − Multi - billion - dollar gross sales potential in hematological diseases − Fully owned asset Strong cash position, with cash runway through the end of 2025 *Also known as JNJ - 77242113 **Completed Protagonist Therapeutics

Discovery, Pre - Clinical & Clinical Development 5 • Primary focus on completing Phase 3 VERIFY study of Rusfertide in polycythemia vera • Separate 2 - year extension (PTG - 300 - 21) study will open this year for patients who have completed participation in the REVIVE study – Total duration of treatment up to 5 years • Discovery projects in progress – Oral hepcidin – New targets in hematology – New targets in immunology focused on ‘oral peptide approach’ as a strong differentiator Ongoing Projects and Future Plans
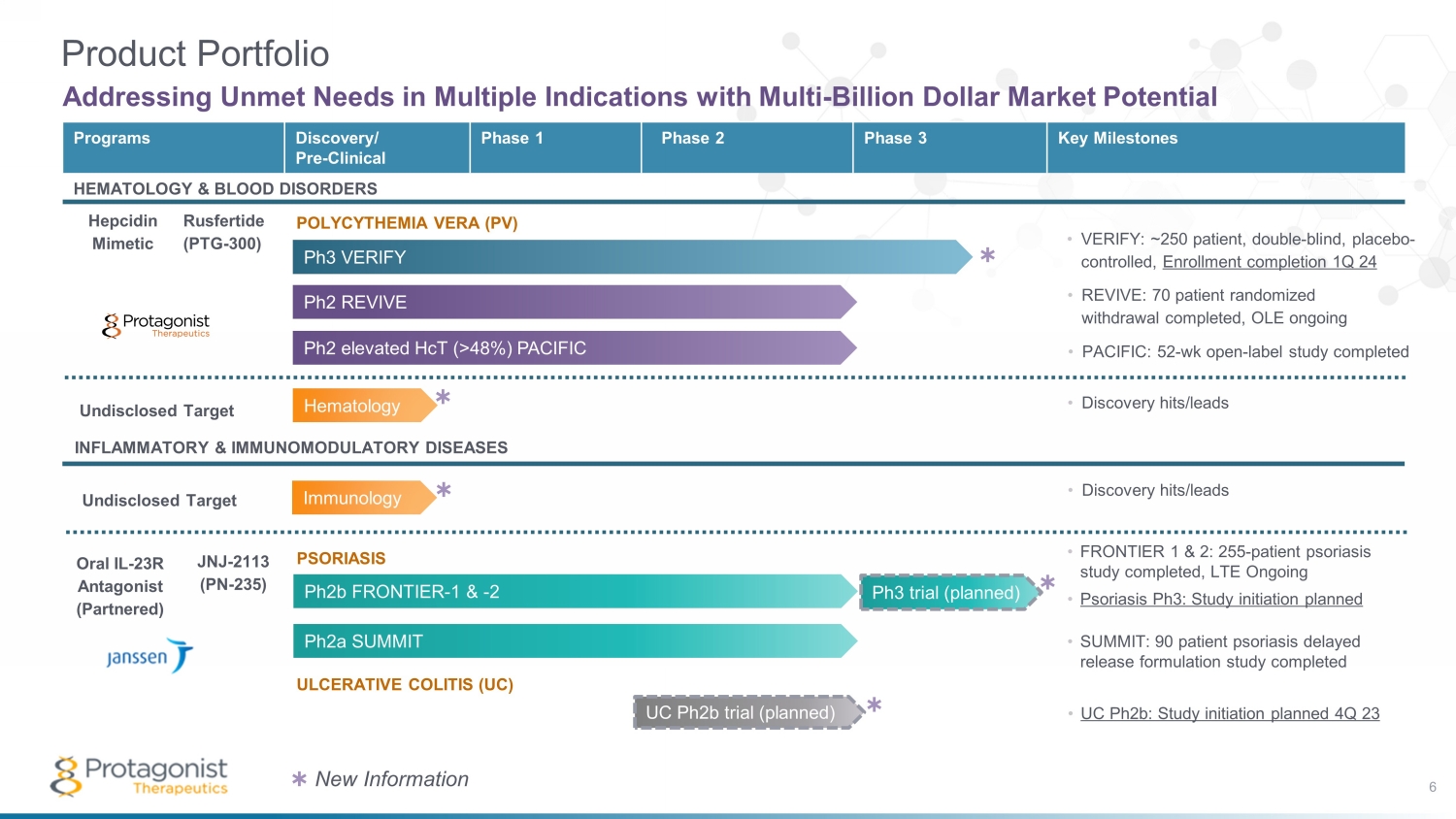
UC Ph2b trial (planned) INFLAMMATORY & IMMUNOMODULATORY DISEASES Oral IL - 23R Antagonist (Partnered) JNJ - 2113 (PN - 235) Hematology Ph3 trial (planned) Programs Discovery/ Pre - Clinical Phase 1 Phase 2 Phase 3 Key Milestones HEMATOLOGY & BLOOD DISORDERS Hepcidin Mimetic Rusfertide (PTG - 300) Ph2b FRONTIER - 1 & - 2 Product Portfolio Addressing Unmet Needs in Multiple Indications with Multi - Billion Dollar Market Potential Ph2a SUMMIT Ph2 elevated HcT (>48%) PACIFIC Ph2 REVIVE Ph3 VERIFY POLYCYTHEMIA VERA (PV) ULCERATIVE COLITIS (UC) PSORIASIS • FRONTIER 1 & 2: 255 - patient psoriasis study completed, LTE Ongoing • Psoriasis Ph3: Study initiation planned • VERIFY: ~250 patient, double - blind, placebo - controlled, Enrollment completion 1Q 24 • REVIVE: 70 patient randomized withdrawal completed, OLE ongoing • PACIFIC: 52 - wk open - label study completed • SUMMIT: 90 patient psoriasis delayed release formulation study completed • UC Ph2b: Study initiation planned 4Q 23 6 • D iscovery hits/leads • Discovery hits/leads Immunology ✱ ✱ ✱ ✱ ✱ New Information ✱ Undisclosed Target Undisclosed Target

Rusfertide (PTG - 300): Hepcidin Mimetic Addressing Unmet Needs in Polycythemia Vera
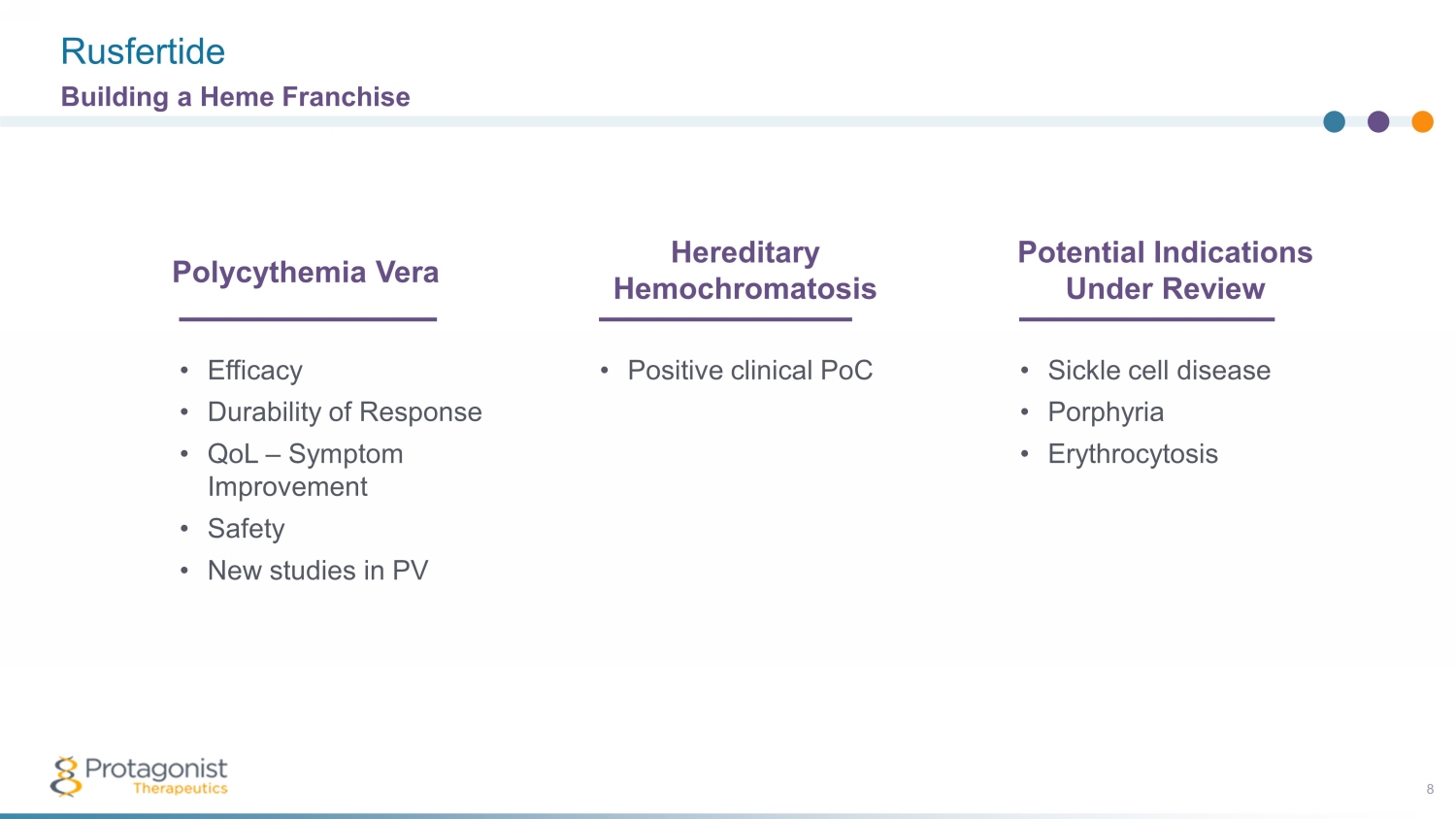
Rusfertide 8 Building a Heme Franchise • Efficacy • Durability of Response • QoL – Symptom Improvement • Safety • New studies in PV Polycythemia Vera • Positive clinical PoC Hereditary Hemochromatosis • Sickle cell disease • Porphyria • Erythrocytosis Potential Indications Under Review
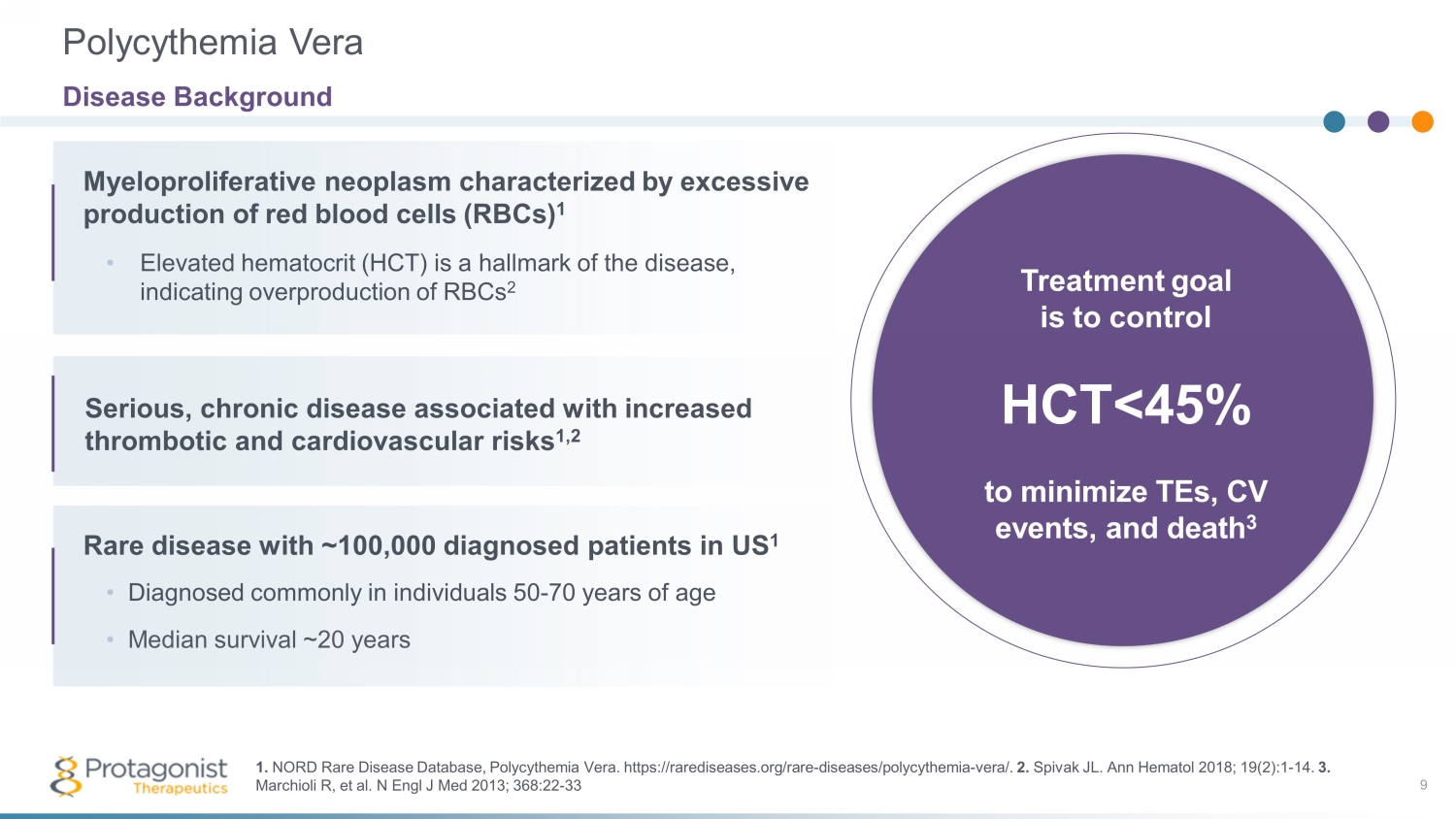
Myeloproliferative neoplasm characterized by excessive production of red blood cells ( RBCs) 1 Treatment goal is to control HCT <45% to minimize TEs, CV events, and death 3 9 • Elevated hematocrit (HCT) is a hallmark of the disease, indicating overproduction of RBCs 2 Serious, chronic disease associated with increased thrombotic and cardiovascular risks 1,2 Rare disease with ~100,000 diagnosed patients in US 1 • Diagnosed commonly in individuals 50 - 70 years of age • Median survival ~20 years Polycythemia Vera Disease Background 1. NORD Rare Disease Database, Polycythemia Vera. https://rarediseases.org/rare - diseases/polycythemia - vera/. 2. Spivak JL. Ann Hematol 2018; 19(2):1 - 14. 3. Marchioli R, et al. N Engl J Med 2013; 368:22 - 33
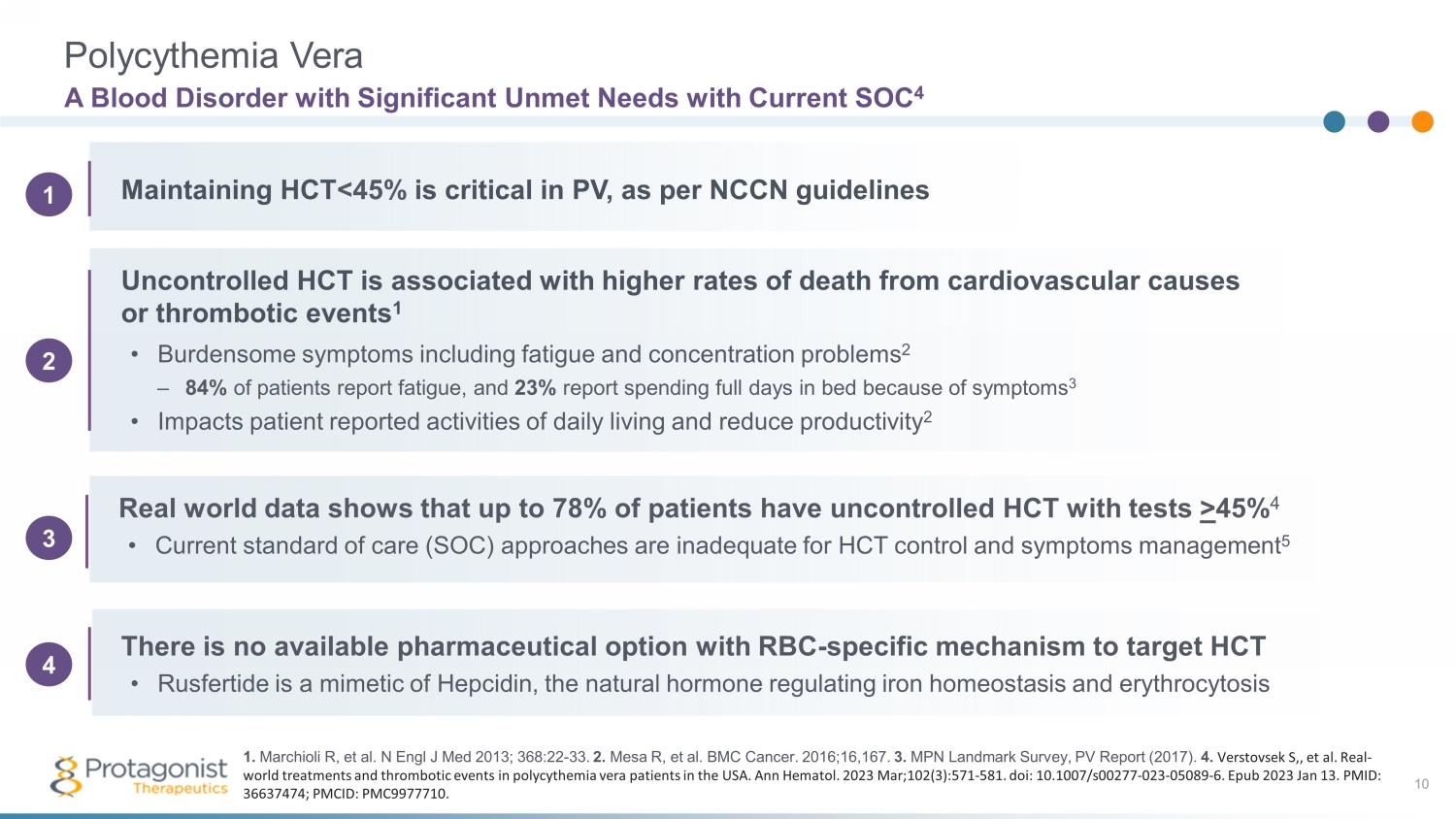
Polycythemia Vera • Burdensome symptoms including fatigue and concentration problems 2 – 84% of patients report fatigue, and 23% report spending full days in bed because of symptoms 3 • Impacts patient reported activities of daily living and reduce productivity 2 A Blood Disorder with Significant Unmet Needs with Current SOC 4 10 Maintaining HCT <45% is critical in PV, as per NCCN guidelines Uncontrolled HCT is associated with higher rates of death from cardiovascular causes or thrombotic events 1 Real world data shows that up to 78% of patients have uncontrolled HCT with tests > 45% 4 • Current standard of care (SOC) approaches are inadequate for HCT control and symptoms management 5 There is no available pharmaceutical option with RBC - specific mechanism to target HCT • Rusfertide is a mimetic of Hepcidin, the natural hormone regulating iron homeostasis and erythrocytosis 1. Marchioli R, et al. N Engl J Med 2013; 368:22 - 33. 2. Mesa R, et al. BMC Cancer. 2016;16,167. 3. MPN Landmark Survey, PV Report (2017). 4. Verstovsek S,, et al. Real - world treatments and thrombotic events in polycythemia vera patients in the USA. Ann Hematol . 2023 Mar;102(3):571 - 581. doi : 10.1007/s00277 - 023 - 05089 - 6. Epub 2023 Jan 13. PMID: 36637474; PMCID: PMC9977710. 2 1 3 4

Current Treatment Options for PV and Potential Limitations 11 Cytoreductive Therapies May Lead to Intolerance and/or Inadequate Hematocrit Control Hydroxyurea Hydrea ® Typically used as a first - line treatment in high - risk patients Patients still experience periods where HCT > 45% , 1 potentially resulting in dose increases and in need for phlebotomies Often discontinued due to blood counts, AEs, disease progression, or poor adherence 2 Risk of skin cancer 3 25% of patients become resistant or intolerant 2,4 Interferon Pegasys ®, Besremi ® Interferons have long been used off - label in treatment; Besremi is the first interferon product approved for PV 5 Slow onset of action, with a median time to response of 1.2 to 1.4 years 6 Failed to show noninferiority to HU at 12 months in the PROUD - PV study 7 Black box warning for serious neuropsychiatric, autoimmune, ischemic, and infectious disorders 6 Ruxolitinib Jakafi® Only approved for hydroxyurea - resistant or intolerant patients 7 Improves splenomegaly, a potential marker of disease progression 8 Potential serious side effects include thrombocytopenia, neutropenia, and anemia 7 Risk of skin cancer 7 23% of patients were found to have discontinued ruxolitinib within a mean of 2 years post treatment initiation 9 1 . Chornenki NLJ, et al. Leuk Res. 2018 Jul;70:62 - 66. 2 . Parasuraman S, et. al . Exp Hematol Oncol. 2016;5:3 3. Hydrea FDA label. 4 . Altomare I, et. al. Clin Lymphoma Myeloma Leuk. 2021;21(11):e915 - e921. 4 . Gilreath JA, et. al. Blood. 2018;132 (Supplement 1). 5 . Besremi FDA label. 6 . Gisslinger H, et. al. Lancet Haematol. 2020;7(3):e196 - e208. 7 . Jakafi FDA label. 8 . Coltoff R, et. al. Clin Lymphoma Myeloma Leuk. 2020;20(10): 697 - 703. 9. Tremblay, D. Leuk Res. 2021;109.
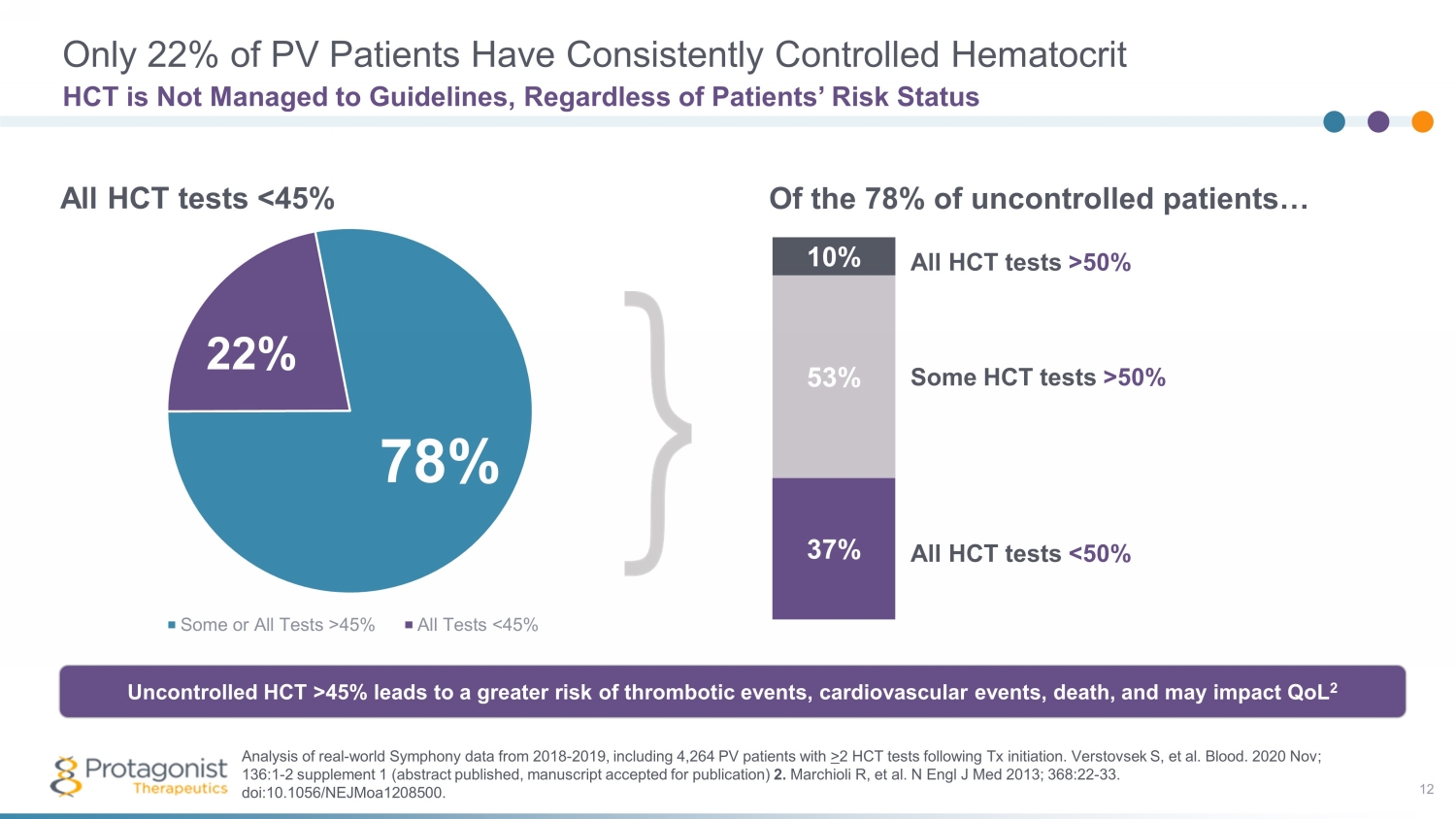
Only 22% of PV Patients Have Consistently Controlled Hematocrit 12 HCT is Not Managed to Guidelines, Regardless of Patients’ Risk Status Some or All Tests >45% All Tests <45% 22% 78% 85% 37% 53% 10% All HCT tests >50% Some HCT tests >50% All HCT tests <50% All HCT tests <45% Of the 78% of uncontrolled patients… Uncontrolled HCT >45% leads to a greater risk of thrombotic events, cardiovascular events, death, and may impact QoL 2 Analysis of real - world Symphony data from 2018 - 2019, including 4,264 PV patients with > 2 HCT tests following Tx initiation. Verstovsek S, et al. Blood. 2020 Nov; 136:1 - 2 supplement 1 (abstract published, m anuscript accepted for publication) 2. Marchioli R, et al. N Engl J Med 2013; 368:22 - 33. doi :10.1056/NEJMoa1208500 .
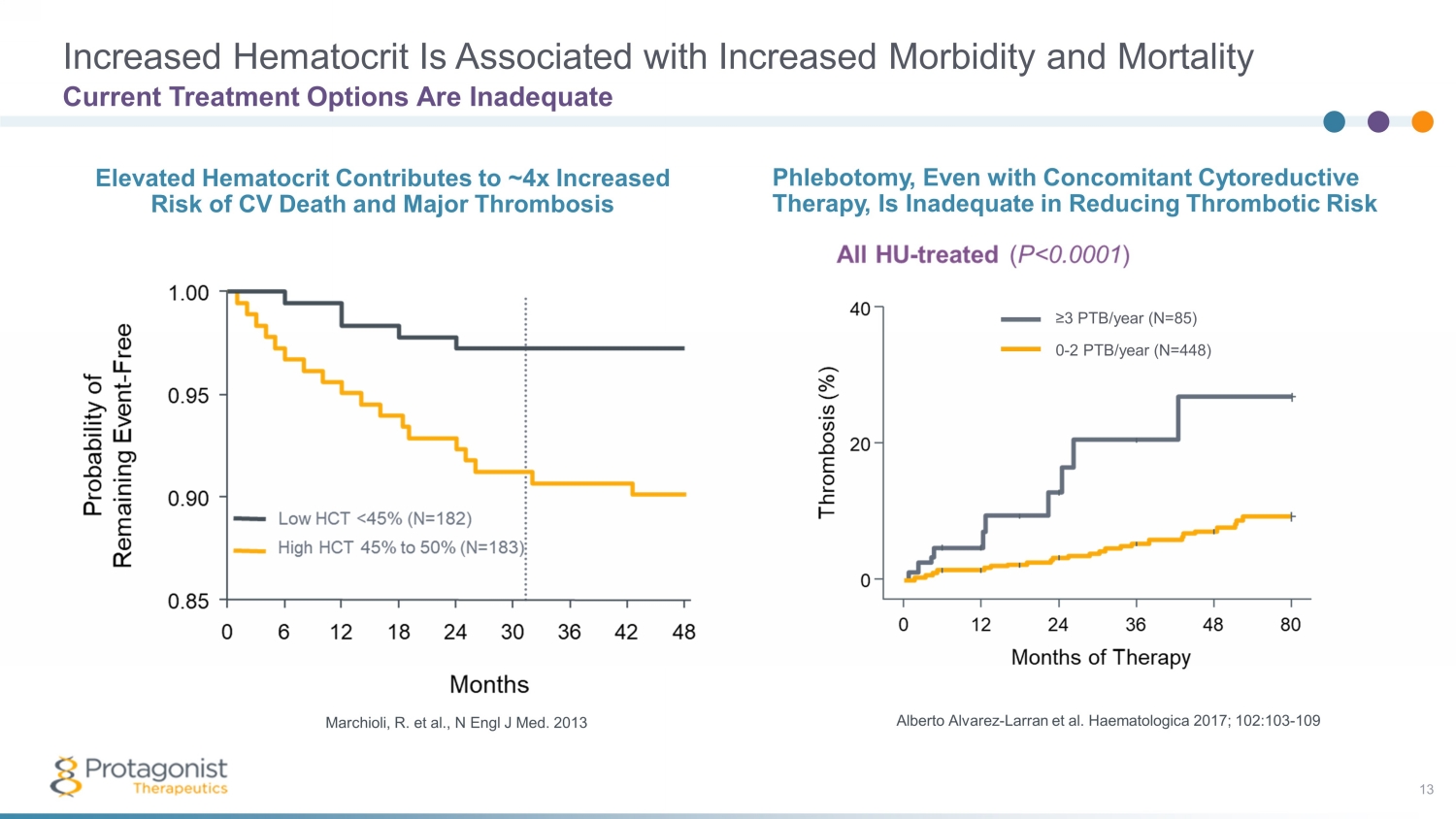
Increased Hematocrit Is Associated with Increased Morbidity and Mortality 13 Alberto Alvarez - Larran et al. Haematologica 2017; 102:103 - 109 Marchioli , R. et al., N Engl J Med. 2013 ≥3 PTB/year (N=85) 0 - 2 PTB/year (N=448) Phlebotomy, Even with Concomitant Cytoreductive Therapy, Is Inadequate in Reducing Thrombotic Risk Elevated Hematocrit Contributes to ~4x Increased Risk of CV Death and Major Thrombosis Current Treatment Options Are Inadequate
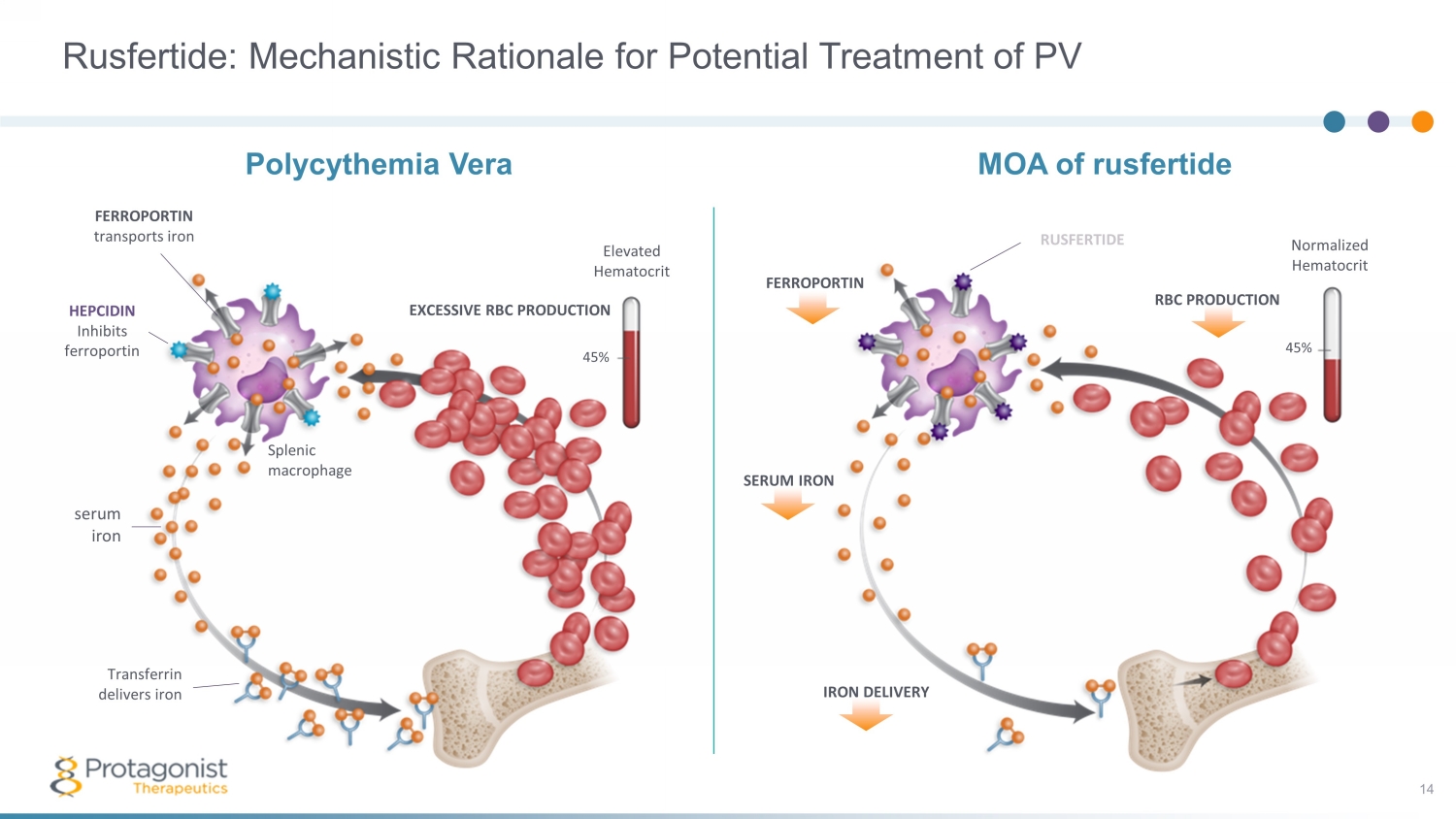
FERROPORTIN Normalized Hematocrit 45% SERUM IRON IRON DELIVERY MOA of rusfertide RBC PRODUCTION RUSFERTIDE Rusfertide: Mechanistic Rationale for Potential Treatment of PV 14 FERROPORTIN transports iron HEPCIDIN Inhibits ferroportin serum iron Transferrin delivers iron EXCESSIVE RBC PRODUCTION 45% Elevated Hematocrit Polycythemia Vera Splenic macrophage
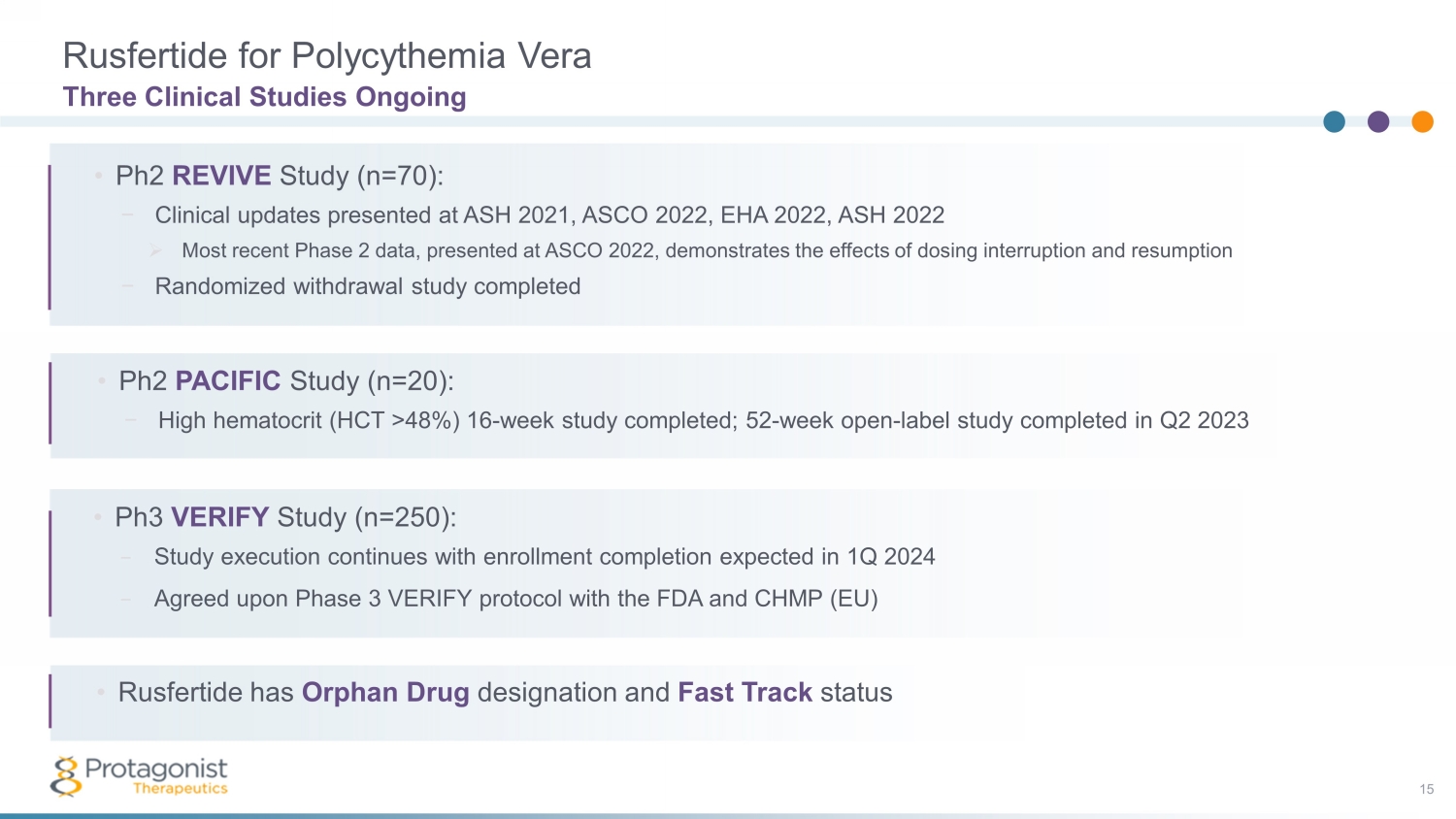
Rusfertide for Polycythemia Vera 15 Three Clinical Studies Ongoing • Ph3 VERIFY Study (n=250): − Study execution continues with enrollment completion expected in 1Q 2024 − Agreed upon Phase 3 VERIFY protocol with the FDA and CHMP (EU) • Phase 2 PACIFIC Study: − High hematocrit (HCT >48%) 16 - week study completed; subjects in • Ph2 REVIVE Study (n=70): − Clinical updates presented at ASH 2021, ASCO 2022, EHA 2022, ASH 2022 » Most recent Phase 2 data, presented at ASCO 2022, demonstrates the effects of dosing interruption and resumption − Randomized withdrawal study completed • Ph2 PACIFIC Study (n=20): − High hematocrit (HCT >48%) 16 - week study completed; 52 - week open - label study completed in Q2 2023 • Rusfertide has Orphan Drug designation and Fast Track status
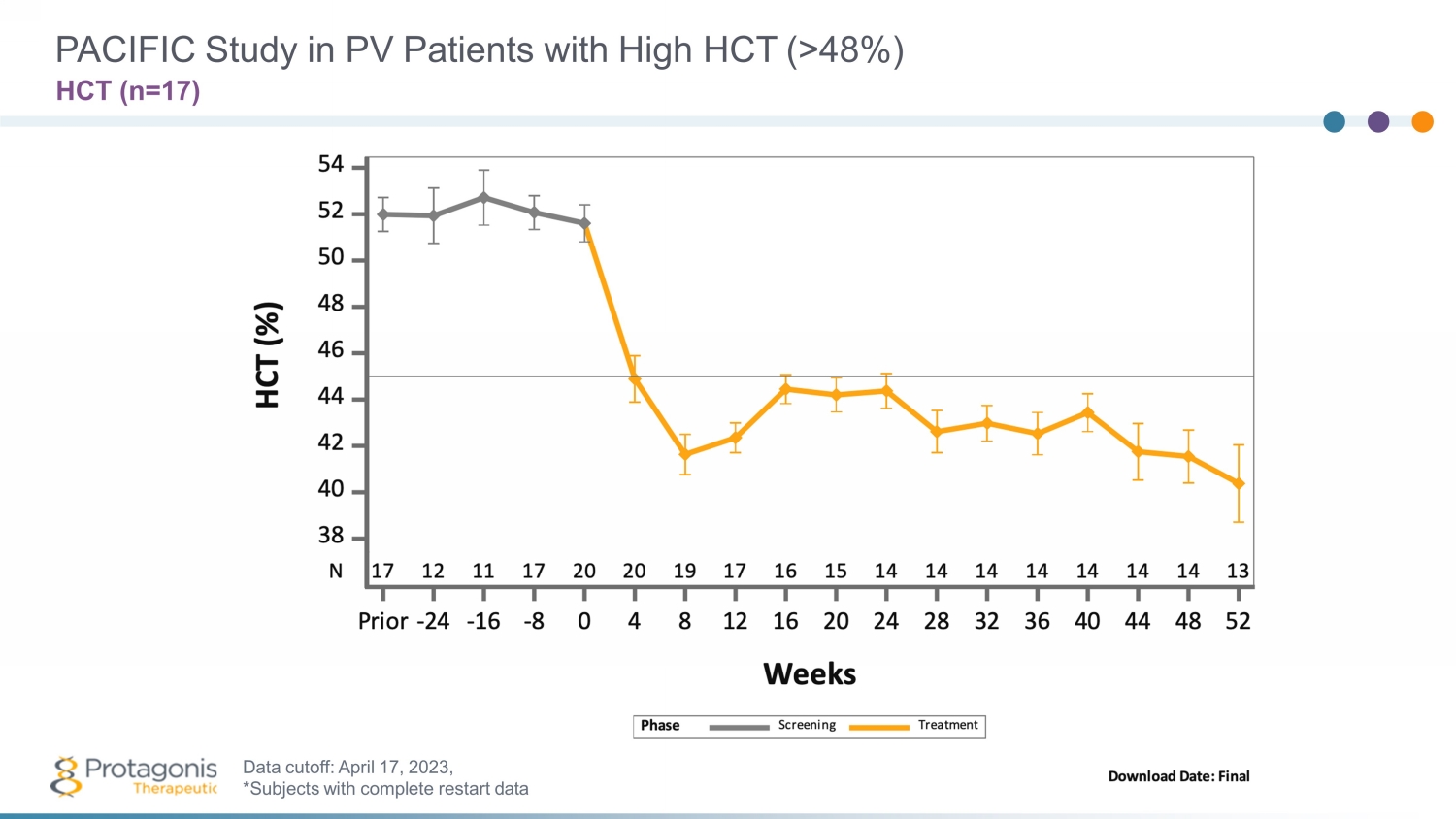
PACIFIC Study in PV Patients with High HCT (>48%) HCT (n=1 7 ) Data cutoff: April 1 7 , 202 3 , *Subjects with complete restart data
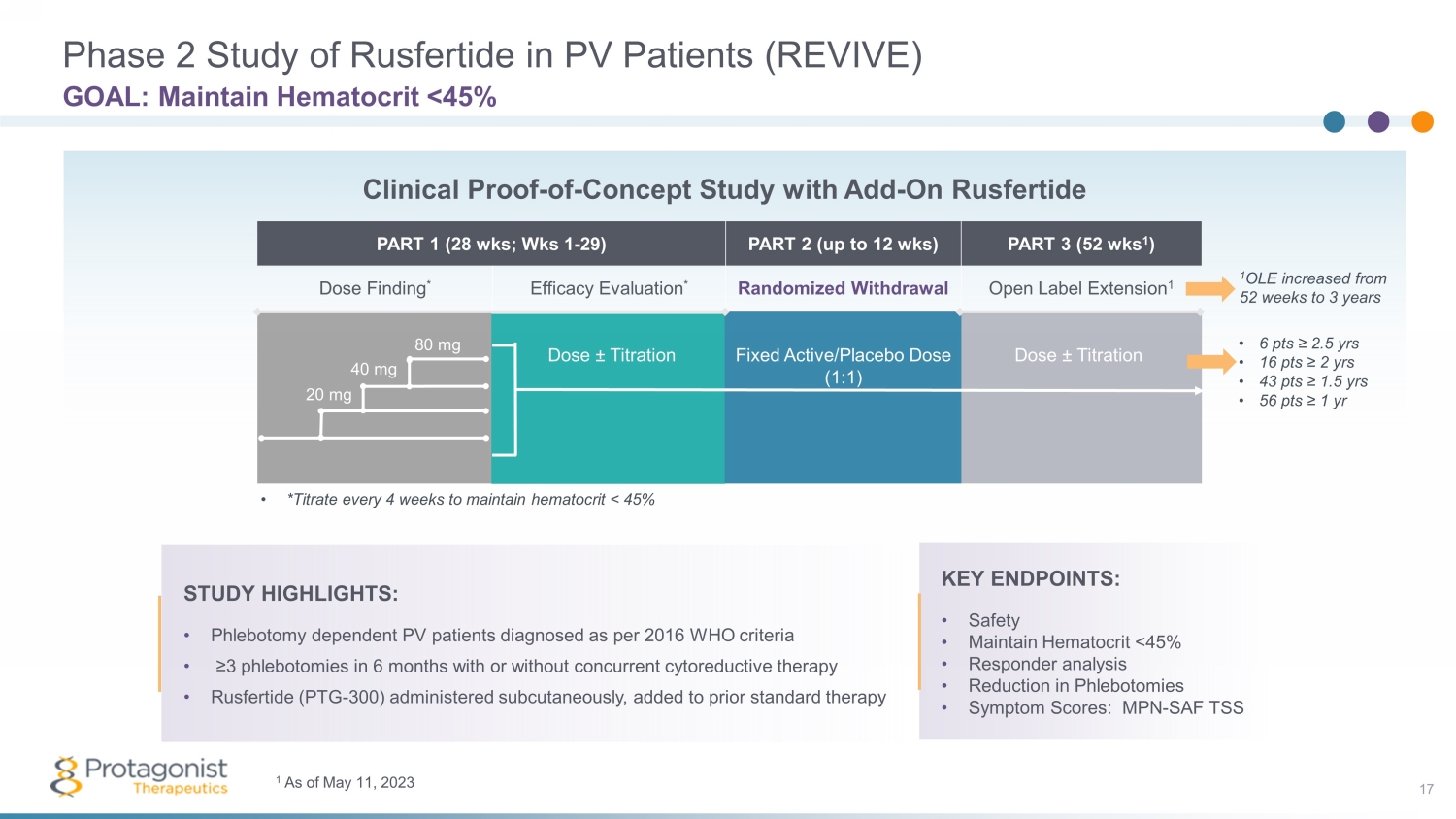
PART 1 (28 wks ; Wks 1 - 29) PART 2 (up to 12 wks ) PART 3 (52 wks 1 ) Dose Finding * Efficacy Evaluation * Randomized Withdrawal Open Label Extension 1 Phase 2 Study of Rusfertide in PV Patients (REVIVE) 17 GOAL: Maintain Hematocrit <45% STUDY HIGHLIGHTS: • Phlebotomy dependent PV patients diagnosed as per 2016 WHO criteria • ≥3 phlebotomies in 6 months with or without concurrent cytoreductive therapy • Rusfertide (PTG - 300) administered subcutaneously, added to prior standard therapy Dose ± Titration Fixed Active/Placebo Dose (1:1) Dose ± Titration • *Titrate every 4 weeks to maintain hematocrit < 45% 20 mg 40 mg 80 mg Clinical Proof - of - Concept Study with Add - On Rusfertide KEY ENDPOINTS: • Safety • Maintain Hematocrit <45% • Responder analysis • Reduction in Phlebotomies • Symptom Scores: MPN - SAF TSS 1 OLE increased from 52 weeks to 3 years • 6 pts ≥ 2.5 yrs • 16 pts ≥ 2 yrs • 43 pts ≥ 1.5 yrs • 56 pts ≥ 1 yr 1 As of May 11, 2023
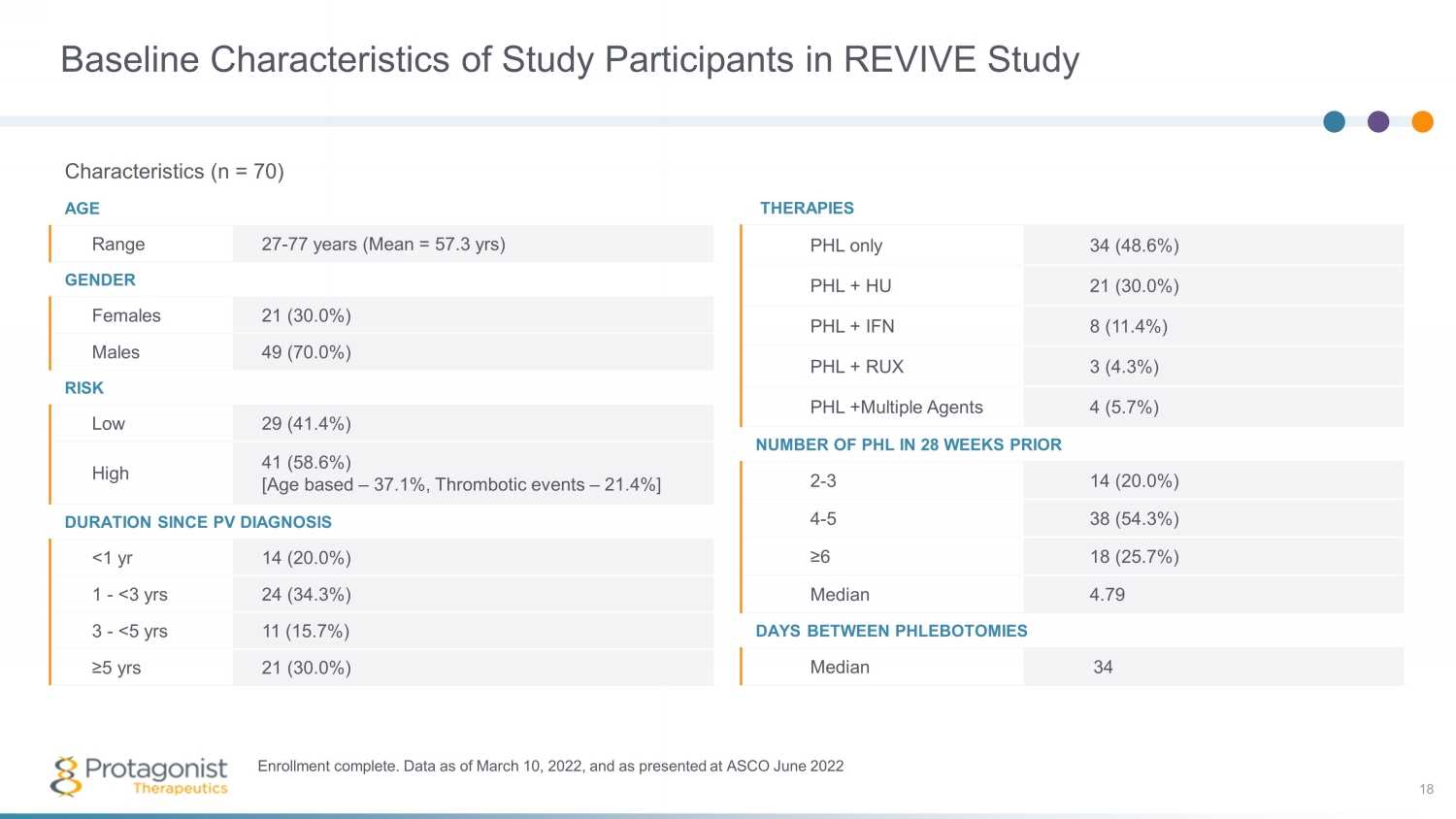
Baseline Characteristics of Study Participants in REVIVE Study Characteristics (n = 70) AGE Range 27 - 77 years (Mean = 57.3 yrs ) GENDER Females 21 (30.0%) Males 49 (70.0%) RISK Low 29 (41.4%) High 41 (58.6%) [Age based – 37.1%, Thrombotic events – 21.4%] DURATION SINCE PV DIAGNOSIS <1 yr 14 (20.0%) 1 - <3 yrs 24 (34.3%) 3 - <5 yrs 11 (15.7%) ≥5 yrs 21 (30.0%) Enrollment complete. Data as of March 10, 2022, and as presented at ASCO June 2022 THERAPIES PHL only 34 (48.6%) PHL + HU 21 (30.0%) PHL + IFN 8 (11.4%) PHL + RUX 3 (4.3%) PHL +Multiple Agents 4 (5.7%) NUMBER OF PHL IN 28 WEEKS PRIOR 2 - 3 14 (20.0%) 4 - 5 38 (54.3%) ≥6 18 (25.7%) Median 4.79 DAYS BETWEEN PHLEBOTOMIES Median 34 18
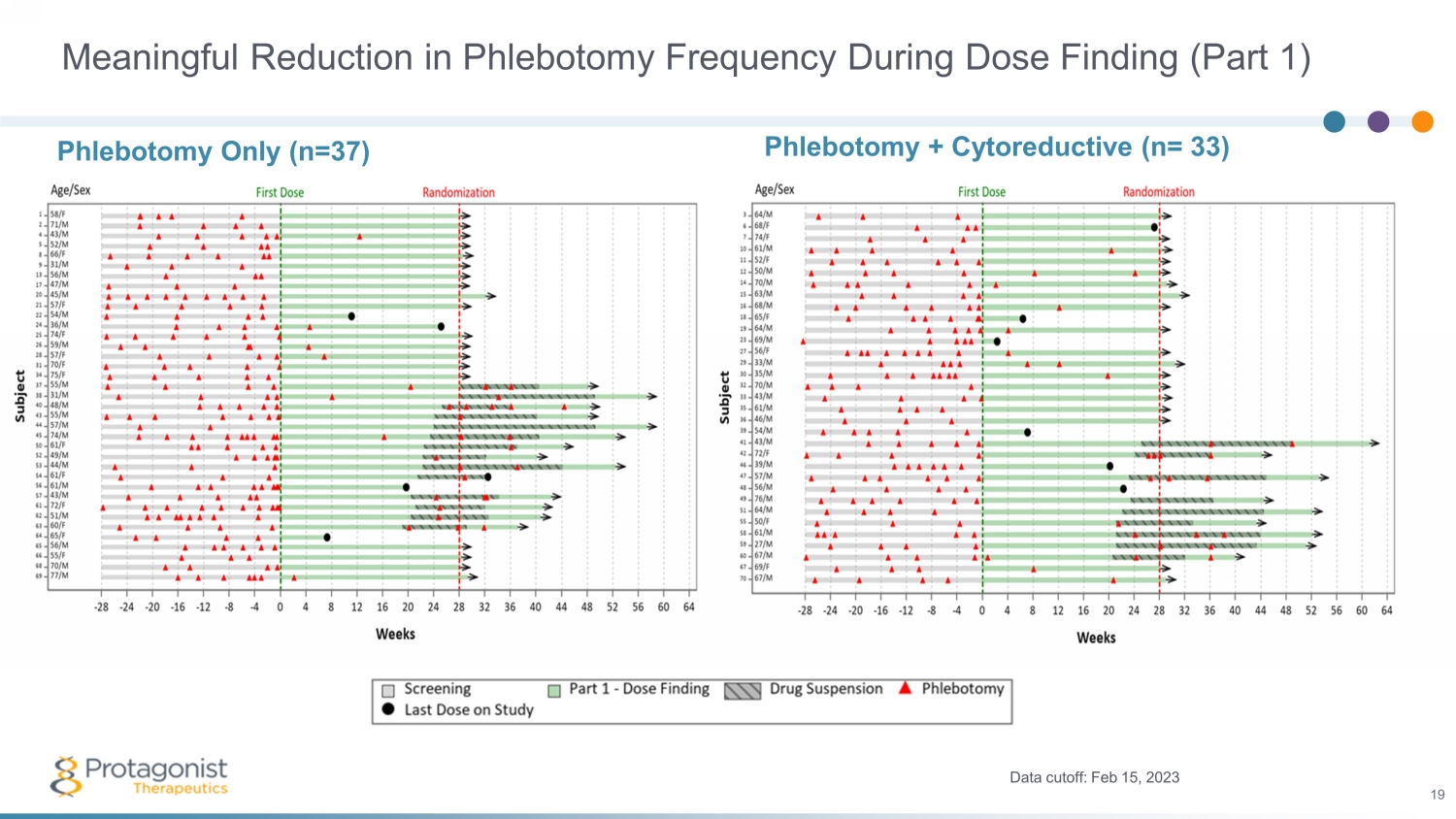
Meaningful Reduction in Phlebotomy Frequency During Dose Finding (Part 1) Phlebotomy + Cytoreductive (n= 33) Data cutoff: Feb 15, 2023 19 Phlebotomy Only (n=37)

PART 1 (28 wks ; Wks 1 - 29) PART 2 (up to 12 wks ) PART 3 (52 wks 1 ) Dose Finding * Efficacy Evaluation * Randomized Withdrawal Open Label Extension 1 Unblinding of the Randomized Withdrawal, Part 2 of the REVIVE Study 20 Highly Statistically Significant Results Dose ± Titration Fixed Active/Placebo Dose (1:1) Dose ± Titration • *Titrate every 4 weeks to maintain hematocrit < 45% 20 mg 40 mg 80 mg Clinical Proof - of - Concept Study with Add - On KEY ENDPOINTS: • Safety • Maintain Hematocrit <45% • Responder analysis • Reduction in Phlebotomies • Symptom Scores: MPN - SAF TSS 1 OLE increased from 52 weeks to 3 years • 6 pts ≥ 2.5 yrs 1 • 16 pts ≥ 2 yrs • 43 pts ≥ 1.5 yrs • 56 pts ≥ 1 yr 1 As of May 11, 2023 STUDY HIGHLIGHTS: • Phlebotomy dependent PV patients diagnosed as per 2016 WHO criteria • ≥3 phlebotomies in 6 months with or without concurrent cytoreductive therapy • Rusfertide (PTG - 300) administered subcutaneously, added to prior standard therapy
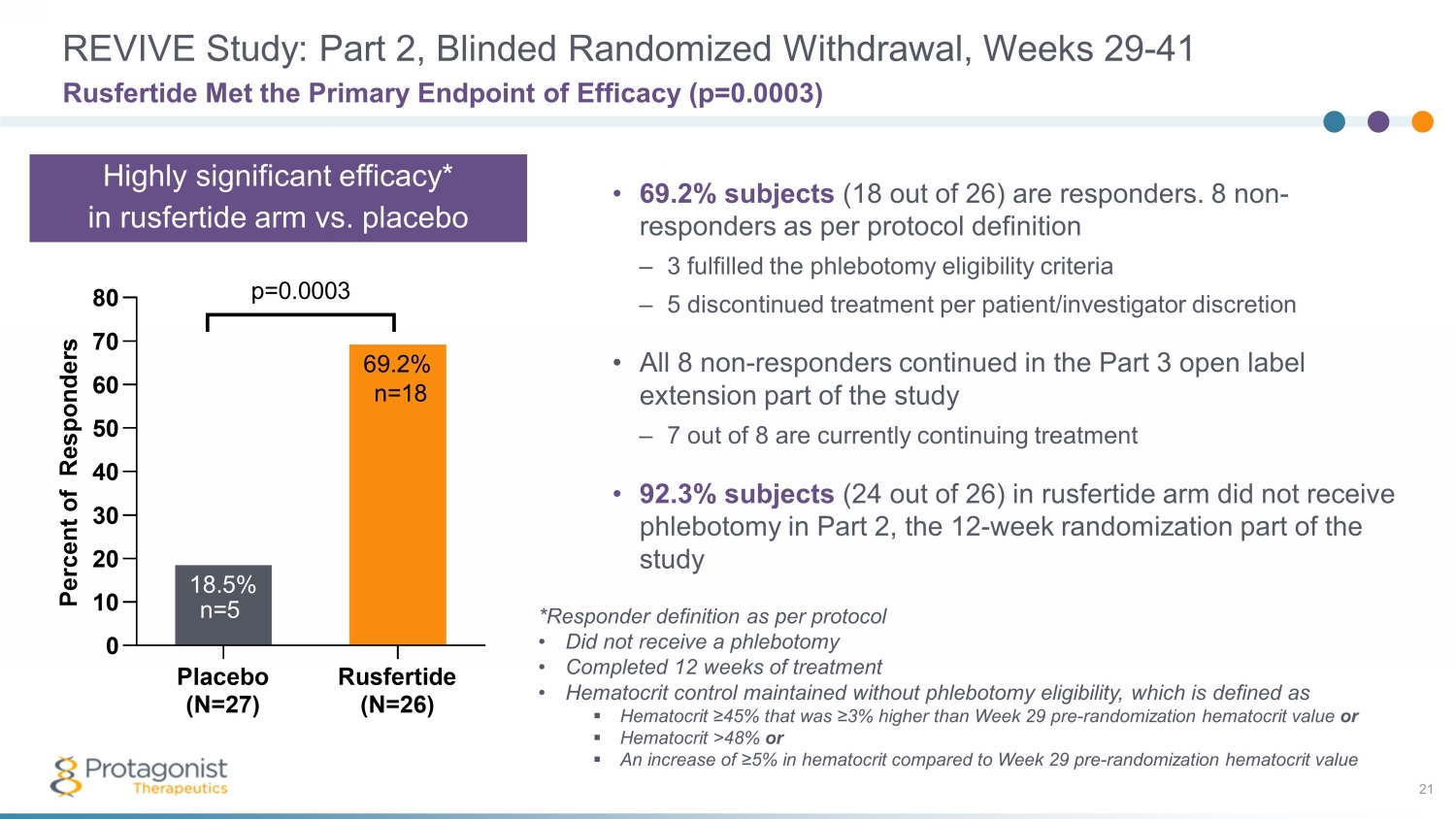
REVIVE Study: Part 2, Blinded Randomized Withdrawal, Weeks 29 - 41 21 • 69.2% subjects (18 out of 26) are responders. 8 non - responders as per protocol definition – 3 fulfilled the phlebotomy eligibility criteria – 5 discontinued treatment per patient/investigator discretion • All 8 non - responders continued in the Part 3 open label extension part of the study – 7 out of 8 are currently continuing treatment • 92.3% subjects (24 out of 26) in rusfertide arm did not receive phlebotomy in Part 2, the 12 - week randomization part of the study Rusfertide Met the Primary Endpoint of Efficacy (p=0.0003) Placebo (N=27) Rusfertide (N=26) 0 10 20 30 40 50 60 70 80 69.2% 18.5% P e r c e n t o f R e s p o n d e r s p=0.0003 n=5 n=18 *Responder definition as per protocol • Did not receive a phlebotomy • Completed 12 weeks of treatment • Hematocrit control maintained without phlebotomy eligibility, which is defined as ▪ Hematocrit ≥45% that was ≥3% higher than Week 29 pre - randomization hematocrit value or ▪ Hematocrit >48% or ▪ An increase of ≥5% in hematocrit compared to Week 29 pre - randomization hematocrit value Highly significant efficacy* in rusfertide arm vs. placebo
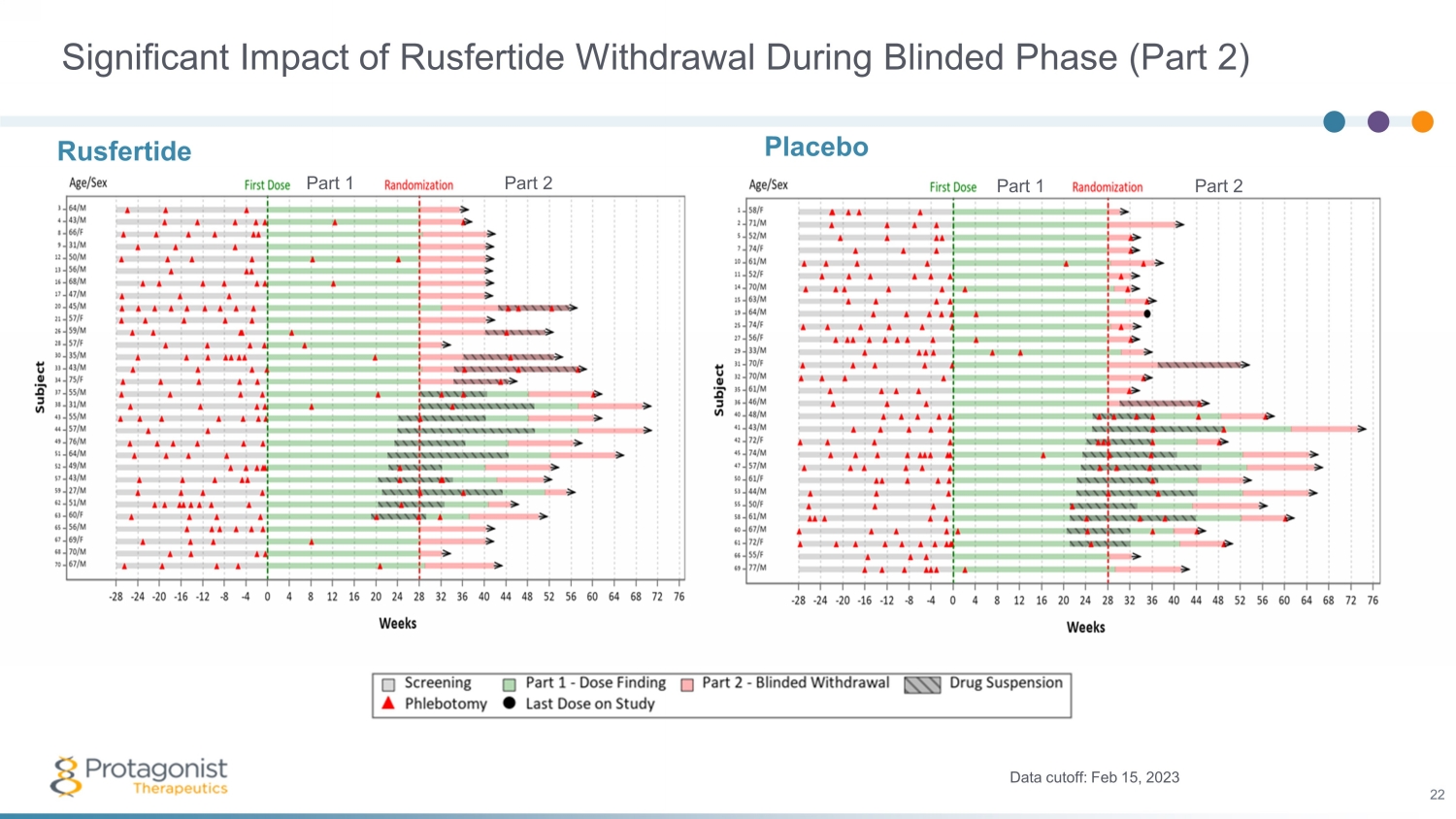
Significant Impact of Rusfertide Withdrawal During Blinded Phase (Part 2) Placebo Data cutoff: Feb 15, 2023 22 Rusfertide Part 1 Part 2 Part 1 Part 2
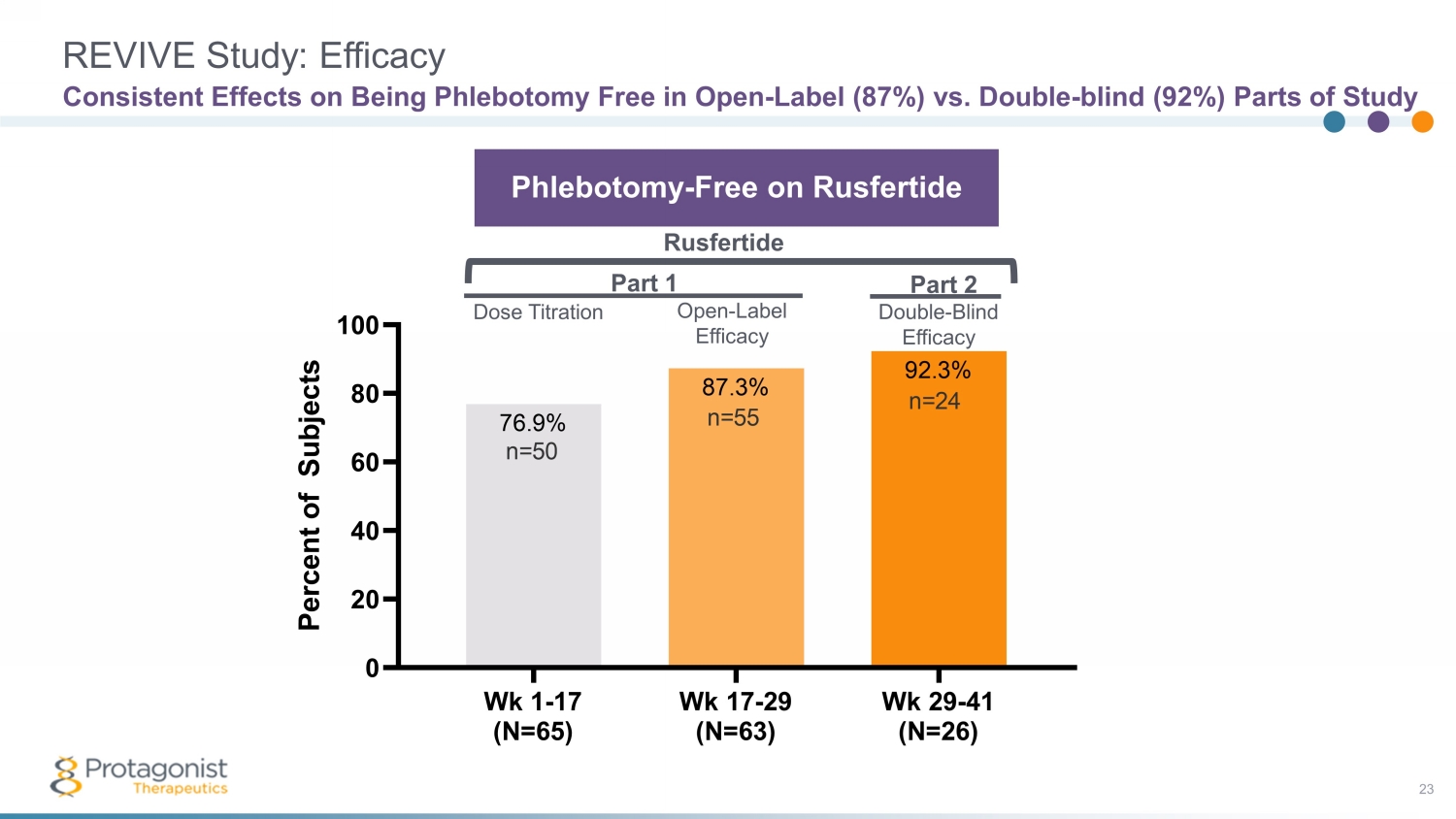
REVIVE Study: Efficacy 23 Consistent Effects on Being Phlebotomy Free in Open - Label (87%) vs. Double - blind (92%) Parts of Study Wk 1-17 (N=65) Wk 17-29 (N=63) Wk 29-41 (N=26) 0 20 40 60 80 100 92.3% 87.3% 76.9% P e r c e n t o f S u b j e c t s n=24 n=50 n=55 Part 1 Part 2 Phlebotomy - Free on Rusfertide Rusfertide Dose Titration Open - Label Efficacy Double - Blind Efficacy

REVIVE Study: Consistent Efficacy 24 Rusfertide Significantly Delays Time to Event on Multiple Outcomes Compared to Placebo 28 30 32 34 36 38 40 42 0 20 40 60 80 100 Responder Weeks P e r c e n t o f S u b j e c t s P <0.0001 P <0.0001 28 30 32 34 36 38 40 42 0 20 40 60 80 100 Absence of PHL Eligibility Weeks P e r c e n t o f S u b j e c t s P <0.0001 Responder Absence of PHL Eligibility HCT <45%
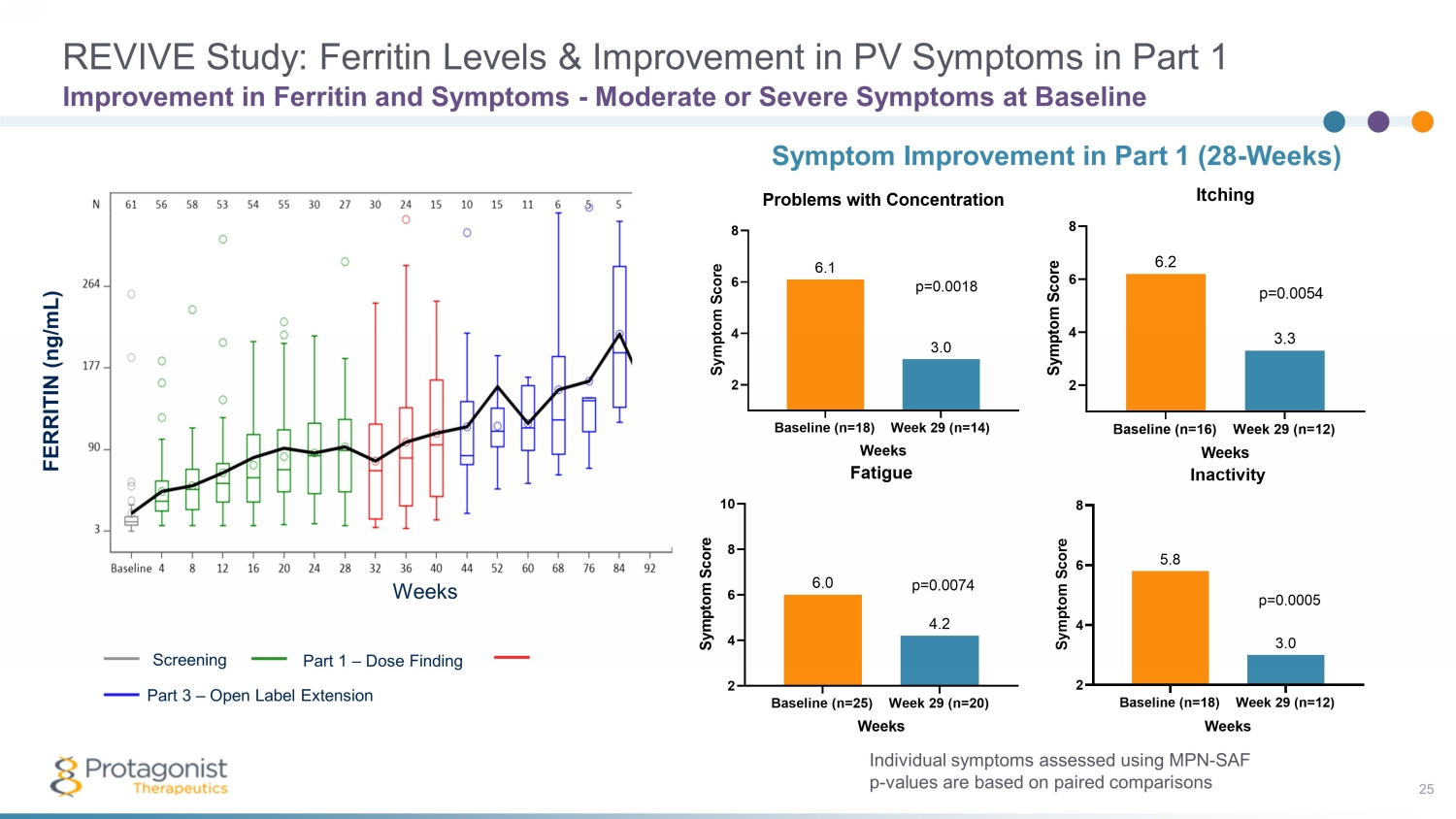
REVIVE Study: Ferritin Levels & Improvement in PV Symptoms in Part 1 25 Improvement in Ferritin and Symptoms - Moderate or Severe Symptoms at Baseline Individual symptoms assessed using MPN - SAF p - values are based on paired comparisons Symptom Improvement in Part 1 (28 - Weeks) Baseline (n=25) Week 29 (n=20) 2 4 6 8 10 4.2 6.0 Fatigue Weeks S y m p t o m S c o r e p=0.0074 Baseline (n=18) Week 29 (n=14) 2 4 6 8 3.0 6.1 Problems with Concentration Weeks S y m p t o m S c o r e p=0.0018 Screening Part 3 – Open Label Extension Part 1 – Dose Finding Weeks FERRITIN (ng/mL)
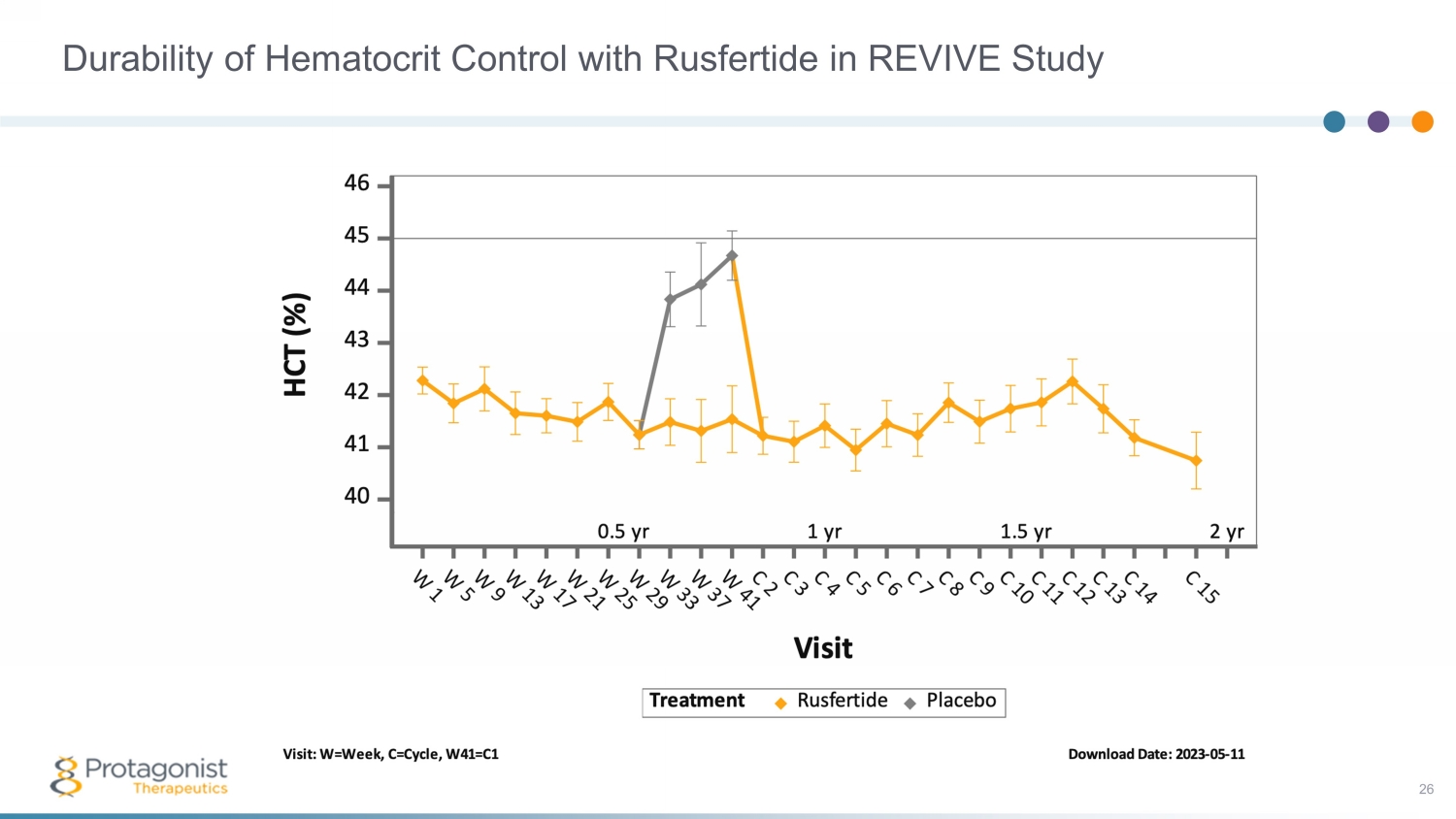
Durability of Hematocrit Control with Rusfertide in REVIVE Study 26
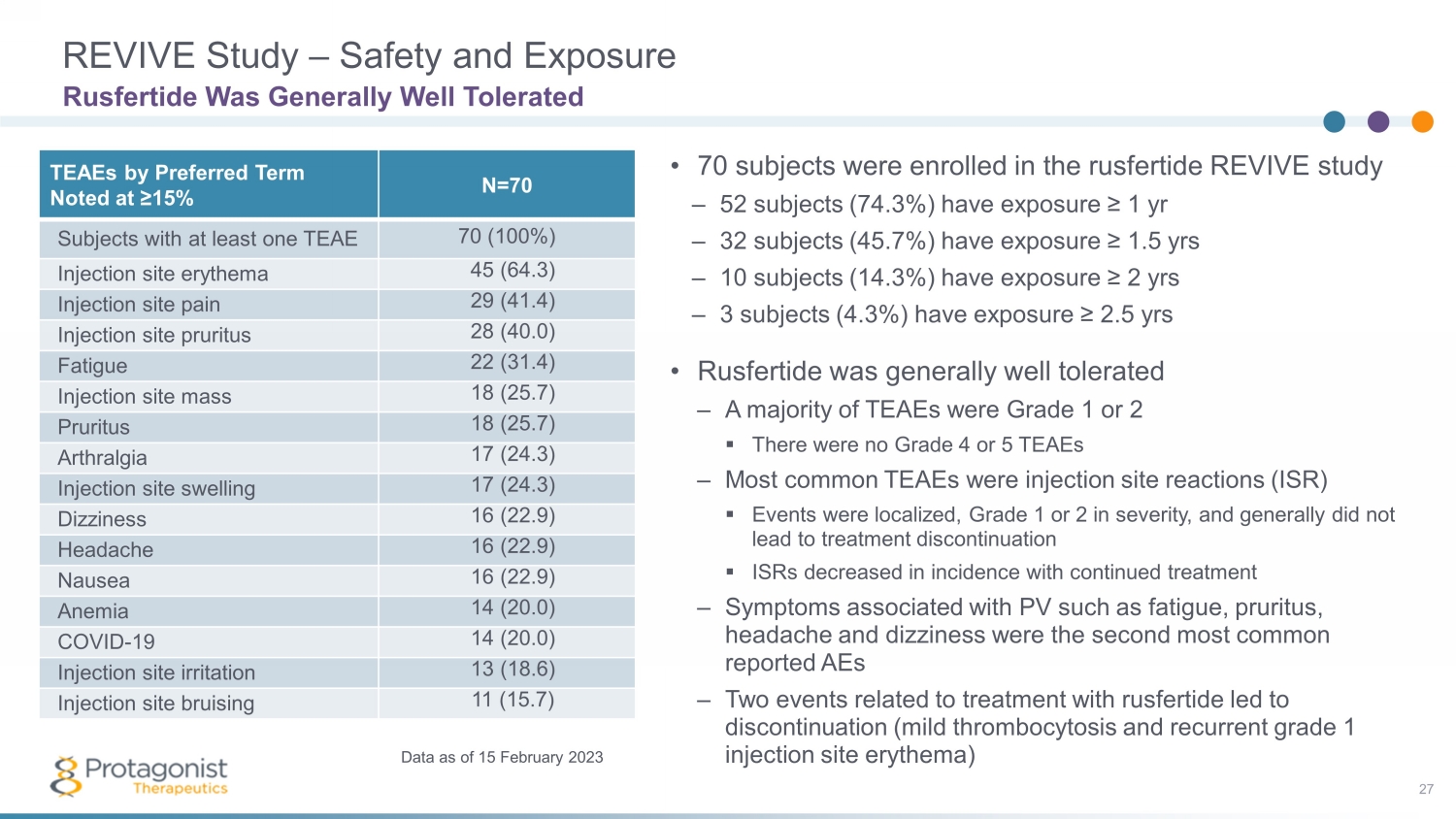
REVIVE Study – Safety and Exposure 27 • 70 subjects were enrolled in the rusfertide REVIVE study – 52 subjects (74.3%) have exposure ≥ 1 yr – 32 subjects (45.7%) have exposure ≥ 1.5 yrs – 10 subjects (14.3%) have exposure ≥ 2 yrs – 3 subjects (4.3%) have exposure ≥ 2.5 yrs • Rusfertide was generally well tolerated – A majority of TEAEs were Grade 1 or 2 ▪ There were no Grade 4 or 5 TEAEs – Most common TEAEs were injection site reactions (ISR) ▪ Events were localized, Grade 1 or 2 in severity, and generally did not lead to treatment discontinuation ▪ ISRs decreased in incidence with continued treatment – Symptoms associated with PV such as fatigue, pruritus, headache and dizziness were the second most common reported AEs – Two events related to treatment with rusfertide led to discontinuation (mild thrombocytosis and recurrent grade 1 injection site erythema) Rusfertide Was Generally Well Tolerated TEAEs by Preferred Term Noted at ≥15% N=70 Subjects with at least one TEAE 70 (100%) Injection site erythema 45 (64.3) Injection site pain 29 (41.4) Injection site pruritus 28 (40.0) Fatigue 22 (31.4) Injection site mass 18 (25.7) Pruritus 18 (25.7) Arthralgia 17 (24.3) Injection site swelling 17 (24.3) Dizziness 16 (22.9) Headache 16 (22.9) Nausea 16 (22.9) Anemia 14 (20.0) COVID - 19 14 (20.0) Injection site irritation 13 (18.6) Injection site bruising 11 (15.7) Data as of 15 February 2023
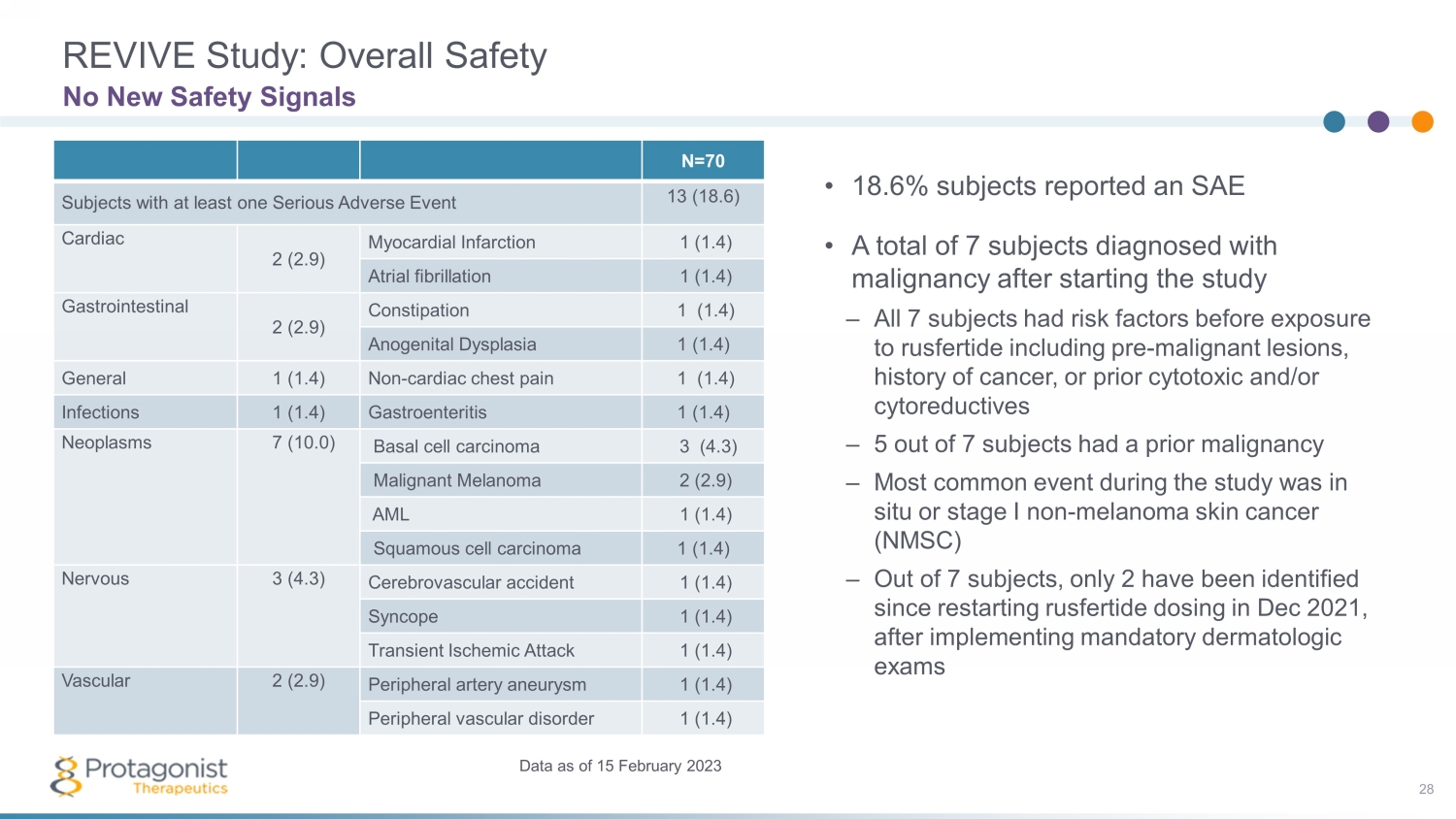
REVIVE Study: Overall Safety 28 No New Safety Signals Data as of 15 February 2023 • 18.6% subjects reported an SAE • A total of 7 subjects diagnosed with malignancy after starting the study – All 7 subjects had risk factors before exposure to rusfertide including pre - malignant lesions, history of cancer, or prior cytotoxic and/or cytoreductives – 5 out of 7 subjects had a prior malignancy – Most common event during the study was in situ or stage I non - melanoma skin cancer (NMSC) – Out of 7 subjects, only 2 have been identified since restarting rusfertide dosing in Dec 2021, after implementing mandatory dermatologic exams N=70 Subjects with at least one Serious Adverse Event 13 (18.6) Cardiac 2 (2.9) Myocardial Infarction 1 (1.4) Atrial fibrillation 1 (1.4) Gastrointestinal 2 (2.9) Constipation 1 (1.4) Anogenital Dysplasia 1 (1.4) General 1 (1.4) Non - cardiac chest pain 1 (1.4) Infections 1 (1.4) Gastroenteritis 1 (1.4) Neoplasms 7 (10.0) Basal cell carcinoma 3 (4.3) Malignant Melanoma 2 (2.9) AML 1 (1.4) Squamous cell carcinoma 1 (1.4) Nervous 3 (4.3) Cerebrovascular accident 1 (1.4) Syncope 1 (1.4) Transient Ischemic Attack 1 (1.4) Vascular 2 (2.9) Peripheral artery aneurysm 1 (1.4) Peripheral vascular disorder 1 (1.4)
• Rapid HCT control (<45%) without phlebotomy in high HCT (>48%) PACIFIC study • Rusfertide treatment with or without cytoreductives appears to be well tolerated – Safety update presented at ASH, December 2022; no new safety signals observed 1 • ~250 patient, randomized, placebo - controlled Ph3 VERIFY study to confirm efficacy and safety – Execution underway, enrollment completion by 1Q 2024 Rusfertide Summary 29 An Investigational Injectable Hepcidin Mimetic for Treatment of Polycythemia Vera • PV patients requiring frequent phlebotomy + cytoreductives have been treated with rusfertide for >2 years in the REVIVE study, with subjects remaining essentially phlebotomy free – Rapid, sustained and durable hematocrit control – Robust efficacy in all categories of patients – Rusfertide dosing was interrupted and led to loss of effect; restart restored therapeutic benefits – Positive improvements in symptom scores – 53 patients, 1:1 randomization part 2 of the study completed 1. Pemmaraju, Naveen, et al. "Subgroup Analysis of Adverse Events Following Rusfertide Dosing in Revive: A Phase 2 Study of Pati ent s with Polycythemia Vera." Blood 140.Supplement 1 (2022): 6835 - 6837.
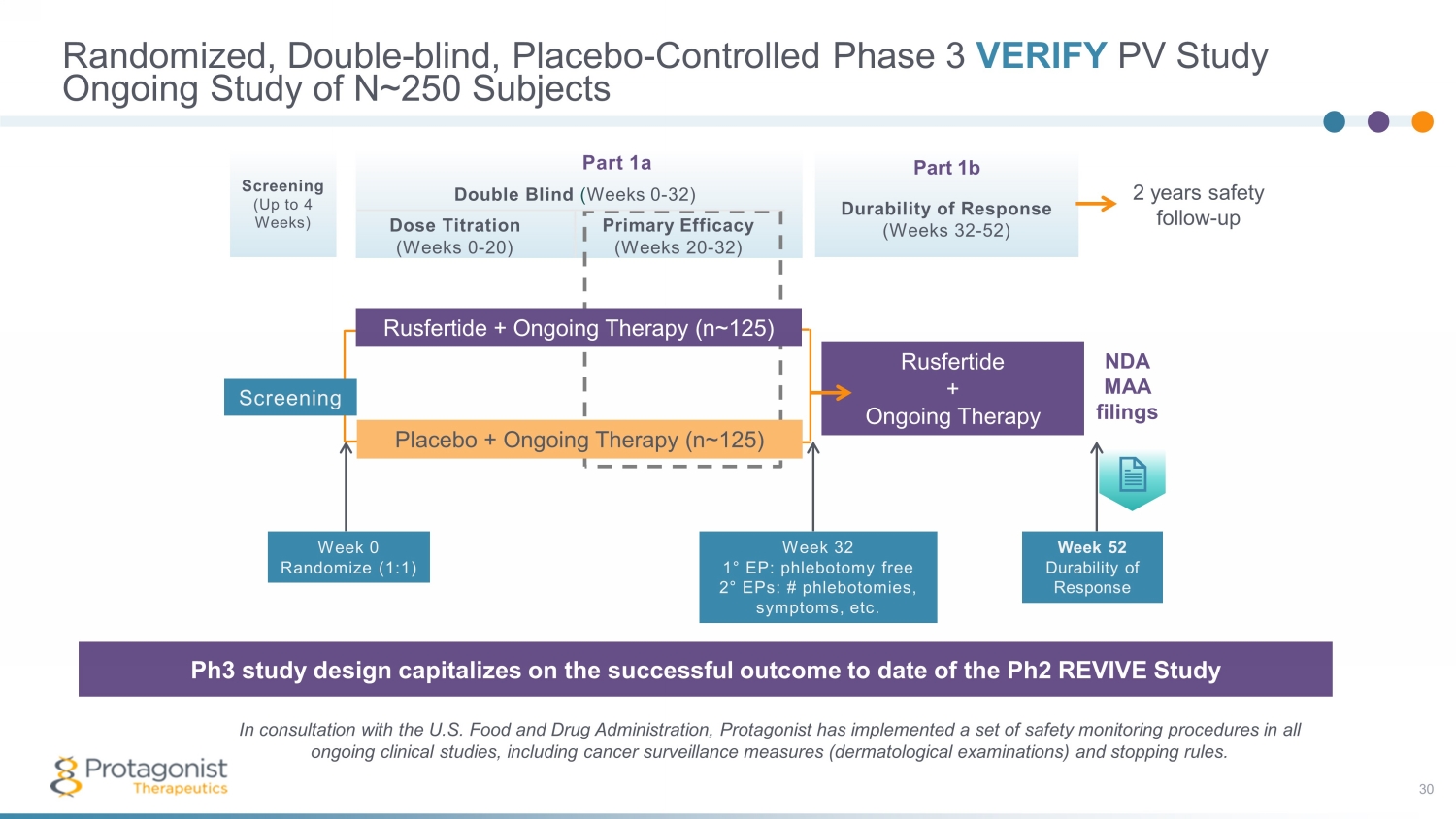
Part 1a Double Blind ( Weeks 0 - 32) Dose Titration Primary Efficacy (Weeks 0 - 20) (Weeks 20 - 32) Part 1b Durability of Response (Weeks 32 - 52) Screening (Up to 4 Weeks) Randomized, Double - blind, Placebo - Controlled Phase 3 VERIFY PV Study 30 Ongoing Study of N~250 Subjects The Phase 3 study design capitalizes on the successful outcome of the 60 - plus patient open - label Phase 2 REVIVE Study In consultation with the U.S. Food and Drug Administration, Protagonist has implemented a set of safety monitoring procedures in all ongoing clinical studies, including cancer surveillance measures (dermatological examinations) and stopping rules. Ph3 study design capitalizes on the successful outcome to date of the Ph2 REVIVE Study Week 32 1 ° EP: phlebotomy free 2 ° EPs: # phlebotomies, symptoms, etc. Placebo + Ongoing Therapy (n~ 125 ) Rusfertide + Ongoing Therapy Rusfertide + Ongoing Therapy (n~ 125 ) Week 52 Durability of Response NDA MAA filings Week 0 Randomize (1:1) Screening 2 years safety follow - up
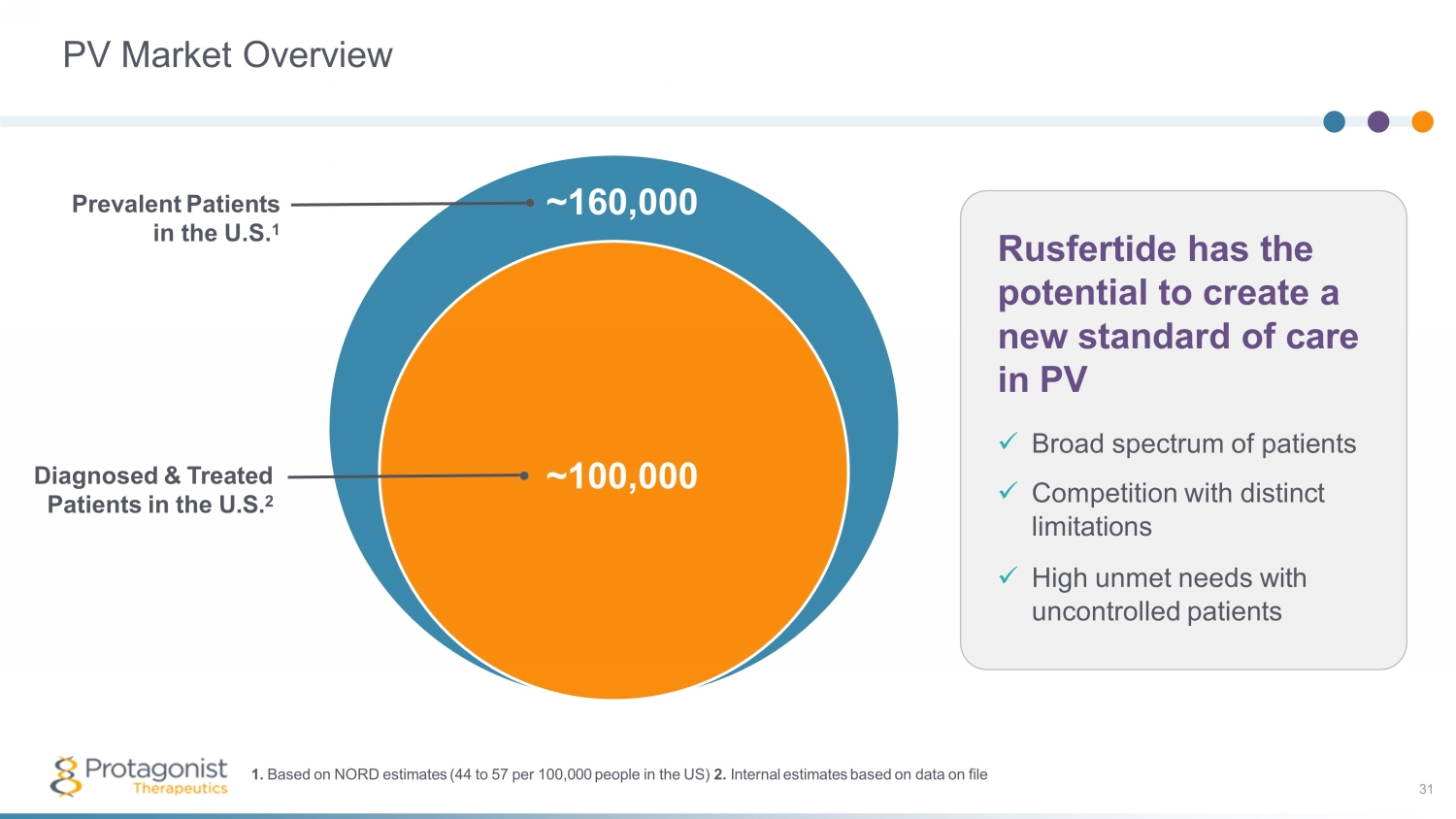
PV Market Overview ~100,000 Diagnosed & Treated Patients in the U.S. 2 Rusfertide has the potential to create a new standard of care in PV x Broad spectrum of patients x Competition with distinct limitations x High unmet needs with uncontrolled patients ~ 160,000 Prevalent Patients in the U.S. 1 1. Based on NORD estimates (44 to 57 per 100,000 people in the US) 2. Internal estimates based on data on file 31
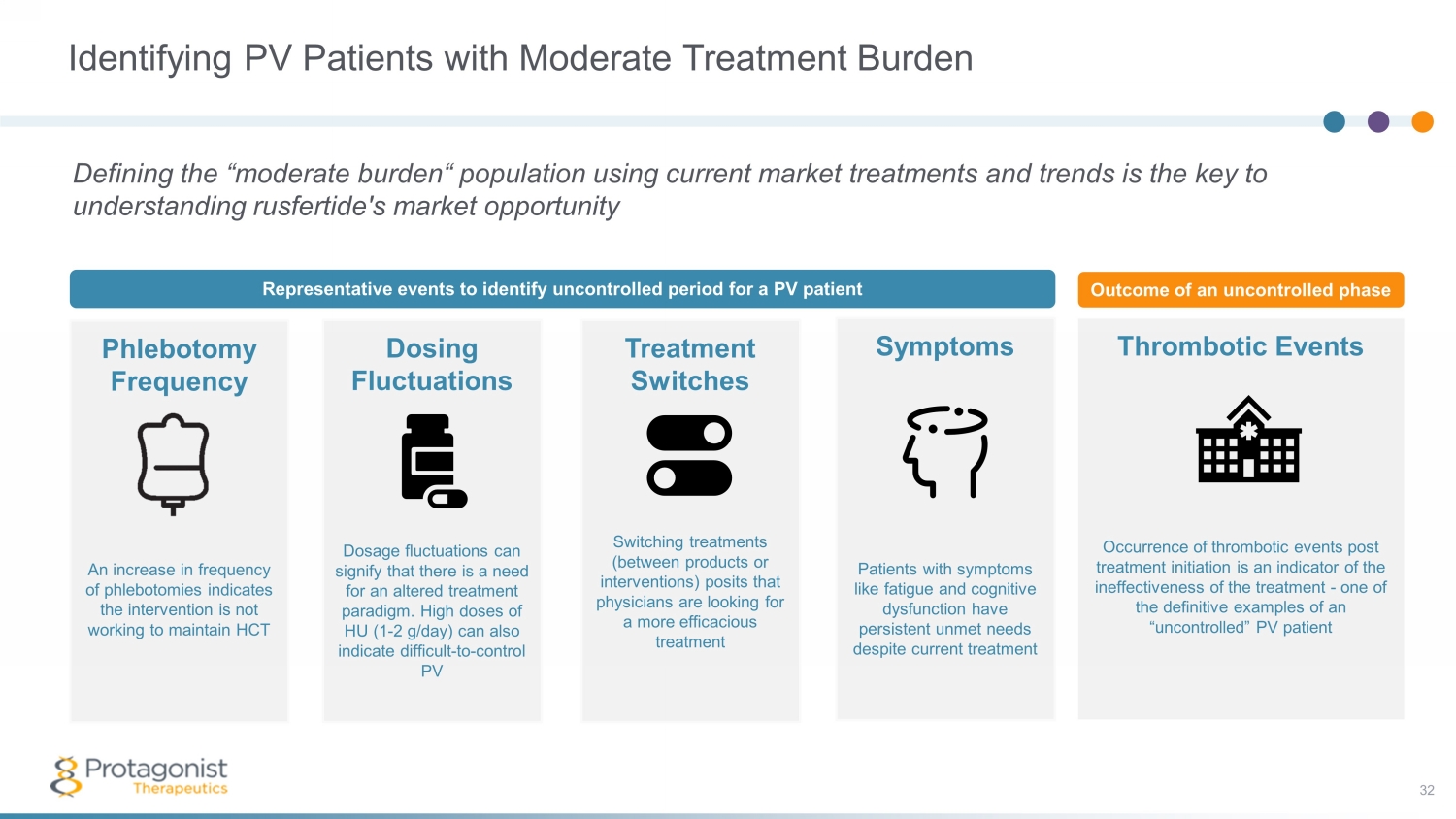
Identifying PV Patients with Moderate Treatment Burden Defining the “moderate burden“ population using current market treatments and trends is the key to understanding rusfertide's market opportunity Phlebotomy Frequency An increase in frequency of phlebotomies indicates the intervention is not working to maintain HCT Dosing Fluctuations Dosage fluctuations can signify that there is a need for an altered treatment paradigm. High doses of HU (1 - 2 g/day) can also indicate difficult - to - control PV Treatment Switches Switching treatments (between products or interventions) posits that physicians are looking for a more efficacious treatment Thrombotic Events Occurrence of thrombotic events post treatment initiation is an indicator of the ineffectiveness of the treatment - one of the definitive examples of an “uncontrolled” PV patient Symptoms Patients with symptoms like fatigue and cognitive dysfunction have persistent unmet needs despite current treatment Representative events to identify uncontrolled period for a PV patient Outcome of an uncontrolled phase 32

Potential Commercial Positioning for Rusfertide Therapy of Choice for Patients Experiencing Moderate Treatment Burden to Achieve HCT Control Low burden Infrequent Phlebotomy (<4/year) High burden Other Agents Moderate burden Frequent Phlebotomy and/or HU Illustrative rusfertide target. Percentages derived from 100,000 diagnosed and treated patients in the US Estimates based on Protagonist internal research, data on file ~30% ~60% ~10% Rusfertide Target Most patients receiving phlebotomy and/or HU have inconsistent and uncontrolled HCT. Rusfertide , a hepcidin mimetic, can potentially benefit a broad spectrum of patients by enabling consistent and continuous control of HCT <45%. 33

JNJ - 2113 (formerly PN - 235): IL - 23 Receptor Antagonist Oral Targeted Investigational Therapy for Psoriasis and Other IL - 23 Mediated Diseases 34

Protagonist - Janssen Oral, IL - 23R Antagonist Collaboration 35 1 Stelara® generated $9.7B in sales, and Tremfya® generated $2.7B in sales in 2022, per Johnson & Johnson 2022 Annual Report. Ste lara® and Tremfaya ® not part of Protagonist - Janssen collaboration. Collaboration overview – Initiated in 2017 with I&I market leader Janssen Biotech 1 – JNJ - 2113 (formerly PN - 235) jointly discovered using Protagonist’s proprietary peptide discovery platform – Protagonist completed pre - clinical and Phase 1 studies – Janssen responsible for further development and commercialization Collaboration economics – Protagonist eligible for up to $855M in development and sales milestones and upward - tiering royalties for JNJ - 2113 and other collaboration compounds Recent clinical data; development status – Phase 2B FRONTIER 1 data presented at WCD 2023 (July 2023) – Phase 3 psoriasis study planned – Phase 2b UC study planned (Q4 2023) JNJ - 2113 potential best - in - class oral agent – Potential to expand IL - 23 market to oral therapy

IL - 23R Antagonist Market Overview 36 Multi - billion Market Opportunity for an Oral Agent Significant market potential for IL - 23R antagonist – Relevant indications include psoriasis, psoriatic arthritis, IBD (ulcerative colitis, Crohn’s disease) – Psoriasis prevalence = 125M WW patients 1 (8M US 1 ) – 30% develop psoriatic arthritis – $13.2B major market sales for psoriasis (2020) 2 – Projected growth to $25.3B in 2030 2 – $14.2B major market sales for IBD (2020) 2 – Projected growth to $24.9B in 2029 2 Oral agents expected to contribute to market growth – Substantial portion of patients untreated with current standard of care – 25% of treated psoriasis patients treated with biologics 1 – Despite strong efficacy, biologics associated with safety concerns, loss of response, inconvenient administration, highlighting the need for safe and effective oral options 3, 4 1. Evaluate Pharma 2022; 2. GlobalData 2020; 3. Levin, E. et al. J Dermatol Treat ; 25(1); 78 - 82; 4. Piragine E., et al. J Clin Med 2022 ; 11(6); 1506
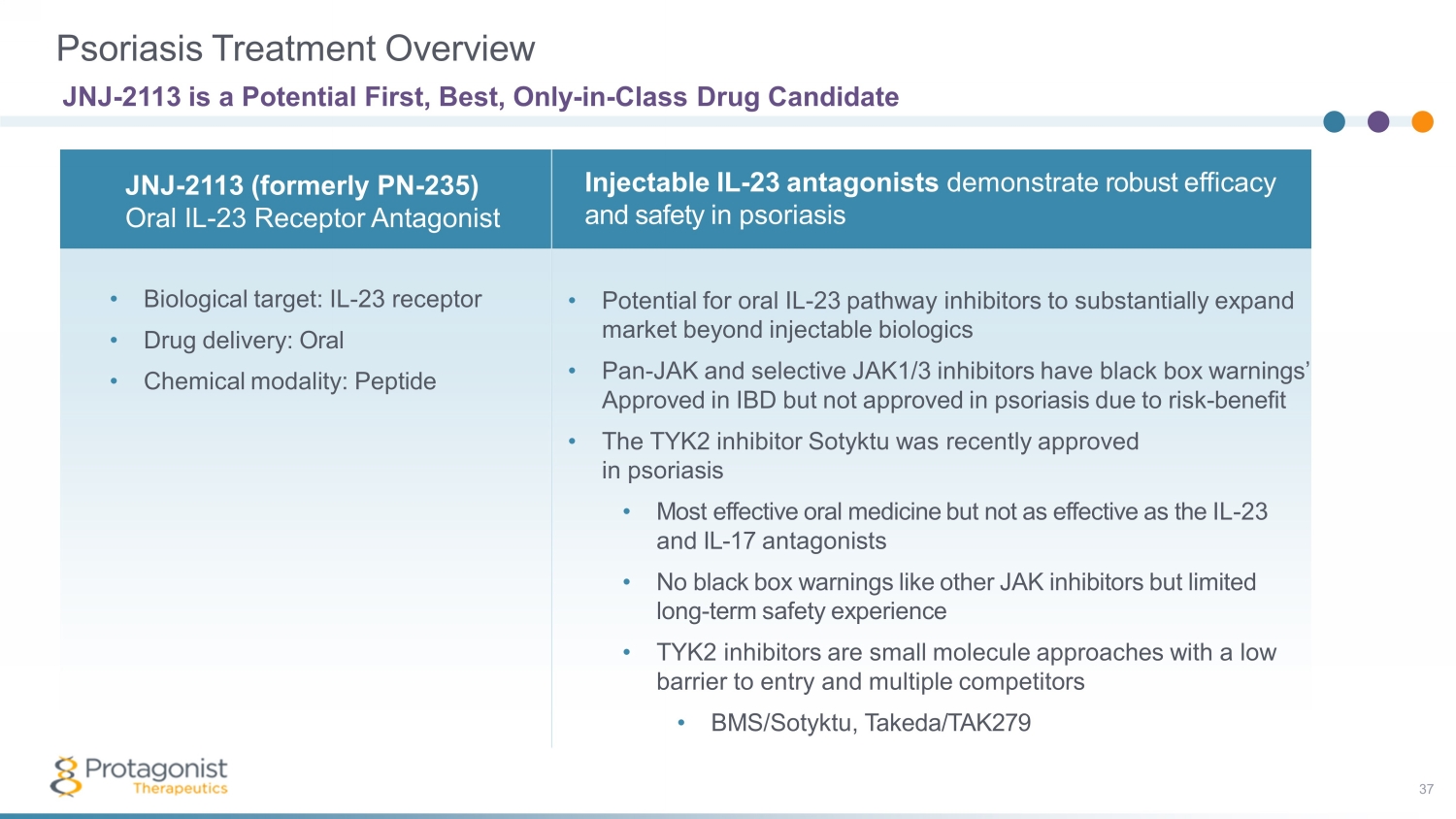
Psoriasis Treatment Overview JNJ - 2113 is a Potential First, Best, Only - in - Class Drug Candidate • Biological target: IL - 23 receptor • Drug delivery: Oral • Chemical modality: Peptide Injectable IL - 23 antagonists demonstrate robust efficacy and safety in psoriasis JNJ - 2113 (formerly PN - 235) Oral IL - 23 Receptor Antagonist • Potential for oral IL - 23 pathway inhibitors to substantially expand market beyond injectable biologics • Pan - JAK and selective JAK1/3 inhibitors have black box warnings’ Approved in IBD but not approved in psoriasis due to risk - benefit • The TYK2 inhibitor Sotyktu was recently approved in psoriasis • Most effective oral medicine but not as effective as the IL - 23 and IL - 17 antagonists • No black box warnings like other JAK inhibitors but limited long - term safety experience • TYK2 inhibitors are small molecule approaches with a low barrier to entry and multiple competitors • BMS/ Sotyktu , Takeda/TAK279 37

JNJ - 2113 (PN - 235) 38 Multiple Clinical Studies in Progress & Planned • Planned – Phase 3 study for moderate - to - severe plaque psoriasis – Phase 2b study for ulcerative colitis • FRONTIER 1 – 255 patient Phase 2b placebo - controlled study in moderate - to - severe plaque psoriasis – Oral tablet dosing, qd and bid – Topline data summary; March 07, 2023 • FRONTIER 2 – Long term extension study; ongoing • SUMMIT – 90 patient Phase 2 placebo - controlled study in moderate - to - severe plaque psoriasis – Oral once daily delayed release tablets – Completed April 10, 2023 • Other – Phase 1 study in healthy volunteers; completed (by Protagonist) – NCT05062200: Phase 1 study in healthy Japanese and Chinese participants; completed – NCT05703841: Phase 1 study in healthy Chinese adult participants; completed

JNJ - 2113 Characteristics • Highly potent (single digit picomolar) oral IL - 23R antagonist: – >1000 - fold more potent vs first - generation candidate (PBMC, phospho - STAT3 assay) – Similar or better target affinity vs. IL - 23 mAbs • High oral stability: Ex - vivo stability: >24hr half - life in feces (human, cyno , and rat) In - vivo stability: >25% fecal recovery after 24hrs in cynos • Pre - clinical Proof - of - Concept: – Achieved pre - clinical PoC with oral dosing in IL - 23 - induced rat ear skin inflammation model – Similar inhibition to systemic IL - 23 mAbs • Phase 1 study in NHVs: – Inhibition of IL - 23 pathway related biomarkers comparable to approved IL - 23 mAbs Preclinical, Phase 1 and Phase 2b Data Supportive of a Robust Clinical Development Program • Phase 2b FRONTIER1 study in Psoriasis : – Potential for best - in - class oral agent for psoriasis 39 ISID – Fourie A, et al. First - in - Class Oral Peptide Systemically Targeting the IL - 23 Pathway. Abstract presented at the Internat ional Societies for Investigative Dermatology; May 2023 WCD – Bissonnette R, et al. A Phase 2, Randomized, Placebo - controlled, Dose Ranging Study of Oral JNJ - 77242113 for the Treatment of Moderate - to - Severe Plaque Psoriasis: FRONTIER 1. Late Breaking Abstract presented at the World Congress of Dermatology; July 2023
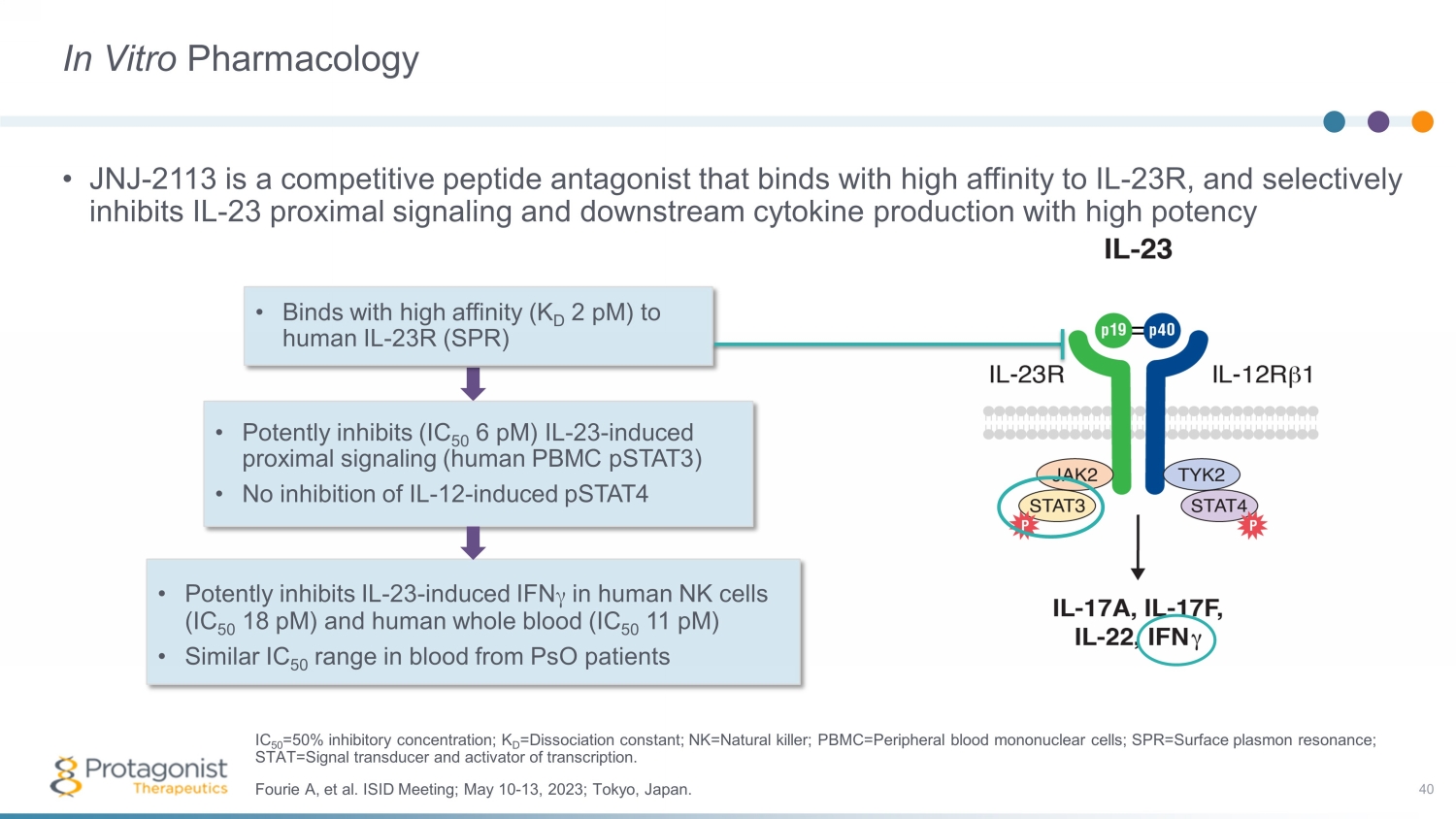
In Vitro Pharmacology • JNJ - 2113 is a competitive peptide antagonist that binds with high affinity to IL - 23R, and selectively inhibits IL - 23 proximal signaling and downstream cytokine production with high potency 40 • Binds with high affinity (K D 2 pM ) to human IL - 23R (SPR) • Potently inhibits (IC 50 6 pM ) IL - 23 - induced proximal signaling (human PBMC pSTAT3) • No inhibition of IL - 12 - induced pSTAT4 • Potently inhibits IL - 23 - induced IFN γ in human NK cells (IC 50 18 pM ) and human whole blood (IC 50 11 pM ) • Similar IC 50 range in blood from PsO patients Fourie A, et al. ISID Meeting; May 10 - 13, 2023; Tokyo, Japan. IC 50 =50% inhibitory concentration; K D =Dissociation constant; NK=Natural killer; PBMC=Peripheral blood mononuclear cells; SPR=Surface plasmon resonance; STAT=Signal transducer and activator of transcription.

Orally Dosed JNJ - 2113 Attenuates Weight Loss and Colon Inflammation in the Rat TNBS - induced Colitis Model • Statistically significant effects seen beginning at doses of 0.1 to 0.3 mg/kg/day • Although exposure in plasma and skin was much lower than GI tissues, exquisite potency of JNJ - 2113 indicated potential for systemic activity beyond the GI tract 41 Fourie A, et al. ISID Meeting; May 10 - 13, 2023; Tokyo, Japan. GI=Gastrointestinal; TNBS= Trinitrobenzenesulfonic acid. ns=not significant, **p <0.01, ****p <0.0001. Graph on right represents median and interquartile range. Data combined from th ree studies.
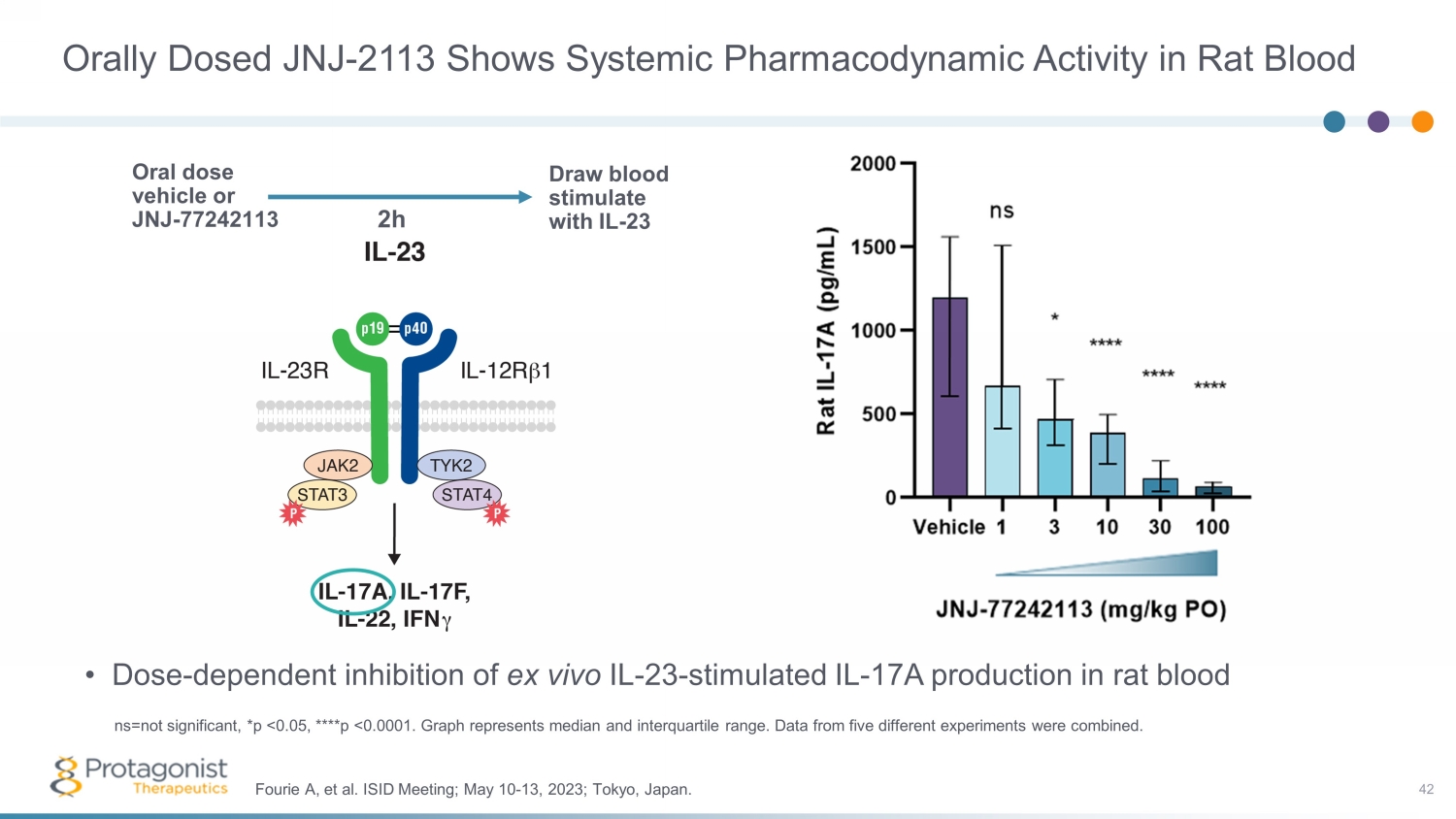
Orally Dosed JNJ - 2113 Shows Systemic Pharmacodynamic Activity in Rat Blood • Dose - dependent inhibition of ex vivo IL - 23 - stimulated IL - 17A production in rat blood 42 Fourie A, et al. ISID Meeting; May 10 - 13, 2023; Tokyo, Japan. ns=not significant, *p <0.05, ****p <0.0001. Graph represents median and interquartile range. Data from five different experi men ts were combined. Oral dose vehicle or JNJ - 77242113 Draw blood stimulate with IL - 23 2h
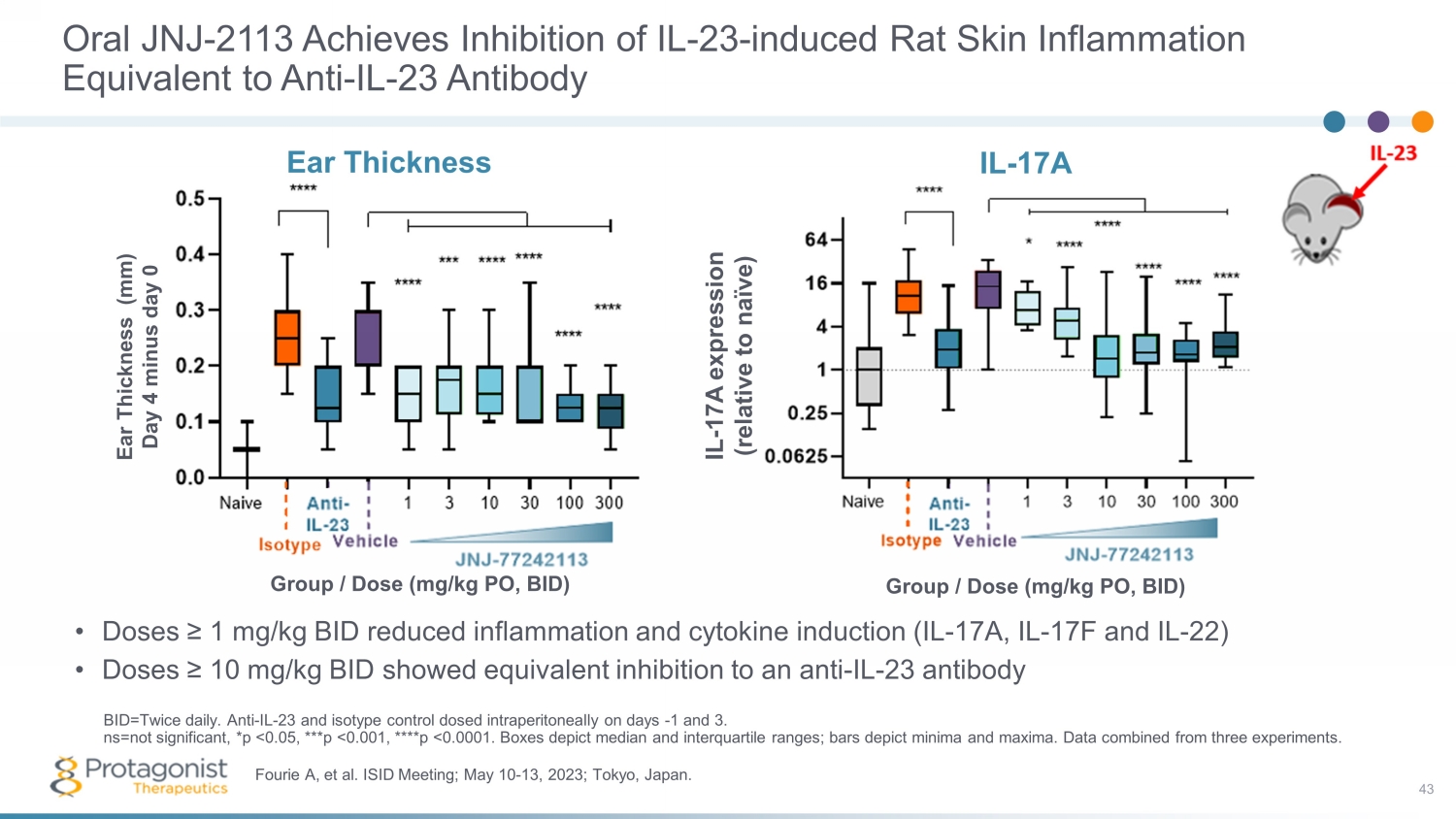
Oral JNJ - 2113 Achieves Inhibition of IL - 23 - induced Rat Skin Inflammation Equivalent to Anti - IL - 23 Antibody • Doses ≥ 1 mg/kg BID reduced inflammation and cytokine induction (IL - 17A, IL - 17F and IL - 22) • Doses ≥ 10 mg/kg BID showed equivalent inhibition to an anti - IL - 23 antibody 43 Fourie A, et al. ISID Meeting; May 10 - 13, 2023; Tokyo, Japan. BID=Twice daily. Anti - IL - 23 and isotype control dosed intraperitoneally on days - 1 and 3. ns=not significant, *p <0.05, ***p <0.001, ****p <0.0001. Boxes depict median and interquartile ranges; bars depict minima an d m axima. Data combined from three experiments. Ear Thickness Ear Thickness (mm) Day 4 minus day 0 Group / Dose (mg/kg PO, BID) IL - 17A IL - 17A expression (relative to naïve) Group / Dose (mg/kg PO, BID)
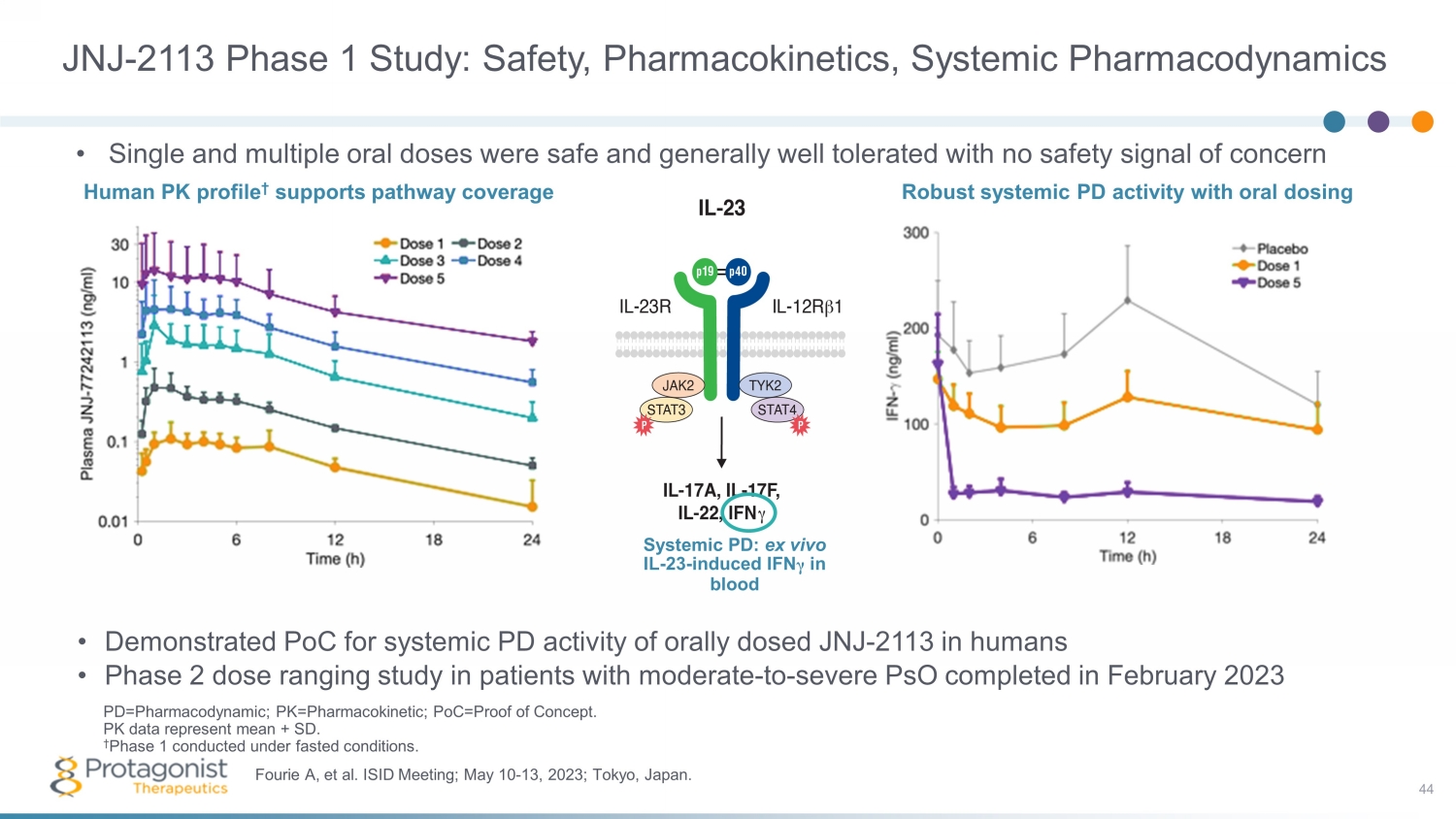
JNJ - 2113 Phase 1 Study: Safety, Pharmacokinetics, Systemic Pharmacodynamics • Demonstrated PoC for systemic PD activity of orally dosed JNJ - 2113 in humans • Phase 2 dose ranging study in patients with moderate - to - severe PsO completed in February 2023 44 Fourie A, et al. ISID Meeting; May 10 - 13, 2023; Tokyo, Japan. PD=Pharmacodynamic; PK=Pharmacokinetic; PoC=Proof of Concept. PK data represent mean + SD. † Phase 1 conducted under fasted conditions. • Single and multiple oral doses were safe and generally well tolerated with no safety signal of concern Human PK profile † supports pathway coverage Systemic P D: ex vivo IL - 23 - induced IFN γ in blood Robust systemic PD activity with oral dosing
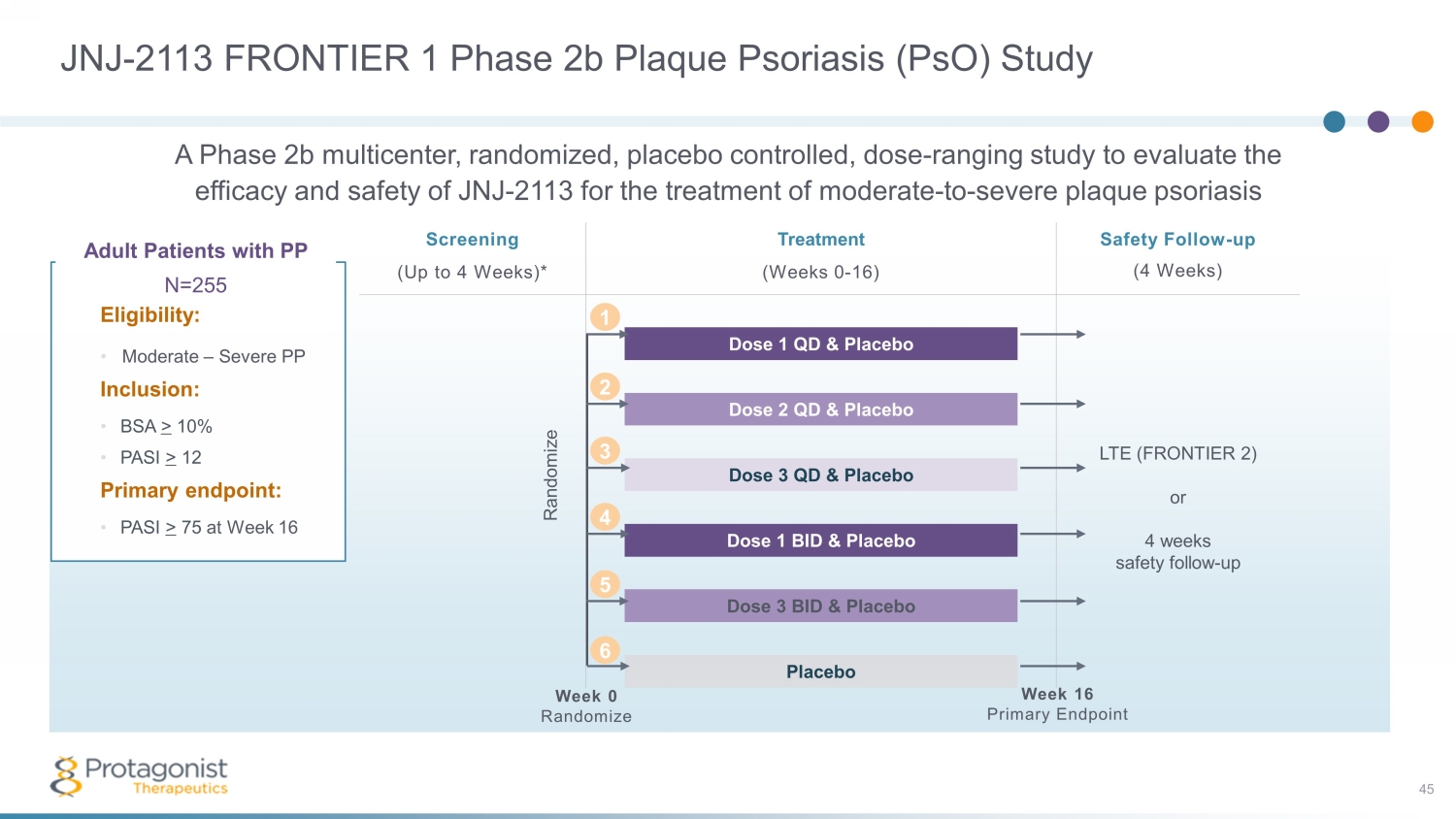
JNJ - 2113 FRONTIER 1 Phase 2b Plaque Psoriasis ( PsO ) Study 45 Adult Patients with PP N=255 Eligibility: • Moderate – Severe PP Inclusion: • BSA > 10% • PASI > 12 Primary endpoint: • PASI > 75 at Week 16 Screening Treatment Safety Follow - up (4 Weeks) (Up to 4 Weeks)* (Weeks 0 - 16) Dose 1 QD & Placebo LTE (FRONTIER 2) or 4 weeks safety follow - up Dose 2 QD & Placebo Dose 3 QD & Placebo Dose 1 BID & Placebo Dose 3 BID & Placebo Placebo Week 0 Randomize Week 16 Primary Endpoint Randomize 2 1 3 4 5 6 A Phase 2b multicenter, randomized, placebo controlled, dose - ranging study to evaluate the efficacy and safety of JNJ - 2113 for the treatment of moderate - to - severe plaque psoriasis
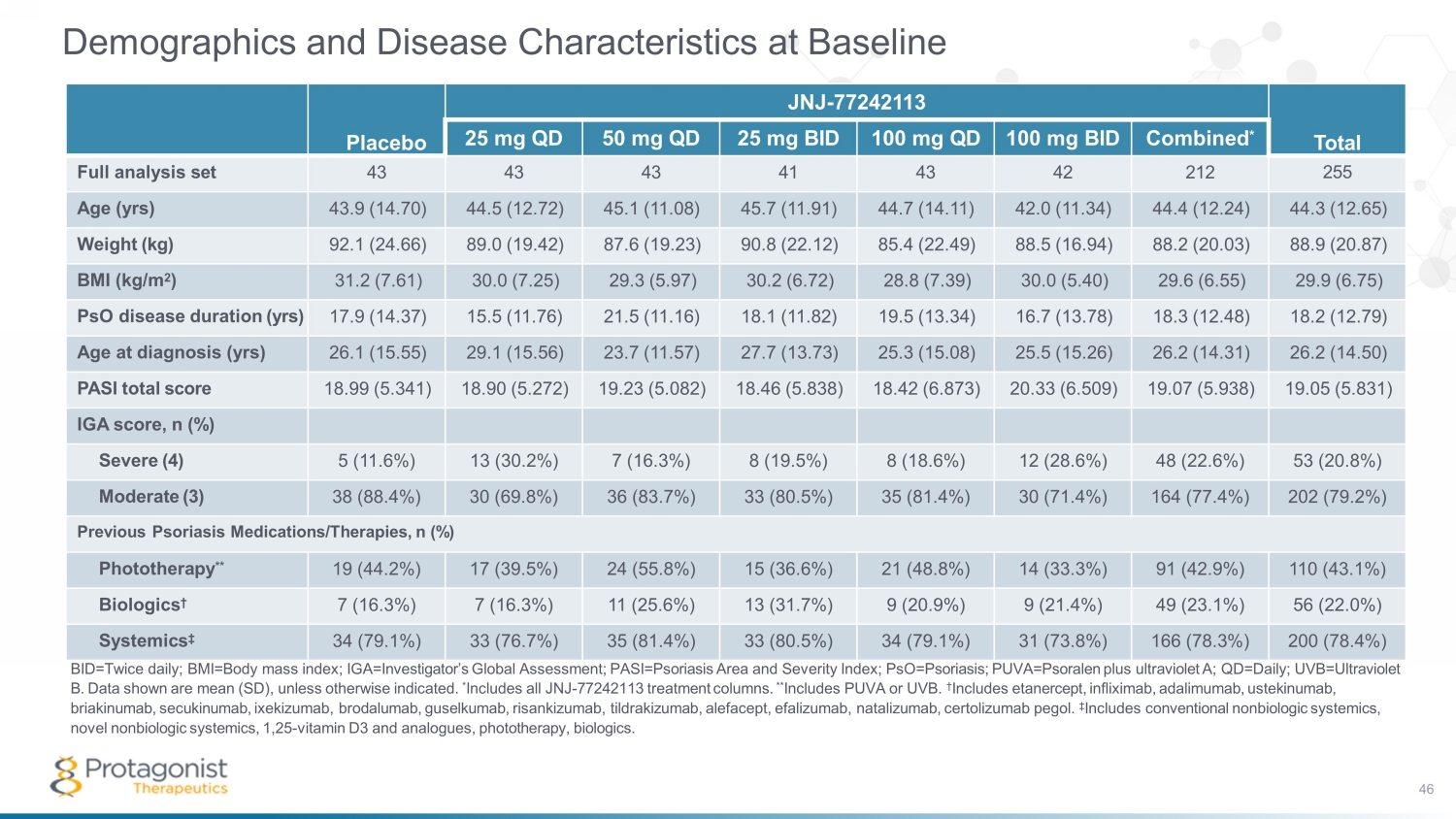
Demographics and Disease Characteristics at Baseline BID=Twice daily; BMI=Body mass index; IGA=Investigator’s Global Assessment; PASI=Psoriasis Area and Severity Index; PsO=Psoriasis; PUVA=Psoralen plus ultraviolet A; QD=Daily; UVB=Ultraviolet B. Data shown are mean (SD), unless otherwise indicated. * Includes all JNJ - 77242113 treatment columns. ** Includes PUVA or UVB. † Includes etanercept, infliximab, adalimumab, ustekinumab, briakinumab, secukinumab , ixekizumab, brodalumab, guselkumab, risankizumab, tildrakizumab, alefacept, efalizumab, natalizumab, certolizumab pegol. ‡ Includes conventional nonbiologic systemics, novel nonbiologic systemics, 1,25 - vitamin D3 and analogues, phototherapy, biologics. Placebo JNJ - 77242113 Total 25 mg QD 50 mg QD 25 mg BID 100 mg QD 100 mg BID Combined * Full analysis set 43 43 43 41 43 42 212 255 Age (yrs) 43.9 (14.70) 44.5 (12.72) 45.1 (11.08) 45.7 (11.91) 44.7 (14.11) 42.0 (11.34) 44.4 (12.24) 44.3 (12.65) Weight (kg) 92.1 (24.66) 89.0 (19.42) 87.6 (19.23) 90.8 (22.12) 85.4 (22.49) 88.5 (16.94) 88.2 (20.03) 88.9 (20.87) BMI (kg/m 2 ) 31.2 (7.61) 30.0 (7.25) 29.3 (5.97) 30.2 (6.72) 28.8 (7.39) 30.0 (5.40) 29.6 (6.55) 29.9 (6.75) PsO disease duration (yrs) 17.9 (14.37) 15.5 (11.76) 21.5 (11.16) 18.1 (11.82) 19.5 (13.34) 16.7 (13.78) 18.3 (12.48) 18.2 (12.79) Age at diagnosis (yrs) 26.1 (15.55) 29.1 (15.56) 23.7 (11.57) 27.7 (13.73) 25.3 (15.08) 25.5 (15.26) 26.2 (14.31) 26.2 (14.50) PASI total score 18.99 (5.341) 18.90 (5.272) 19.23 (5.082) 18.46 (5.838) 18.42 (6.873) 20.33 (6.509) 19.07 (5.938) 19.05 (5.831) IGA score, n (%) Severe (4) 5 (11.6%) 13 (30.2%) 7 (16.3%) 8 (19.5%) 8 (18.6%) 12 (28.6%) 48 (22.6%) 53 (20.8%) Moderate (3) 38 (88.4%) 30 (69.8%) 36 (83.7%) 33 (80.5%) 35 (81.4%) 30 (71.4%) 164 (77.4%) 202 (79.2%) Previous Psoriasis Medications/Therapies, n (%) Phototherapy ** 19 (44.2%) 17 (39.5%) 24 (55.8%) 15 (36.6%) 21 (48.8%) 14 (33.3%) 91 (42.9%) 110 (43.1%) Biologics † 7 (16.3%) 7 (16.3%) 11 (25.6%) 13 (31.7%) 9 (20.9%) 9 (21.4%) 49 (23.1%) 56 (22.0%) Systemics ‡ 34 (79.1%) 33 (76.7%) 35 (81.4%) 33 (80.5%) 34 (79.1%) 31 (73.8%) 166 (78.3%) 200 (78.4%) 46
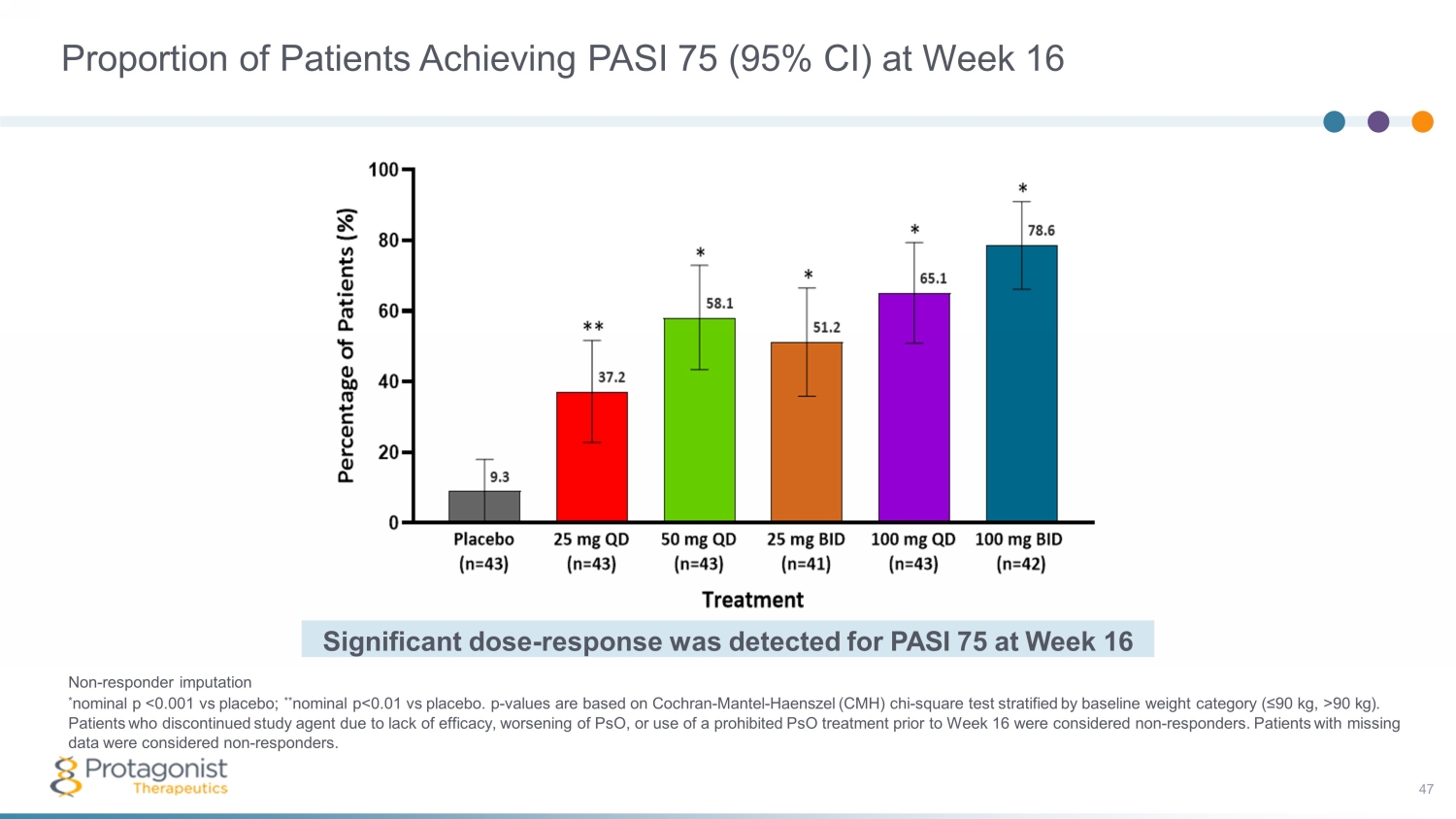
Non - responder imputation * nominal p <0.001 vs placebo; ** nominal p<0.01 vs placebo. p - values are based on Cochran - Mantel - Haenszel (CMH) chi - square test stratified by baseline weight category (≤90 kg, >90 kg). Patients who discontinued study agent due to lack of efficacy, worsening of PsO, or use of a prohibited PsO treatment prior to Week 16 were considered non - responders. Patients with missing data were considered non - responders. Significant dose - response was detected for PASI 75 at Week 16 Proportion of Patients Achieving PASI 75 (95% CI) at Week 16 47
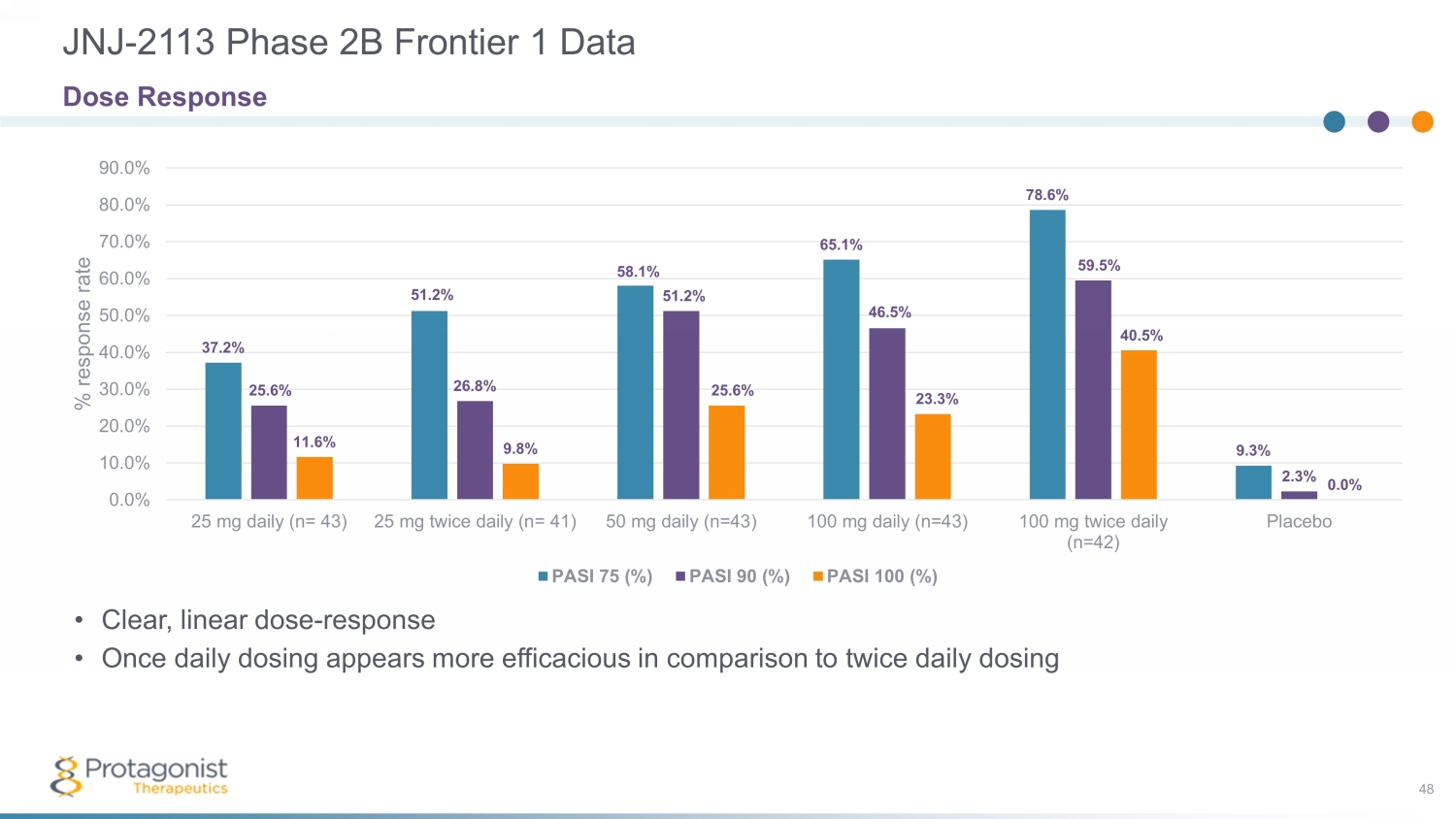
JNJ - 2113 Phase 2B Frontier 1 Data 48 37.2% 51.2% 58.1% 65.1% 78.6% 9.3% 25.6% 26.8% 51.2% 46.5% 59.5% 2.3% 11.6% 9.8% 25.6% 23.3% 40.5% 0.0% 0.0% 10.0% 20.0% 30.0% 40.0% 50.0% 60.0% 70.0% 80.0% 90.0% 25 mg daily (n= 43) 25 mg twice daily (n= 41) 50 mg daily (n=43) 100 mg daily (n=43) 100 mg twice daily (n=42) Placebo % response rate PASI 75 (%) PASI 90 (%) PASI 100 (%) Dose Response • Clear, linear dose - response • Once daily dosing appears more efficacious in comparison to twice daily dosing
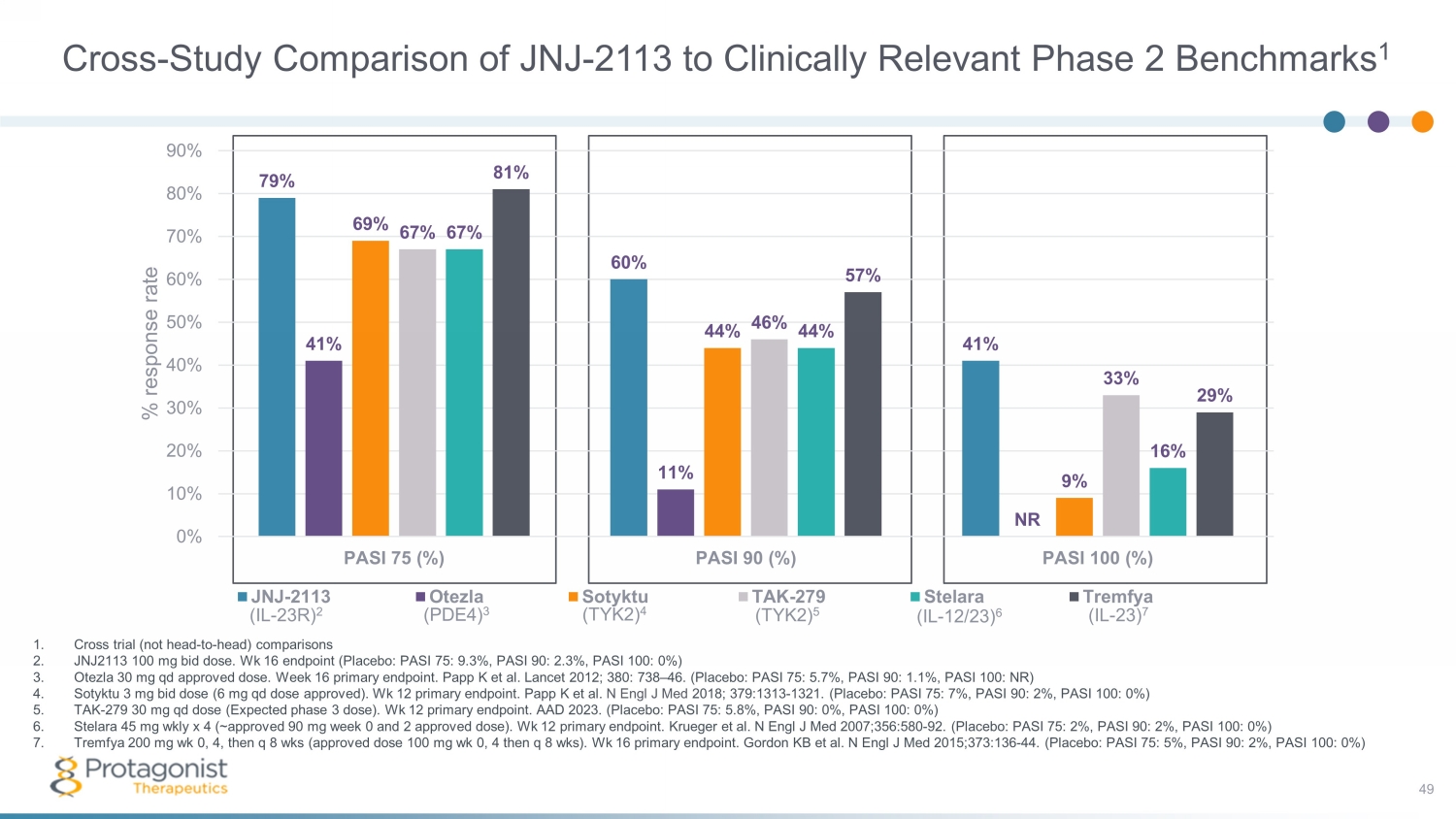
Cross - Study Comparison of JNJ - 2113 to Clinically Relevant Phase 2 Benchmarks 1 49 1. Cross trial (not head - to - head) comparisons 2. J NJ2113 100 mg bid dose. Wk 16 endpoint (Placebo: PASI 75: 9.3%, PASI 90: 2.3%, PASI 100: 0%) 3. Otezla 30 mg qd approved dose. Week 16 primary endpoint. Papp K et al. Lancet 2012; 380: 738 – 46. (Placebo: PASI 75: 5.7%, PASI 90: 1.1%, PASI 100: NR) 4. Sotyktu 3 mg bid dose (6 mg qd dose approved). Wk 12 primary endpoint. Papp K et al. N Engl J Med 2018; 379:1313 - 1321. (Placebo: PASI 75: 7%, PASI 90: 2%, PASI 100: 0%) 5. TAK - 279 30 mg qd dose (Expected phase 3 dose). Wk 12 primary endpoint. AAD 2023. (Placebo: PASI 75: 5.8%, PASI 90: 0%, PASI 100: 0%) 6. Stelara 45 mg wkly x 4 (~approved 90 mg week 0 and 2 approved dose). Wk 12 primary endpoint. Krueger et al. N Engl J Med 2007;356:580 - 92. (Placebo: PASI 75: 2%, PASI 90: 2%, PASI 100: 0%) 7. Tremfya 200 mg wk 0, 4, then q 8 wks (approved dose 100 mg wk 0, 4 then q 8 wks ). Wk 16 primary endpoint. Gordon KB et al. N Engl J Med 2015;373:136 - 44. (Placebo: PASI 75: 5%, PASI 90: 2%, PASI 100: 0%) (IL - 23R) 2 (PDE4) 3 (TYK2) 4 (TYK2) 5 (IL - 12/23) 6 (IL - 23) 7 79% 60% 41% 41% 11% NR 69% 44% 9% 67% 46% 33% 67% 44% 16% 81% 57% 29% 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% PASI 75 (%) PASI 90 (%) PASI 100 (%) % response rate JNJ-2113 Otezla Sotyktu TAK-279 Stelara Tremfya
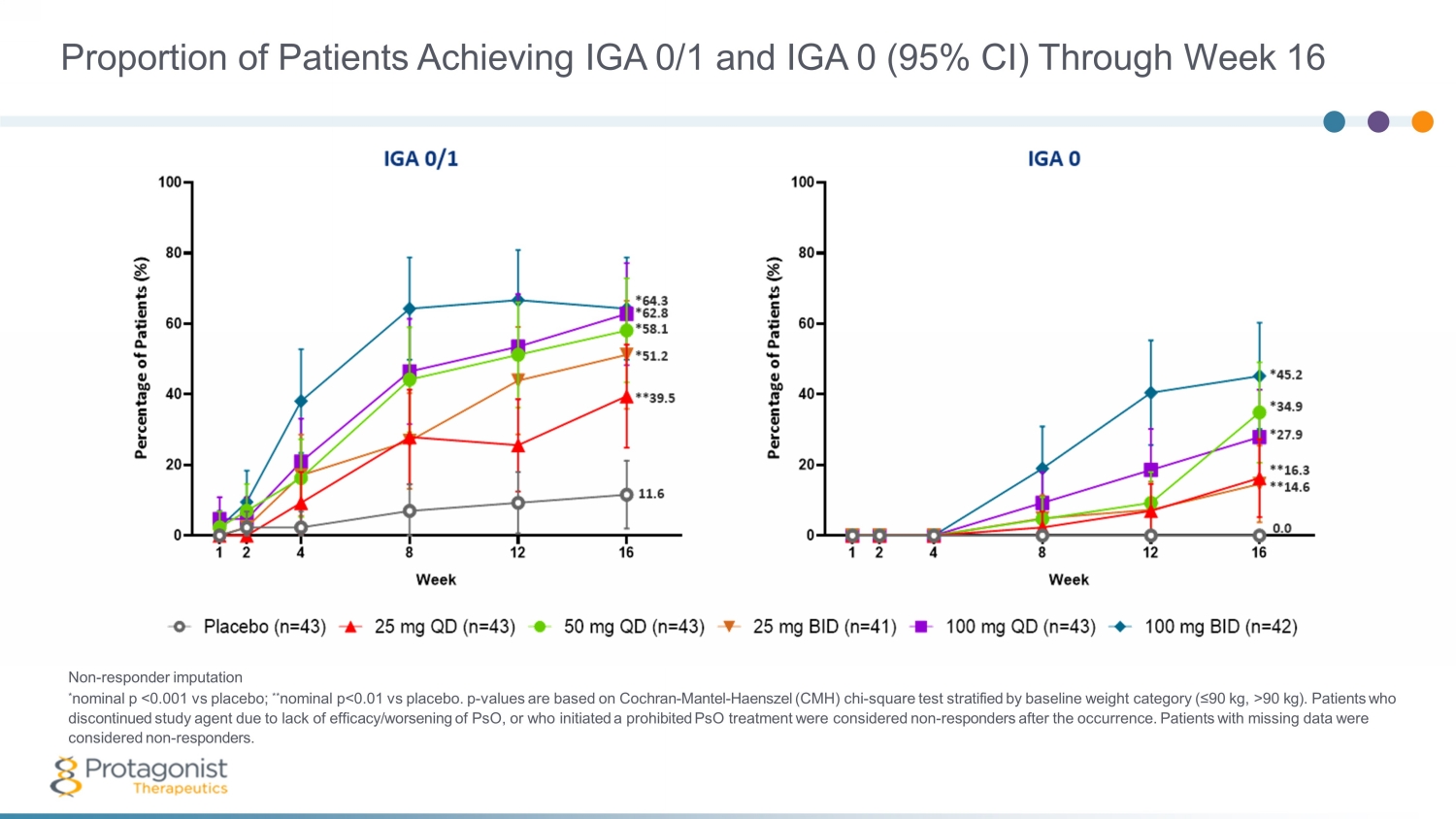
Proportion of Patients Achieving IGA 0/1 and IGA 0 (95% CI) Through Week 16 Non - responder imputation * nominal p <0.001 vs placebo; ** nominal p<0.01 vs placebo. p - values are based on Cochran - Mantel - Haenszel (CMH) chi - square test stratified by baseline weight category (≤90 kg, >90 kg). Patients who discontinued study agent due to lack of efficacy/worsening of PsO, or who initiated a prohibited PsO treatment were considered non - responders after the occurrence. Patients with missing data were considered non - responders.

Number of Patients With ≥1 TEAE With Frequency of ≥5% of Preferred Terms in Any Treatment Group Through End of Study by System Organ Class and Preferred Term AE=Adverse event; BID=Twice daily; QD=Daily; TEAE=Treatment - Emergent Adverse Events. * Includes all JNJ - 2113 treatment columns. Patients are counted only once for any given event, regardless of the number of times they actually experienced the event. Adverse events are coded using MedDRA Version 25.1. Placebo JNJ - 77242113 25 mg QD 50 mg QD 25 mg BID 100 mg QD 100 mg BID Combined * Safety analysis set, n 43 43 43 41 43 42 212 Avg duration of follow - up (weeks) 15.03 15.70 15.75 16.20 16.07 15.81 15.90 Patients with ≥1 AE, n (%) 22 (51.2%) 20 (46.5%) 26 (60.5%) 20 (48.8%) 19 (44.2%) 26 (61.9%) 111 (52.4%) System organ class/Preferred term, n (%) Infections and infestations 12 (27.9%) 15 (34.9%) 17 (39.5%) 14 (34.1%) 7 (16.3%) 11 (26.2%) 64 (30.2%) COVID - 19 5 (11.6%) 5 (11.6%) 3 (7.0%) 8 (19.5%) 3 (7.0%) 4 (9.5%) 23 (10.8%) Nasopharyngitis 2 (4.7%) 1 (2.3%) 8 (18.6%) 3 (7.3%) 1 (2.3%) 2 (4.8%) 15 (7.1%) Upper respiratory tract infection 1 (2.3%) 3 (7.0%) 0 0 0 2 (4.8%) 5 (2.4%) Gastrointestinal disorders 5 (11.6%) 3 (7.0%) 6 (14.0%) 4 (9.8%) 4 (9.3%) 7 (16.7%) 24 (11.3%) Diarrhoea 1 (2.3%) 2 (4.7%) 4 (9.3%) 2 (4.9%) 1 (2.3%) 1 (2.4%) 10 (4.7%) Nervous system disorders 1 (2.3%) 0 3 (7.0%) 2 (4.9%) 3 (7.0%) 2 (4.8%) 10 (4.7%) Headache 1 (2.3%) 0 1 (2.3%) 1 (2.4%) 3 (7.0%) 1 (2.4%) 6 (2.8%) Respiratory, thoracic and mediastinal disorders 1 (2.3%) 1 (2.3%) 1 (2.3%) 0 3 (7.0%) 2 (4.8%) 7 (3.3%) Cough 0 1 (2.3%) 1 (2.3%) 0 3 (7.0%) 1 (2.4%) 6 (2.8%) 51
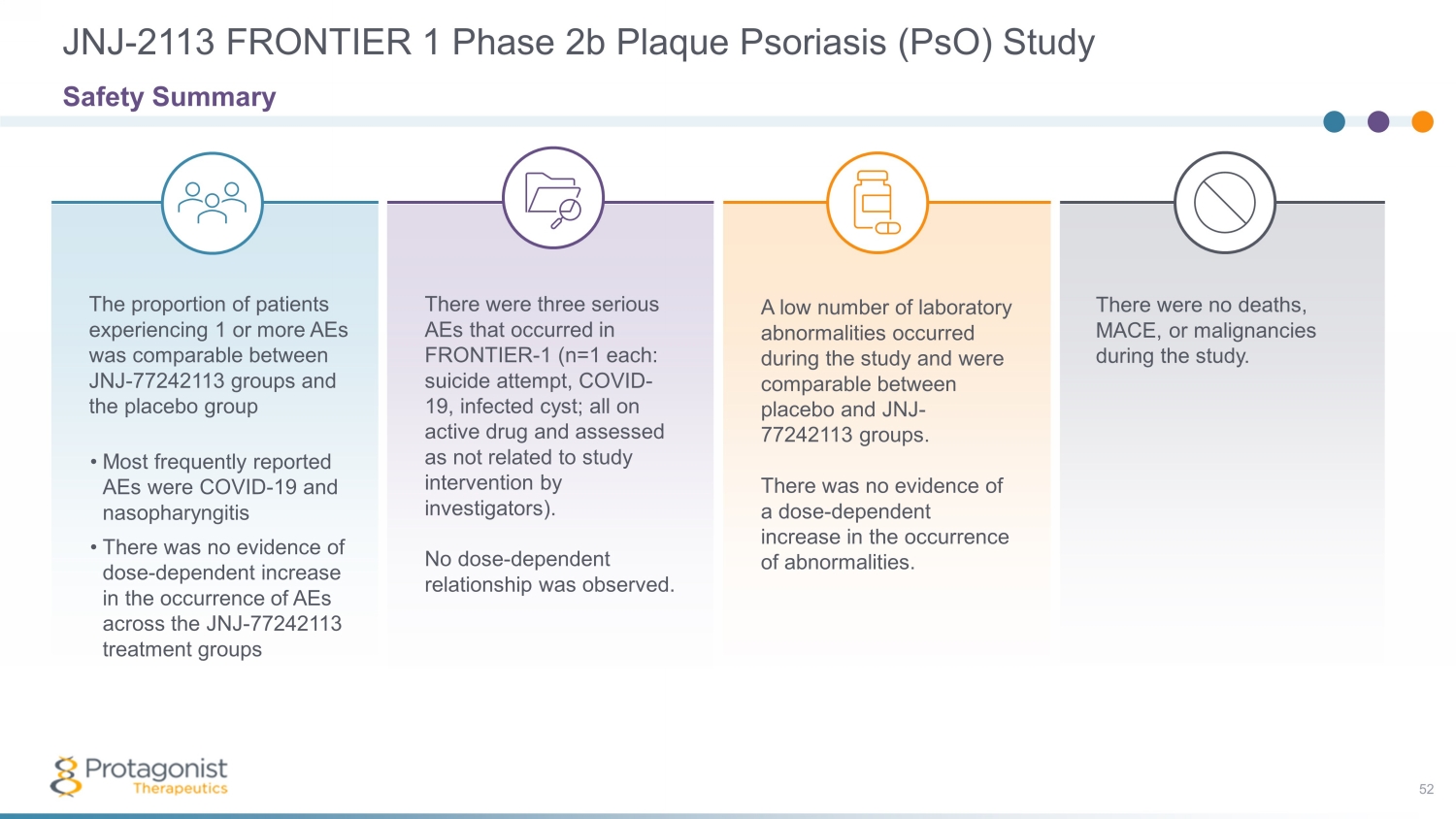
There were no deaths, MACE, or malignancies during the study. A low number of laboratory abnormalities occurred during the study and were comparable between placebo and JNJ - 77242113 groups. There was no evidence of a dose - dependent increase in the occurrence of abnormalities. There were three serious AEs that occurred in FRONTIER - 1 (n=1 each: suicide attempt, COVID - 19, infected cyst; all on active drug and assessed as not related to study intervention by investigators). No dose - dependent relationship was observed. The proportion of patients experiencing 1 or more AEs was comparable between JNJ - 77242113 groups and the placebo group • Most frequently reported AEs were COVID - 19 and nasopharyngitis • There was no evidence of dose - dependent increase in the occurrence of AEs across the JNJ - 77242113 treatment groups JNJ - 2113 FRONTIER 1 Phase 2b Plaque Psoriasis ( PsO ) Study 52 Safety Summary
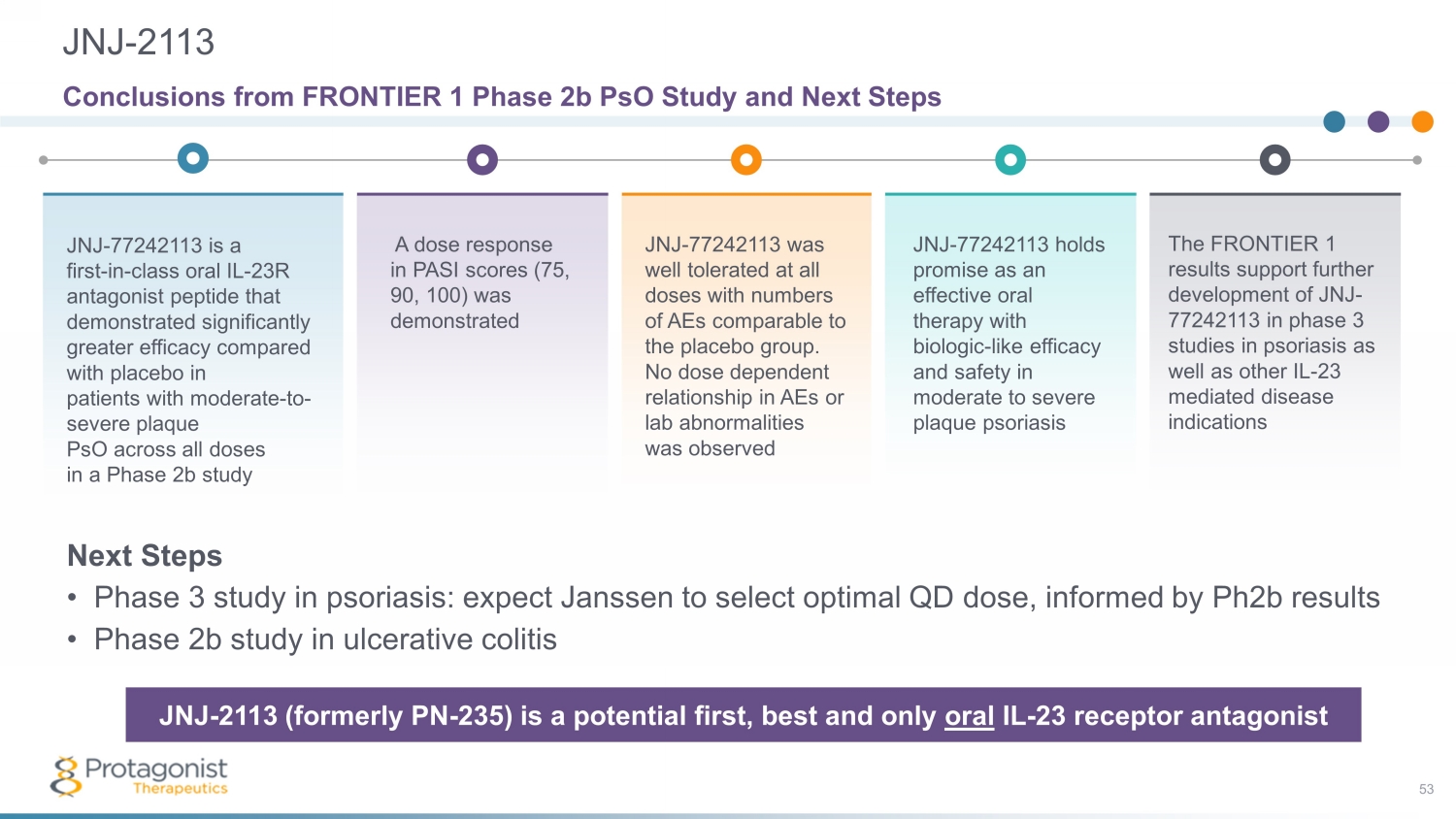
JNJ - 2113 53 The FRONTIER 1 results support further development of JNJ - 77242113 in phase 3 studies in psoriasis as well as other IL - 23 mediated disease indications Conclusions from FRONTIER 1 Phase 2b PsO Study and Next Steps A dose response in PASI scores (75, 90, 100) was demonstrated JNJ - 77242113 is a first - in - class oral IL - 23R antagonist peptide that demonstrated significantly greater efficacy compared with placebo in patients with moderate - to - severe plaque PsO across all doses in a Phase 2b study JNJ - 77242113 was well tolerated at all doses with numbers of AEs comparable to the placebo group. No dose dependent relationship in AEs or lab abnormalities was observed JNJ - 77242113 holds promise as an effective oral therapy with biologic - like efficacy and safety in moderate to severe plaque psoriasis JNJ - 2113 (formerly PN - 235) is a potential first, best and only oral IL - 23 receptor antagonist Next Steps • Phase 3 study in psoriasis: expect Janssen to select optimal QD dose, informed by Ph2b results • Phase 2b study in ulcerative colitis
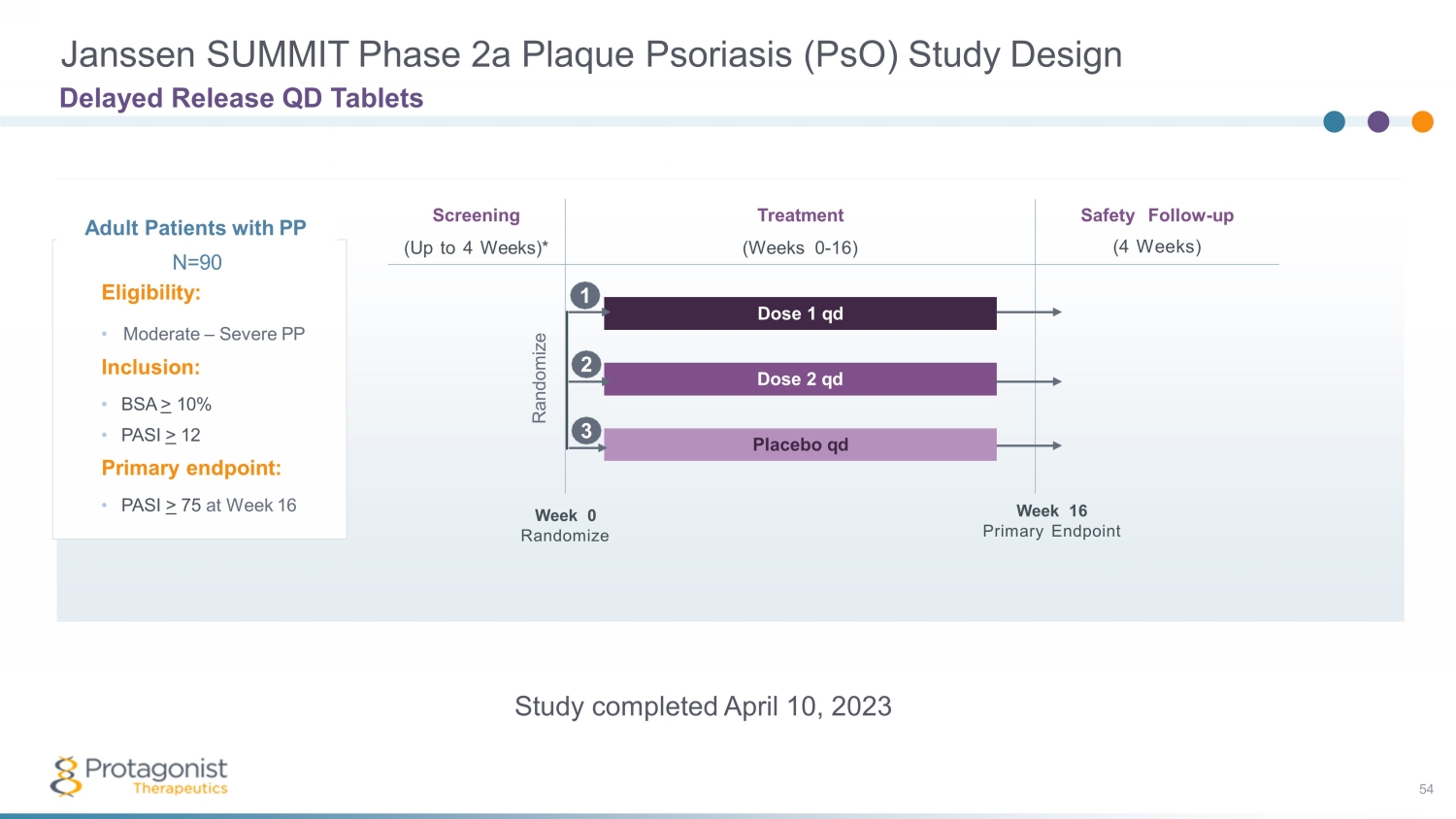
Janssen SUMMIT Phase 2a Plaque Psoriasis ( PsO ) Study Design Delayed Release QD Tablets Adult Patients with PP N =9 0 Eligibility: • Moderate – Severe PP Inclusion: • BSA > 10% • PASI > 12 Primary endpoint: • PASI > 75 at Week 16 Safety Follow - up (4 Weeks) Screening (Up to 4 Weeks)* Treatment (Weeks 0 - 16) Dose 1 qd Dose 2 qd Placebo qd Week 0 Randomize Week 16 Primary Endpoint Randomize 2 1 3 Study completed April 10, 2023 54
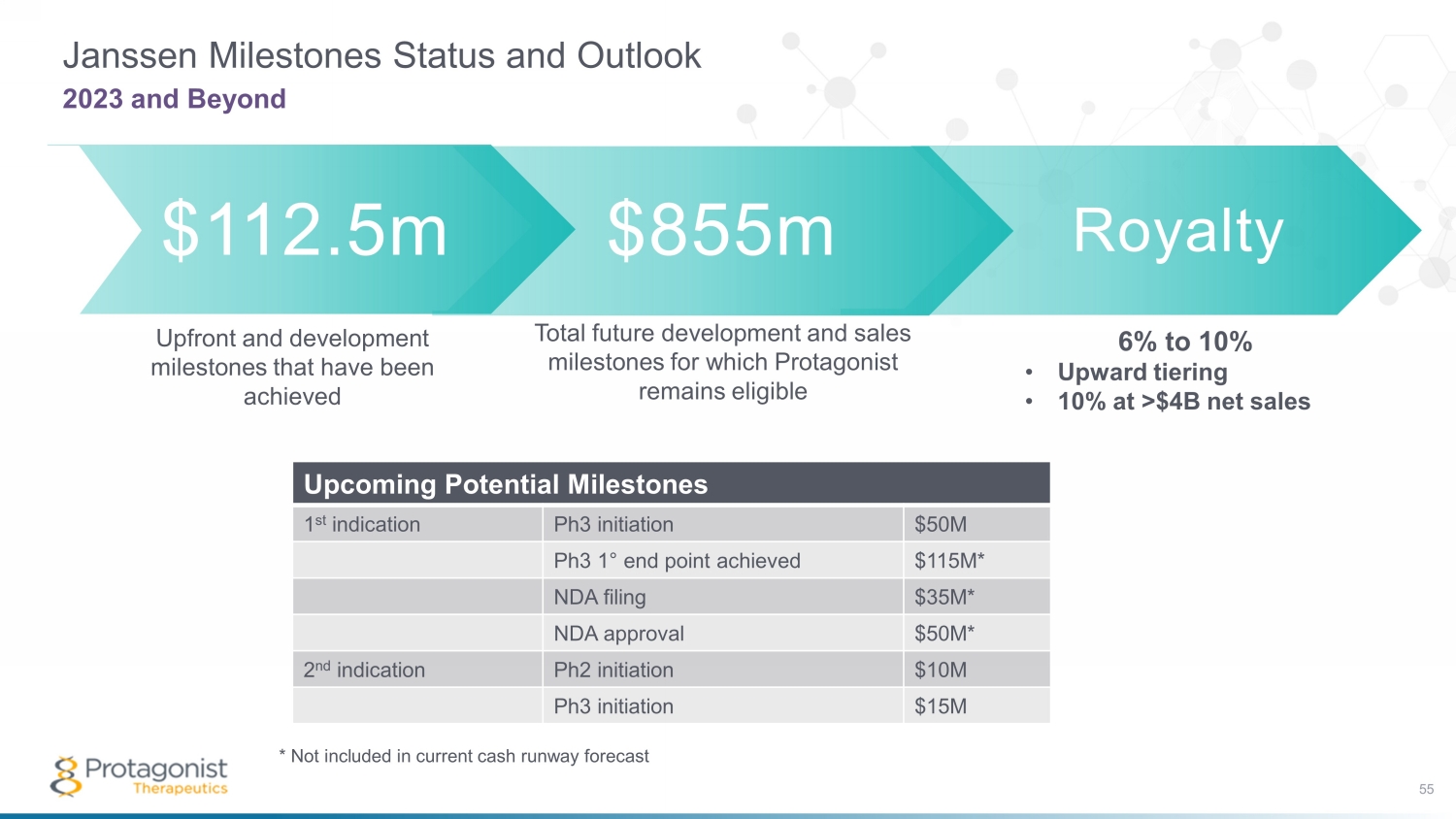
Royalty Janssen Milestones Status and Outlook 55 2023 and Beyond $112.5m $855m Total future development and sales milestones for which Protagonist remains eligible Upfront and development milestones that have been achieved 6% to 10% • Upward tiering • 10% at >$4B net sales Upcoming Potential Milestones 1 st indication Ph3 initiation $50M Ph3 1 ° end point achieved $115M* NDA filing $35M* NDA approval $50M* 2 nd indication Ph2 initiation $10M Ph3 initiation $15M * Not included in current cash runway forecast

56 Financials
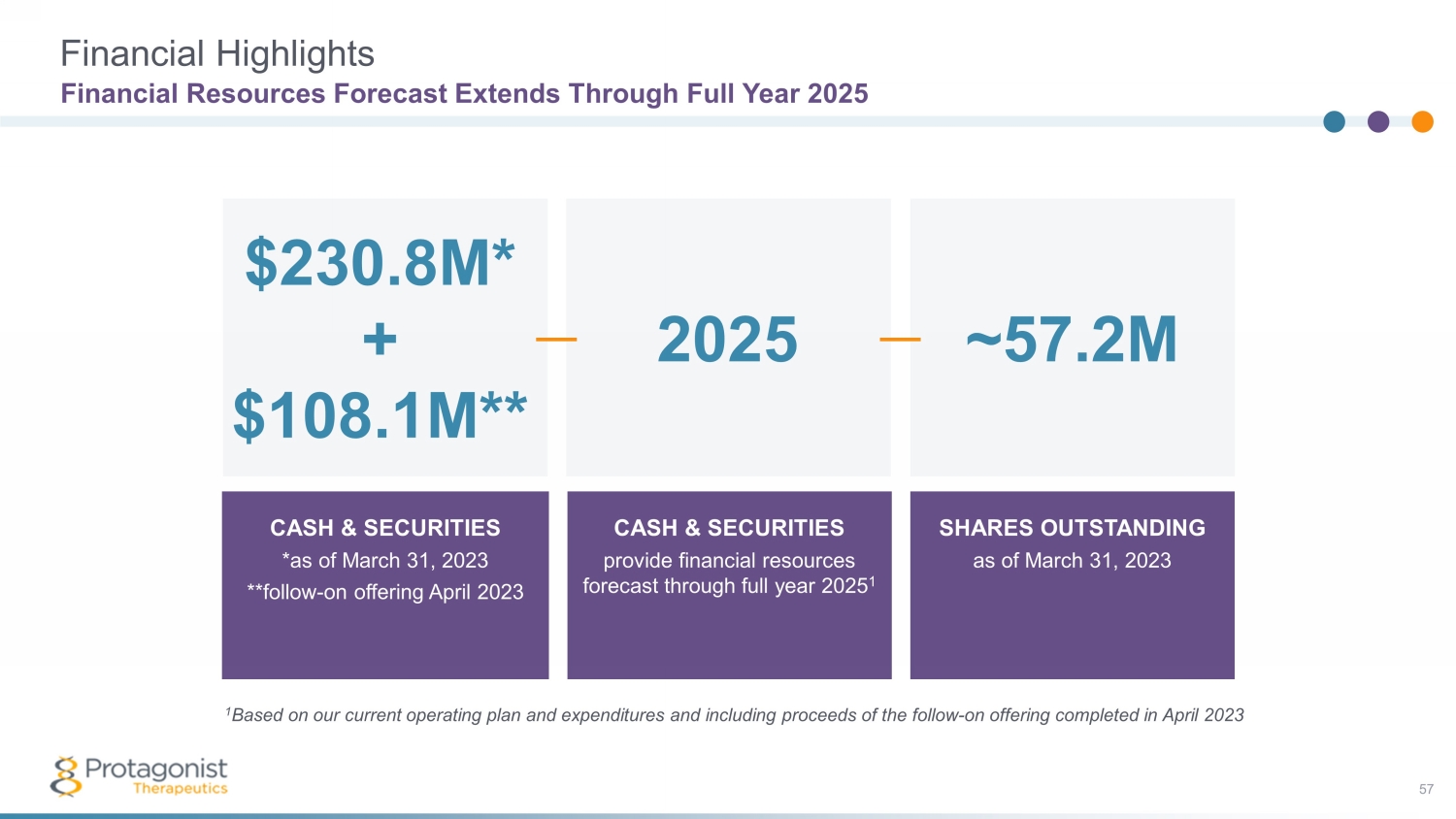
Financial Highlights Financial Resources Forecast Extends Through Full Year 2025 CASH & SECURITIES *as of March 31, 2023 **follow - on offering April 2023 CASH & SECURITIES provide financial resources forecast through full year 2025 1 SHARES OUTSTANDING as of March 31, 2023 2025 ~57.2M $230.8M* + $108.1M** 1 Based on our current operating plan and expenditures and including proceeds of the follow - on offering completed in April 2023 57

58 Thank you
v3.23.2
Cover
|
Jul. 04, 2023 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Jul. 04, 2023
|
| Entity File Number |
001-37852
|
| Entity Registrant Name |
PROTAGONIST THERAPEUTICS, INC.
|
| Entity Central Index Key |
0001377121
|
| Entity Tax Identification Number |
98-0505495
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Address, Address Line One |
7707 Gateway Blvd.
|
| Entity Address, Address Line Two |
Suite 140
|
| Entity Address, City or Town |
Newark
|
| Entity Address, State or Province |
CA
|
| Entity Address, Postal Zip Code |
94560-1160
|
| City Area Code |
510
|
| Local Phone Number |
474-0170
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Common Stock, par value $0.00001
|
| Trading Symbol |
PTGX
|
| Security Exchange Name |
NASDAQ
|
| Entity Emerging Growth Company |
false
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Protagonist Therapeutics (NASDAQ:PTGX)
Historical Stock Chart
Von Apr 2024 bis Mai 2024

Protagonist Therapeutics (NASDAQ:PTGX)
Historical Stock Chart
Von Mai 2023 bis Mai 2024
