UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM 10-Q
☒ QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the quarterly period ended June 30, 2023
OR
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from
to .
Commission File Number: 001-40060
Longeveron Inc.
(Exact name of registrant as specified in its
charter)
| Delaware | | | | 47-2174146 |
(State or Other Jurisdiction of Incorporation) | | | | (IRS Employer Identification No.) |
| 1951 NW 7th Avenue, Suite 520, Miami, Florida | | 33136 |
| (Address of principal executive offices) | | (Zip Code) |
(305) 909-0840
(Registrant’s
telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | | Trading Symbol | | Name of each exchange on which registered |
| Common Stock, par value $0.001 per share | | LGVN | | The NASDAQ Capital Market |
Indicate
by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities
Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports),
and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate
by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule
405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant
was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant
is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company.
See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,”
and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ | | Accelerated filer | ☐ |
| Non-accelerated filer | ☒ | | Smaller reporting company | ☒ |
| Emerging growth company | ☒ | | | |
If an emerging growth company, indicate by check mark if the registrant
has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant
to Section 13(a) of the Exchange Act. ☐
Indicate
by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
As of August 9, 2023, the registrant had 6,311,725 shares of Class
A common stock, $0.001 par value per shares, and 14,855,539 shares of Class B common stock, $0.001 par value per share, outstanding.
LONGEVERON INC.
TABLE OF CONTENTS
PART I. FINANCIAL INFORMATION
Item 1. Condensed Financial Statements.
Longeveron Inc.
Condensed Balance Sheets
(In thousands, except share and per share data)
| | |
June 30,
2023 | | |
December 31,
2022 | |
| | |
(Unaudited) | | |
| |
| Assets | |
| | |
| |
| Current assets: | |
| | |
| |
| Cash and cash equivalents | |
$ | 2,747 | | |
$ | 10,503 | |
| Marketable securities | |
| 5,910 | | |
| 9,155 | |
| Prepaid expenses and other current assets | |
| 1,539 | | |
| 404 | |
| Accounts and grants receivable | |
| 96 | | |
| 218 | |
| Total current assets | |
| 10,292 | | |
| 20,280 | |
| Property and equipment, net | |
| 2,717 | | |
| 2,949 | |
| Intangible assets, net | |
| 2,482 | | |
| 2,409 | |
| Operating lease asset | |
| 1,379 | | |
| 1,531 | |
| Other assets | |
| 223 | | |
| 244 | |
| Total assets | |
$ | 17,093 | | |
$ | 27,413 | |
| Liabilities and stockholders’ equity | |
| | | |
| | |
| Current liabilities: | |
| | | |
| | |
| Accounts payable | |
$ | 1,700 | | |
$ | 1,751 | |
| Accrued expenses | |
| 1,308 | | |
| 650 | |
| Current portion of lease liability | |
| 578 | | |
| 564 | |
| Estimated lawsuit liability | |
| - | | |
| 1,398 | |
| Deferred revenue | |
| 496 | | |
| 506 | |
| Total current liabilities | |
| 4,082 | | |
| 4,869 | |
| Long-term liabilities: | |
| | | |
| | |
| Lease liability | |
| 1,748 | | |
| 2,041 | |
| Total long-term liabilities | |
| 1,748 | | |
| 2,041 | |
| Total liabilities | |
| 5,830 | | |
| 6,910 | |
| Commitments and contingencies (Note 9) | |
| | | |
| | |
| Stockholders’ equity: | |
| | | |
| | |
| Preferred stock, $0.001 par value per share, 5,000,000 shares authorized, no shares issued and outstanding at June 30, 2023 and December 31, 2022 | |
| - | | |
| - | |
| Class A Common Stock, $0.001 par value per share, 84,295,000 shares authorized, 6,314,225 shares issued and outstanding at June 30, 2023: 6,127,320 issued and outstanding at December 31, 2022 | |
| 6 | | |
| 6 | |
| Class B Common Stock, $0.001 par value per share, 15,705,000 shares authorized, 14,855,539 shares issued and outstanding at June 30, 2023; 14,891,085 issued and outstanding, at December 31, 2022 | |
| 15 | | |
| 15 | |
| Additional paid-in capital | |
| 84,729 | | |
| 83,712 | |
| Stock subscription receivable | |
| (100 | ) | |
| (100 | ) |
| Accumulated deficit | |
| (73,052 | ) | |
| (62,773 | ) |
| Accumulated other comprehensive loss | |
| (335 | ) | |
| (357 | ) |
| Total stockholders’ equity | |
| 11,263 | | |
| 20,503 | |
| Total liabilities and stockholders’ equity | |
$ | 17,093 | | |
$ | 27,413 | |
See accompanying notes to unaudited condensed
financial statements.
Longeveron Inc.
Condensed Statements of Operations
(In thousands, except per share data)
(Unaudited)
| | |
Three months ended
June 30, | | |
Six months ended
June 30, | |
| | |
2023 | | |
2022 | | |
2023 | | |
2022 | |
| Revenues | |
| | |
| | |
| | |
| |
| Clinical trial revenue | |
$ | 217 | | |
$ | 340 | | |
$ | 455 | | |
$ | 650 | |
| Grant revenue | |
| - | | |
| 126 | | |
| 41 | | |
| 186 | |
| Total revenues | |
| 217 | | |
| 466 | | |
| 496 | | |
| 836 | |
| Cost of revenues | |
| 124 | | |
| 306 | | |
| 327 | | |
| 376 | |
| Gross profit | |
| 93 | | |
| 160 | | |
| 169 | | |
| 460 | |
| | |
| | | |
| | | |
| | | |
| | |
| Operating expenses | |
| | | |
| | | |
| | | |
| | |
| General and administrative | |
| 3,375 | | |
| 2,427 | | |
| 5,230 | | |
| 4,407 | |
| Research and development | |
| 2,287 | | |
| 1,720 | | |
| 5,067 | | |
| 3,147 | |
| Selling and marketing | |
| 143 | | |
| 234 | | |
| 300 | | |
| 521 | |
| Total operating expenses | |
| 5,805 | | |
| 4,381 | | |
| 10,597 | | |
| 8,075 | |
| Loss from operations | |
| (5,712 | ) | |
| (4,221 | ) | |
| (10,428 | ) | |
| (7,615 | ) |
| Other income and (expenses) | |
| | | |
| | | |
| | | |
| | |
| Non-operating lawsuit expense | |
| - | | |
| (1,398 | ) | |
| - | | |
| (1,398 | ) |
| Other income and (expenses), net | |
| 80 | | |
| (5 | ) | |
| 149 | | |
| (121 | ) |
| Total other income and (expenses), net | |
| 80 | | |
| (1,403 | ) | |
| 149 | | |
| (1,519 | ) |
| Net loss | |
$ | (5,632 | ) | |
$ | (5,624 | ) | |
$ | (10,279 | ) | |
$ | (9,134 | ) |
Basic and diluted net loss per share | |
$ | (0.27 | ) | |
$ | (0.27 | ) | |
$ | (0.49 | ) | |
$ | (0.44 | ) |
Basic and diluted weighted average common shares outstanding | |
| 21,105,420 | | |
| 20,943,897 | | |
| 21,069,714 | | |
| 20,927,640 | |
See accompanying notes to unaudited condensed
financial statements.
Longeveron Inc.
Condensed Statements of Comprehensive Loss
(In thousands, except per share data)
(Unaudited)
| | |
Three months ended
June 30, | | |
Six months ended
June 30, | |
| | |
2023 | | |
2022 | | |
2023 | | |
2022 | |
| | |
| | |
| | |
| | |
| |
| Net loss | |
$ | (5,632 | ) | |
$ | (5,624 | ) | |
$ | (10,279 | ) | |
$ | (9,134 | ) |
| Other comprehensive loss: | |
| | | |
| | | |
| | | |
| | |
| Net unrealized (loss) gain on available-for-sale securities | |
| (36 | ) | |
| - | | |
| 22 | | |
| - | |
| Total comprehensive loss | |
$ | (5,668 | ) | |
$ | (5,624 | ) | |
$ | (10,257 | ) | |
$ | (9,134 | ) |
See accompanying notes to unaudited condensed
financial statements.
Longeveron Inc.
Condensed Statements of Stockholders’
Equity
(In thousands, except share amounts)
(Unaudited)
| | |
Class A
Common Stock | | |
Class B
Common Stock | | |
Subscription | | |
Additional
Paid-In | | |
Accumulated | | |
Accumulated
Other
Comprehensive | | |
Total
Stockholder’s | |
| | |
Number | | |
Amount | | |
Number | | |
Amount | | |
Receivable | | |
Capital | | |
Deficit | | |
Loss | | |
Equity | |
| Balance at December 31, 2022 | |
| 6,127,320 | | |
$ | 6 | | |
| 14,891,085 | | |
$ | 15 | | |
$ | (100 | ) | |
$ | 83,712 | | |
$ | (62,773 | ) | |
$ | (357 | ) | |
$ | 20,503 | |
| Conversion of Class B common stock for Class A common stock | |
| 35,546 | | |
| - | | |
| (35,546 | ) | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | |
| Class A Common Stock, issued for RSUs vested | |
| 179,723 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | |
| Class A Common Stock, held for taxes on RSUs vested | |
| (28,364 | ) | |
| - | | |
| - | | |
| - | | |
| - | | |
| (103 | ) | |
| - | | |
| - | | |
| (103 | ) |
| Equity-based compensation | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 1,120 | | |
| - | | |
| - | | |
| 1,120 | |
| Unrealized loss attributable to change in market value of available for sale investments | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 22 | | |
| 22 | |
| Net loss | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (10,279 | ) | |
| - | | |
| (10,279 | ) |
| Balance at June 30, 2023 | |
| 6,314,225 | | |
$ | 6 | | |
| 14,855,539 | | |
$ | 15 | | |
$ | (100 | ) | |
$ | 84,729 | | |
$ | (73,052 | ) | |
$ | (335 | ) | |
$ | 11,263 | |
| | |
Class A
Common Stock | | |
Class B
Common Stock | | |
Subscription | | |
Additional
Paid-In | | |
Accumulated | | |
Accumulated
Other
Comprehensive | | |
Total
Stockholder’s | |
| | |
Number | | |
Amount | | |
Number | | |
Amount | | |
Receivable | | |
Capital | | |
Deficit | | |
Loss | | |
Equity | |
| Balance at December 31, 2021 | |
| 5,175,361 | | |
$ | 5 | | |
| 15,702,834 | | |
$ | 16 | | |
$ | (100 | ) | |
$ | 81,470 | | |
$ | (43,938 | ) | |
$ | - | | |
$ | 37,453 | |
| Conversion of Units into Class A and B common stock | |
| 641,749 | | |
| 1 | | |
| (641,749 | ) | |
| (1 | ) | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | |
| Class A Common Stock, issued for RSUs vested | |
| 131,959 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | |
| Class A Common Stock, held for taxes on RSUs vested | |
| (23,507 | ) | |
| - | | |
| - | | |
| - | | |
| - | | |
| (294 | ) | |
| - | | |
| - | | |
| (294 | ) |
| Class A Common Stock Options Exercised | |
| 374 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | |
| Equity-based compensation | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 1,356 | | |
| - | | |
| - | | |
| 1,356 | |
| Net loss | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (9,134 | ) | |
| - | | |
| (9,134 | ) |
| Balance at June 30, 2022 | |
| 5,925,935 | | |
$ | 6 | | |
| 15,061,085 | | |
$ | 15 | | |
$ | (100 | ) | |
$ | 82,532 | | |
$ | (53,072 | ) | |
$ | - | | |
$ | 29,381 | |
See
accompanying notes to unaudited condensed financial statements.
Longeveron Inc.
Condensed Statements of Stockholders’
Equity
(In thousands, except share amounts)
(Unaudited)
| | |
Class A
Common Stock | | |
Class B
Common Stock | | |
Subscription | | |
Additional
Paid-In | | |
Accumulated | | |
Accumulated
Other
Comprehensive | | |
Total
Stockholder’s | |
| | |
Number | | |
Amount | | |
Number | | |
Amount | | |
Receivable | | |
Capital | | |
Deficit | | |
Loss | | |
Equity | |
| Balance at March 31, 2023 | |
| 6,163,050 | | |
$ | 6 | | |
| 14,871,085 | | |
$ | 15 | | |
$ | (100 | ) | |
$ | 84,116 | | |
$ | (67,420 | ) | |
$ | (299 | ) | |
$ | 16,318 | |
| Conversion of Class B common stock for Class A common stock | |
| 15,546 | | |
| - | | |
| (15,546 | ) | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | |
| Class A Common Stock, issued for RSUs vested | |
| 159,562 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | |
| Class A Common Stock, held for taxes on RSUs vested | |
| (23,933 | ) | |
| - | | |
| - | | |
| - | | |
| - | | |
| (86 | ) | |
| - | | |
| - | | |
| (86 | ) |
| Equity-based compensation | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 699 | | |
| - | | |
| - | | |
| 699 | |
| Unrealized loss attributable to change in market value of available for sale investments | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (36 | ) | |
| (36 | ) |
| Net loss | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (5,632 | ) | |
| - | | |
| (5,632 | ) |
| Balance at June 30, 2023 | |
| 6,314,225 | | |
$ | 6 | | |
| 14,855,539 | | |
$ | 15 | | |
$ | (100 | ) | |
$ | 84,729 | | |
$ | (73,052 | ) | |
$ | (335 | ) | |
$ | 11,263 | |
| | |
Class A
Common Stock | | |
Class B
Common Stock | | |
Subscription | | |
Additional
Paid-In | | |
Accumulated | | |
Accumulated
Other
Comprehensive | | |
Total
Stockholder’s | |
| | |
Number | | |
Amount | | |
Number | | |
Amount | | |
Receivable | | |
Capital | | |
Deficit | | |
Loss | | |
Equity | |
| Balance at March 31, 2022 | |
| 5,326,512 | | |
$ | 5 | | |
| 15,585,062 | | |
$ | 16 | | |
$ | (100 | ) | |
$ | 81,833 | | |
$ | (47,448 | ) | |
$ | - | | |
$ | 34,306 | |
| Conversion of Units into Class A and B common stock | |
| 523,977 | | |
| 1 | | |
| (523,977 | ) | |
| (1 | ) | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | |
| Class A Common Stock, issued for RSUs vested | |
| 87,953 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | |
| Class A Common Stock, held for taxes on RSUs vested | |
| (12,881 | ) | |
| - | | |
| - | | |
| - | | |
| - | | |
| (166 | ) | |
| - | | |
| - | | |
| (166 | ) |
| Class A Common Stock Options Exercised | |
| 374 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | |
| Equity-based compensation | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 865 | | |
| - | | |
| - | | |
| 865 | |
| Net loss | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (5,624 | ) | |
| - | | |
| (5,624 | ) |
| Balance at June 30, 2022 | |
| 5,925,935 | | |
$ | 6 | | |
| 15,061,085 | | |
$ | 15 | | |
$ | (100 | ) | |
$ | 82,532 | | |
$ | (53,072 | ) | |
$ | - | | |
$ | 29,381 | |
See
accompanying notes to unaudited condensed financial statements.
Longeveron Inc.
Condensed Statements of Cash Flows
(In thousands)
(Unaudited)
| | |
Six months ended
June 30, | |
| | |
2023 | | |
2022 | |
| Cash flows from operating activities | |
| | |
| |
| Net loss | |
$ | (10,279 | ) | |
$ | (9,134 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: | |
| | | |
| | |
| Depreciation and amortization | |
| 478 | | |
| 417 | |
| Interest earned on marketable securities | |
| 151 | | |
| 123 | |
| Equity issued for consulting services | |
| - | | |
| 170 | |
| Equity-based compensation | |
| 1,120 | | |
| 1,186 | |
| Non-operating lawsuit expense | |
| - | | |
| 1,398 | |
| Changes in operating assets and liabilities: | |
| | | |
| | |
| Accounts and grants receivable | |
| 122 | | |
| (82 | ) |
| Prepaid expenses and other current assets | |
| (1,136 | ) | |
| (759 | ) |
| Other assets | |
| 23 | | |
| (42 | ) |
| Accounts payable | |
| (51 | ) | |
| (241 | ) |
| Deferred revenue | |
| (10 | ) | |
| 321 | |
| Estimated lawsuit liability | |
| (1,398 | ) | |
| - | |
| Accrued expenses | |
| 658 | | |
| (504 | ) |
| Lease asset and lease liability | |
| (127 | ) | |
| (280 | ) |
| Net cash used in operating activities | |
| (10,449 | ) | |
| (7,427 | ) |
| Cash flows from investing activities | |
| | | |
| | |
| Proceeds from the sale of marketable securities | |
| 3,116 | | |
| 2,497 | |
| Acquisition of property and equipment | |
| (134 | ) | |
| (125 | ) |
| Acquisition of intangible assets | |
| (186 | ) | |
| (90 | ) |
| Net cash provided by investing activities | |
| 2,796 | | |
| 2,282 | |
| Cash flows from financing activities | |
| | | |
| | |
| Payments for taxes on RSUs vested | |
| (103 | ) | |
| (289 | ) |
| Net cash used in financing activities | |
| (103 | ) | |
| (289 | ) |
| Change in cash and cash equivalents | |
| (7,756 | ) | |
| (5,434 | ) |
| Cash and cash equivalents at beginning of the period | |
| 10,503 | | |
| 25,658 | |
| Cash and cash equivalents at end of the period | |
$ | 2,747 | | |
$ | 20,224 | |
| Supplement Disclosure of Non-cash Investing and Financing Activities: | |
| | | |
| | |
| Vesting of RSUs into Class A Common Stock | |
$ | (575 | ) | |
$ | (1,017 | ) |
See accompanying notes to unaudited condensed
financial statements.
Longeveron Inc.
Notes to the Condensed Financial Statements
(Unaudited)
Three and Six Month Periods Ended June 30, 2023
and 2022
1. Nature of Business, Basis of Presentation, and Liquidity
Nature of business:
Longeveron was formed as a Delaware limited liability company on October
9, 2014, and was authorized to transact business in Florida on December 15, 2014. On February 12, 2021, Longeveron, LLC converted its
corporate form (the “Corporate Conversion”) from a Delaware limited liability company (Longeveron, LLC) to a Delaware corporation,
Longeveron Inc. (the “Company,” “Longeveron” or “we,” “us,” or “our”). The
Company is a clinical stage biotechnology company developing cellular therapies for specific aging-related and life-threatening
conditions and operates out of its leased facilities in Miami, Florida.
The Company’s product candidates are currently in development.
There can be no assurance that the Company’s research and development will be successfully completed, that adequate protection
for the Company’s intellectual property will be obtained, that any products developed will obtain necessary government regulatory
approval or that any approved products will be commercially viable. Even if the Company’s product development efforts are successful,
it is uncertain when, if ever, the Company will generate significant revenue from product sales. The Company operates in an environment
of rapid technological change and substantial competition from, among others, existing pharmaceutical and biotechnology companies. In
addition, the Company is dependent upon the services of its employees, partners and consultants.
The accompanying interim condensed balance sheet as of June 30, 2023,
and the condensed statements of operations, statement of comprehensive loss, stockholders’ equity, and cash flows for the three
and six months ended June 30, 2023 and 2022, are unaudited. The unaudited condensed financial statements have been prepared according
to the rules and regulations of the Securities and Exchange Commission (“SEC”) and, therefore, certain information and disclosures
normally included in financial statements prepared in accordance with accounting principles generally accepted in the United States of
America (“US GAAP”) have been omitted. In the opinion of management, the accompanying unaudited condensed financial statements
for the periods presented reflect all adjustments which are normal and recurring, and necessary to fairly state the financial position,
results of operations, and cash flows of the Company. These unaudited condensed financial statements and notes should be read in conjunction
with the audited financial statements and notes thereto in the Company’s 2022 Annual Report on Form 10-K filed with the SEC on
March 14, 2023.
Liquidity:
Since inception, the Company has primarily been engaged in organizational
activities, including raising capital, and research and development activities. The Company does not yet have a product that has been
approved by the U.S. Food and Drug Administration (“FDA”), and has only generated revenues from grants, clinical trials and
contract manufacturing. The Company has not yet achieved profitable operations or generated positive cash flows from operations. The
Company intends to continue its efforts to raise additional equity financing, develop its intellectual property, and secure regulatory
approvals to commercialize its products. There is no assurance that profitable operations, if achieved, could be sustained on a continuing
basis. Further, the Company’s future operations are dependent on the success of the Company’s efforts to raise additional
capital, its research and commercialization efforts, regulatory approval, and, ultimately, the market acceptance of the Company’s
approved products, if any. These financial statements do not include adjustments that might result from the outcome of these uncertainties.
The Company has incurred recurring losses from operations since its
inception, including a net loss of $10.3 million and $9.1 million for the six months ended June 30, 2023 and 2022, respectively. In addition,
as of June 30, 2023, the Company had an accumulated deficit of $73.1 million. The Company expects to continue to generate operating losses
for the foreseeable future.
As of June 30, 2023, the Company had cash, and cash equivalents of
$2.7 million and marketable securities of $5.9 million. The Company has prepared a cash flow forecast which indicates that it does not
have sufficient cash to meet its minimum expenditure commitments for one year from the date these condensed financial statements are
available to be issued and therefore needs to raise additional funds to continue as a going concern. As a result, there is substantial
doubt about the Company’s ability to continue as a going concern. To address the future funding requirements, management has undertaken
the following initiatives:
| ● | On June 27,2023 the Company filed a registration statement
with the SEC to conduct a tradeable subscription rights offering for up to $30.0 million of shares of Class A common stock to its stockholders
and holders of warrants to purchase common stock as of a future record date to be determined. The Company expects to undertake and close
the offering as outlined in the registration statement. On July 28, 2023 the Company filed a first amendment to the registration statement.
The Company may seek additional capital in the private and/or public equity markets, to continue its operations, respond to competitive
pressures, develop new products and services, and to support new strategic partnerships. The Company is evaluating additional equity/debt
financing opportunities on an ongoing basis and may execute them when appropriate. However, there can be no assurances that the Company
can consummate the rights offering or any transaction or consummate a transaction at favorable pricing or at all; |
| ● | the Company will attempt to use equity instruments to provide
a portion of the compensation due to vendors and collaboration partners; |
| ● | the Company plans to pursue potential partnerships for pipeline
programs, however, there can be no assurances that it can consummate such transactions; |
| ● | the Company will continue to support its Bahamas Registry
to generate revenue; and |
| ● | since 2016 our clinical programs have received over $16.0
million in competitive extramural grant awards ($11.5 million which has been directly awarded to us and which are recognized as revenue
when the performance obligations are met) from the National Institutes of Health (NIH), Alzheimer’s Association, and Maryland Stem
Cell Research Fund (MSCRF), and the Company plans to submit additional contract and grant applications for further support of its programs
with various funding agencies. |
The Company’s condensed financial statements do not include
any adjustments to the assets carrying amount, to the expenses presented and to the reclassification of the condensed balance sheets
items that could be necessary should the Company be unable to continue its operations.
2. Summary
of Significant Accounting Policies
Basis of presentation:
The financial statements of the Company were prepared in accordance
with accounting principles generally accepted in the U.S. (“U.S. GAAP”).
Certain reclassifications have been made to prior year financial statements
to conform to classifications used in the current year. These reclassifications had no impact on net loss, stockholders’ equity
or cash flows as previously reported.
Use of estimates:
The presentation of financial statements in conformity with U.S. GAAP
requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent
assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting
period. Actual results could differ from those estimates.
Accounting Standard Updates
A variety of proposed or otherwise potential accounting standards
are currently under consideration by standard-setting organizations and certain regulatory agencies. Because of the tentative and preliminary
nature of such proposed standards, management has not yet determined the effect, if any, that the implementation of such proposed standards
would have on the Company’s condensed financial statements.
In June 2016, the FASB issued ASU 2016-13, Financial Instruments -
Credit Losses: Measurement of Credit Losses on Financial Instruments. The standard requires that credit losses be reported using an expected
losses model rather than the incurred losses model that is currently used, and establishes additional disclosures related to credit risks.
For available-for-sale debt securities with unrealized losses, these standards now require allowances to be recorded instead of reducing
the amortized cost of the investment. These standards limit the amount of credit losses to be recognized for available-for-sale debt
securities to the amount by which carrying value exceeds fair value and requires the reversal of previously recognized credit losses
if fair value increases. The adoption of the standard as of January 1, 2023 did not have a material impact on the Company’s condensed
financial statements; however, the Company did record net unrealized gains and losses in the condensed statement of comprehensive loss
for the three and six month periods ended June 30, 2023.
Cash and cash equivalents:
The Company considers cash to consist of cash on hand and temporary
investments having an original maturity of 90 days or less that are readily convertible into cash.
Marketable securities:
Marketable securities at June 30, 2023 and December 31, 2022 consisted
of marketable fixed income securities, primarily corporate bonds, as well as U.S. Government and agency obligations which are categorized
as trading securities and are thus marked to market and stated at fair value in accordance with ASC 820 Fair Value Measurement. These
investments are considered Level 1 and Level 2 investments within the ASC 820 fair value hierarchy. The fair value of Level 1 investments,
including cash equivalents, money funds and U.S. government securities, are substantially based on quoted market prices. The fair value
of corporate bonds is determined using standard market valuation methodologies, including discounted cash flows, matrix pricing and/or
other similar techniques. The inputs to these valuation techniques include but are not limited to market interest rates, credit
rating of the issuer or counterparty, industry sector of the issuer, coupon rate, call provisions, maturity, estimated duration and assumptions
regarding liquidity and estimated future cash flows. In addition to bond characteristics, the valuation methodologies incorporate
market data, such as actual trades completed, bids and actual dealer quotes, where such information is available. Accordingly, the estimated
fair values are based on available market information and judgments about financial instruments categorized within Level 1 and Level
2 of the fair value hierarchy. Interest and dividends are recorded when earned. Realized gains and losses on investments are
determined by specific identification and are recognized as incurred in the statement of operations. Changes in net unrealized gains
and losses are reported in other comprehensive loss and represent the change in the fair value of investment holdings during the reporting
period. Changes in net unrealized gains and losses were less than $0.1 million for the three and six months ended June 30, 2023
and 2022.
Accounts and grants receivable:
Accounts and grants receivable include amounts due from customers,
granting institutions and others. The amounts as of June 30, 2023 and December 31, 2022 are certain to be collected, and no amount has
been recognized for doubtful accounts. In addition, for the Clinical trial revenue, most participants pay in advance of treatment. Advanced
grant funds and prepayments for the Clinical trial revenue are recorded to deferred revenue.
Accounts and grants receivable by source, as
of (in thousands):
| | |
June 30,
2023 | | |
December 31,
2022 | |
| National Institutes of Health – Grant | |
$ | 96 | | |
$ | 218 | |
| Total | |
$ | 96 | | |
$ | 218 | |
Deferred offering costs:
The Company recorded certain legal, professional and other third-party
fees that were directly associated with in-process equity financings as deferred offering costs until the applicable equity financing
was consummated. After consummation of an equity financing, these costs are recorded in stockholders’ equity as a reduction of
proceeds generated as a result of the offering.
Property and equipment:
Property and equipment, including improvements that extend useful
lives of related assets, are recorded at cost, while maintenance and repairs are charged to operations as incurred. Depreciation is calculated
using the straight-line method based on the estimated useful lives of the assets. Leasehold improvements are amortized over the shorter
of the estimated useful life of the asset or the original term of the lease. Depreciation expense is recorded in the research and development
line of the condensed statements of operations as the assets are primarily related to the Company’s clinical programs.
Intangible assets:
Intangible assets include payments on license agreements with the
Company’s co-founder and chief scientific officer (“CSO”) and the University of Miami (“UM”) (see Note
9) and legal costs incurred related to patents and trademarks. License agreements have been recorded at the value of cash consideration,
common stock and membership units transferred to the respective parties when acquired.
Payments for license agreements are amortized using the straight-line
method over the estimated term of the agreements, which range from 5-20 years. Patents are amortized over their estimated useful life,
once issued. The Company considers trademarks to have an indefinite useful life and evaluates them for impairment on an annual basis.
Amortization expense is recorded in the research and development line of the condensed statements of operations as the assets are primarily
related to the Company’s clinical programs.
Impairment of Long-Lived Assets:
The Company evaluates long-lived assets for impairment, including
property and equipment and intangible assets, when events or changes in circumstances indicate that the carrying value of such assets
may not be recoverable. Upon the occurrence of a triggering event, the asset is reviewed to assess whether the estimated undiscounted
cash flows expected from the use of the asset plus the residual value from the ultimate disposal exceeds the carrying value of the asset.
If the carrying value exceeds the estimated recoverable amounts, the asset is written down to the estimated fair value. Any resulting
impairment loss is reflected on the condensed statements of operations. Upon evaluation, management determined that there was no impairment
of long-lived assets during the three and six months ended June 30, 2023 and 2022.
Deferred revenue:
The unearned portion of advanced grant funds and prepayments for clinical
trial revenue, which will be recognized as revenue when the Company meets the respective performance obligations, has been presented
as deferred revenue in the accompanying condensed balance sheets. For the six months ended June 30, 2023 and 2022, the Company recognized
less than $0.1 million and $0.1 million, respectively of funds that were previously classified as deferred revenue ($0 and $0.1 million,
respectively for the three months ended June 30, 2023 and 2022).
Revenue recognition:
The Company recognizes revenue when performance obligations related
to respective revenue streams are met. For grant revenue, the Company considers the performance obligation met when the grant related
expenses are incurred or supplies and materials are received. The Company is paid in tranches pursuant to terms of the related grant
agreements, and then applies payments based on regular expense reimbursement submissions to grantors. There are no remaining performance
obligations or variable consideration once grant expense reporting to the grantor is complete. For clinical trial revenue, the Company
considers the performance obligation met when the participant has received the treatment. The Company usually receives prepayment for
these services or receives payment at the time the treatment is provided, and there are no remaining performance obligations or variable
consideration once the participant receives the treatment. For contract manufacturing revenue, the Company considers the performance
obligation met when the contractual obligation and/or statement of work has been satisfied. Payment terms may vary depending on specific
contract terms. There are no significant judgments affecting the determination of the amount and timing of revenue recognition.
Revenue by source (in thousands):
| | |
Three months ended
June 30, | | |
Six months ended
June 30, | |
| | |
2023 | | |
2022 | | |
2023 | | |
2022 | |
| National Institute of Health - grant | |
$ | - | | |
$ | 41 | | |
$ | 41 | | |
$ | 82 | |
| Clinical trial revenue | |
| 217 | | |
| 340 | | |
| 455 | | |
| 650 | |
| MSCRF – TEDCO1 - grant | |
| - | | |
| 85 | | |
| - | | |
| 104 | |
| Total | |
$ | 217 | | |
$ | 466 | | |
$ | 496 | | |
$ | 836 | |
| 1 | Maryland Stem Cell Research Fund (MSCRF) - Maryland Technology
Development Corporation (TEDCO) |
The Company records cost of revenues based on expenses directly related
to revenue. For Grants, the Company records allocated expenses for Research and development costs to a grant as a cost of revenues. For
the Clinical trial revenue, directly related expenses for that program are expensed as incurred. These expenses are similar to those
described under “Research and development expense” below.
Research and development expense:
Research and development costs are charged to expense when incurred
in accordance with ASC 730 Research and Development. ASC 730 addresses the proper accounting and reporting for research and development
costs. It identifies: 1) those activities that should be identified as research and development; 2) the elements of costs that should
be identified with research and development activities, and the accounting for these costs; and 3) the financial statement disclosures
related to them. Research and development costs include costs such as clinical trial expenses, contracted research and license agreement
fees with no alternative future use, supplies and materials, salaries, share-based compensation, employee benefits, property and equipment
depreciation and allocation of various corporate costs. The Company accrues for costs incurred by external service providers, including
contract research organizations and clinical investigators, based on its estimates of service performed and costs incurred. These estimates
include the level of services performed by the third parties, patient enrollment in clinical trials, administrative costs incurred by
the third parties, and other indicators of the services completed. Based on the timing of amounts invoiced by service providers, the
Company may also record payments made to those providers as prepaid expenses that will be recognized as expense in future periods as
the related services are rendered.
Concentrations of credit risk:
Financial instruments which potentially subject the Company to credit
risk consist principally of cash and cash equivalents, short-term investments and accounts and grants receivable. Cash and cash equivalents
are held in U.S. financial institutions. At times, the Company may maintain balances in excess of the federally insured amounts.
Income taxes:
Prior to its Corporate Conversion, the Company was treated as a partnership
for U.S. federal and state income tax purposes. Consequently, the Company passed its earnings and losses through to its members based
on the terms of the Company’s Operating Agreement. Accordingly, no provision for income taxes is recorded in the financial statements
for periods prior to the conversion.
Following the Corporate Conversion, the Company’s tax provision consists
of taxes currently payable or receivable, plus any change during the period in deferred tax assets and liabilities. The Company uses
the asset and liability method of accounting for income taxes. Under this method, deferred tax assets and liabilities are recognized
for the future tax consequences attributable to differences between the financial statement carrying amounts of assets and liabilities
and their respective tax basis. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable
income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and
liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. In addition, a valuation
allowance is established to reduce any deferred tax asset for which it is determined that it is more likely than not that some portion
of the deferred tax asset will not be realized. The Company’s tax provision was $0 for the three and six months ended June 30, 2023 and
2022 due to net operating losses. The Company has not recorded any tax benefit for the net operating losses incurred due to the uncertainty
of realizing a benefit in the future.
The Company recognizes the tax benefits from uncertain tax positions
that the Company has taken or expects to take on a tax return. In the unlikely event an uncertain tax position exists in which the Company
could incur income taxes, the Company would evaluate whether there is a probability that the uncertain tax position taken would be sustained
upon examination by a taxing authority. Reserves for uncertain tax positions would then be recorded if the Company determined it is probable
that either a position would not be sustained upon examination or a payment would have to be made to a taxing authority and the amount
was reasonably estimable. As of June 30, 2023 and December 31, 2022, the Company does not believe it has any uncertain tax positions
that would result in the Company having a liability to a taxing authority. It is the Company’s policy to expense any interest and
penalties associated with its tax obligations when they are probable and estimable.
Equity-based compensation:
The Company accounts for equity-based compensation expense by the
measurement and recognition of compensation expense for stock-based awards based on estimated fair values on the date of grant. The fair
value of the stock options is estimated at the date of the grant using the Black-Scholes option-pricing model.
The Black-Scholes option-pricing model requires the input of highly
subjective assumptions, the most significant of which are the expected share price volatility, the expected life of the option award,
the risk-free rate of return, and dividends during the expected term. Because the option-pricing model is sensitive to changes in the
input assumptions, different determinations of the required inputs may result in different fair value estimates of the options.
Neither the Company’s stock options nor its restricted stock
units (“RSUs”) trade on an active market. Volatility is a measure of the amount by which a financial variable, such as a
stock price, has fluctuated (historical volatility) or is expected to fluctuate (expected volatility) during a period. Given the Company’s
limited historical data, the Company utilizes the average historical volatility of similar publicly traded companies that are in the
same industry. The risk-free interest rate is the average U.S. treasury rate (having a term that most closely approximates the expected
life of the option) for the period in which the option was granted. The expected life is the period of time that the options granted
are expected to remain outstanding. Options granted have a maximum term of ten years. The Company has insufficient historical data to
utilize in determining its expected life assumptions and, therefore, uses the simplified method for determining expected life.
3. Marketable
securities
The following is summary of marketable securities
that the Company measures at fair value (in thousands):
| | |
Fair Value at June 30, 2023 | |
| | |
Level 1 | | |
Level 2 | | |
Level 3 | | |
Total | |
| | |
| | |
| | |
| | |
| |
| U.S. Treasury obligations | |
$ | 99 | | |
$ | - | | |
$ | - | | |
$ | 99 | |
| U.S. government agencies | |
| - | | |
| 1,284 | | |
| - | | |
| 1,284 | |
| Corporate and foreign bonds | |
| - | | |
| 4,527 | | |
| - | | |
| 4,527 | |
| Money market funds(1) | |
| 1,027 | | |
| - | | |
| - | | |
| 1,027 | |
| Accrued income | |
| 42 | | |
| - | | |
| - | | |
| 42 | |
| Total marketable securities | |
$ | 1,168 | | |
$ | 5,811 | | |
$ | - | | |
$ | 6,979 | |
| | |
Fair Value at December 31, 2022 | |
| | |
Level 1 | | |
Level 2 | | |
Level 3 | | |
Total | |
| | |
| | |
| | |
| | |
| |
| U.S. Treasury obligations | |
$ | 97 | | |
$ | - | | |
$ | - | | |
$ | 97 | |
| U.S. government agencies | |
| - | | |
| 1,250 | | |
| - | | |
| 1,250 | |
| Corporate and foreign bonds | |
| - | | |
| 7,808 | | |
| - | | |
| 7,808 | |
| Money market funds(1) | |
| 607 | | |
| - | | |
| - | | |
| 607 | |
| Accrued income | |
| 65 | | |
| - | | |
| - | | |
| 65 | |
| Total marketable
securities | |
$ | 769 | | |
$ | 9,058 | | |
$ | - | | |
$ | 9,827 | |
| (1) | Money market funds are included in cash and cash equivalents
in the condensed balance sheet. |
As of June 30, 2023 and December 31, 2022, the Company reported accrued
interest receivable related to marketable securities of less than $0.1 million and $0.1 million, respectively. These amounts are recorded
in other assets on the condensed balance sheets and are not included in the carrying value of the marketable securities.
4. Property
and equipment, net
Major components of property and equipment are as follows (in thousands):
| | |
Useful Lives | |
June 30,
2023 | | |
December 31,
2022 | |
| Leasehold improvements | |
10 years | |
$ | 4,328 | | |
$ | 4,328 | |
| Furniture/Lab equipment | |
7 years | |
| 2,366 | | |
| 2,264 | |
| Computer equipment | |
5 years | |
| 77 | | |
| 46 | |
| Software/Website | |
3 years | |
| 38 | | |
| 38 | |
| Total property and equipment | |
| |
| 6,809 | | |
| 6,676 | |
| Less accumulated depreciation and amortization | |
| |
| 4,092 | | |
| 3,727 | |
| Property and equipment, net | |
| |
$ | 2,717 | | |
$ | 2,949 | |
Depreciation and amortization expense amounted to approximately $0.2
million for each of the three-month periods ended June 30, 2023 and 2022, and $0.4 million and $0.3 million for the six months ended
June 30, 2023 and 2022, respectively.
5. Intangible
assets, net
Major components of intangible assets as of June
30, 2023 are as follows (in thousands):
| | |
Useful Lives | |
Cost | | |
Accumulated
Amortization | | |
Total | |
| License agreements | |
20 years | |
$ | 2,043 | | |
$ | (797 | ) | |
$ | 1,246 | |
| Patent Costs | |
| |
| 1,046 | | |
| - | | |
| 1,046 | |
| Trademark costs | |
| |
| 190 | | |
| - | | |
| 190 | |
| Total | |
| |
$ | 3,279 | | |
$ | (797 | ) | |
$ | 2,482 | |
Major components of intangible assets as of December
31, 2022 are as follows:
| | |
Useful Lives | |
Cost | | |
Accumulated
Amortization | | |
Total | |
| License agreements | |
20 years | |
$ | 2,043 | | |
$ | (685 | ) | |
$ | 1,358 | |
| Patent Costs | |
| |
| 887 | | |
| - | | |
| 887 | |
| Trademark costs | |
| |
| 164 | | |
| - | | |
| 164 | |
| Total | |
| |
$ | 3,094 | | |
$ | (685 | ) | |
$ | 2,409 | |
Amortization expense related to intangible assets amounted to approximately
$0.1 million for each of the three- and six-month periods ended June 30, 2023 and 2022.
Future amortization
expense for intangible assets as of June 30, 2023 is approximately as follows (in thousands):
| Year Ending December 31, | |
Amount | |
| 2023 (remaining six months) | |
$ | 112 | |
| 2024 | |
| 224 | |
| 2025 | |
| 224 | |
| 2026 | |
| 224 | |
| 2027 | |
| 224 | |
| Thereafter | |
| 238 | |
| Total | |
$ | 1,246 | |
6. Leases
The Company records an operating lease asset and an operating lease
liability related to its operating leases (there are no finance leases). The Company’s corporate office lease expires in March
2027. As of June 30, 2023, the operating lease asset and operating lease liability were approximately $1.4 million and $2.3 million,
respectively. As of December 31, 2022, the operating lease asset and operating lease liability were approximately $1.5 million and $2.6
million, respectively.
Future minimum
payments under the operating leases as of June 30, 2023 are as follows (in thousands):
| Year Ending December 31, | |
Amount | |
| 2023 (remaining six months) | |
$ | 341 | |
| 2024 | |
| 682 | |
| 2025 | |
| 682 | |
| 2026 | |
| 682 | |
| 2027 | |
| 169 | |
| Total | |
| 2,556 | |
| Less: Interest | |
| 230 | |
| Present value of operating lease liability | |
$ | 2,326 | |
During each of the three month periods ended June 30, 2023 and 2022,
the Company incurred approximately $0.1 million of total lease costs and for the six month periods ended June 30, 2023 and 2022, the
Company incurred approximately $0.2 million and $0.1 million of total lease costs, respectively, that are included in the general and
administrative expenses in the statements of operations.
7. Stockholders’ Equity
Class A Common Stock
On April 19, 2023, the Company finalized the Separation Agreement
effective June 9, 2023, for James Clavijo, the Company’s former Chief Financial Officer. In part for his agreement to a general
release the Company agreed to pay Mr. Clavijo $275,000 as severance compensation, three months of payment for COBRA insurance coverage
and the immediate acceleration and vesting of 6,690 RSUs that were previously granted. Mr. Clavijo entered into a concurrent consulting
agreement with the Company to continue as interim Chief Financial Officer until a permanent successor joined the Company.
On April 18, 2023, the Company finalized the Separation Agreement
dated March 31, 2023, for Dr. Christopher Min, the Company’s former Chief Medical Officer. In part for his agreement to a general
release the Company agreed to pay Dr. Min: $112,000 as severance compensation and allowed for the immediate acceleration and vesting
of 40,000 RSUs that were previously granted.
RSUs are taxable upon vesting based on the market value on the date
of vesting. The Company is required to make mandatory tax withholding for the payment and satisfaction of income tax, social security
tax, payroll tax, or payment on account of other tax related to withholding obligations that arise by reason of vesting of an RSU. The
taxable income is calculated by multiplying the number of vested RSUs for each individual by the closing share price as of the vesting
date ($2.61 on April 3, 2023) and a tax liability is calculated based on each individual’s tax bracket.
On April 3, 2023, a total of 10,648 RSUs granted in connection with
the Company’s IPO vested, of which 9,570 were held by Company employees. Based on the closing price of $2.61 on April 3, 2023,
the Company recorded a tax liability of $7,000 for the employees and a corresponding tax liability for the Company of $2,000. In total,
the Company paid $9,000 for employee and employer taxes that resulted from the vesting of RSUs. In order to cover the employee tax liability,
the Company withheld 2,514 shares of Class A Common Stock owned by the Company’s employees upon vesting. The shares withheld are
available for reissuance pursuant to the Company’s 2021 Incentive Award Plan, as amended (the “2021 Incentive Plan”).
On April 3, 2023, a total of 12,500 RSUs vested that had been granted
to Wa’el Hashad, the Company’s Chief Executive Officer. Based on the closing price of $2.61 on April 3, 2023, the Company
recorded a tax liability of $10,000 for the employee and a corresponding tax liability for the Company of $2,000. In total, the Company
paid $12,000 for employee and employer taxes that resulted from the vesting of RSUs. In order to cover the employee tax liability, the
Company withheld 3,819 shares of Class A Common Stock shares owned by Mr. Hashad upon vesting. The shares withheld are available for
reissuance pursuant to the 2021 Incentive Plan.
On January 3, 2023, a total of 20,161 RSUs granted in connection with
the Company’s IPO vested, of which 18,005 were held by Company employees. Based on the closing price of $3.37 on January 3, 2023,
the Company recorded a tax liability of $15,000 for the employees and a corresponding tax liability for the Company of $2,000. In total,
the Company paid $17,000 for employee and employer taxes that resulted from the vesting of RSUs. In order to cover the employee tax liability,
the Company withheld 4,431 shares of Class A Common Stock owned by the employees upon vesting. The shares withheld are available for
reissuance pursuant to the 2021 Incentive Plan.
On November 16, 2022, the Company accounted for but had not issued
48,140 RSUs convertible to shares of Class A Common Stock, with an aggregate value of $207,000 as payment for accrued expenses under
a consulting agreement with Dr. Hare. These shares were issued to Dr. Hare on May 24, 2023.
On October 3, 2022, a total of 20,157 RSUs granted in connection with
the Company’s IPO vested, of which 18,001 were held by Company employees. Based on the closing price of $3.75 on October 3, 2022,
the Company recorded a tax liability of $16,000 for the employees and a corresponding tax liability for the Company of $2,000. In total,
the Company paid $18,000 for employee and employer taxes that resulted from the vesting of RSUs. In order to cover the employee tax liability,
the Company withheld 4,204 shares of Class A Common Stock owned by the Company’s employees upon vesting. The shares withheld are
available for reissuance pursuant to the 2021 Incentive Plan.
On July 1, 2022, a total of 20,158 RSUs granted in connection with
the Company’s IPO vested, of which 18,002 were held by Company employees. Based on the closing price of $5.94 on July 1, 2022,
the Company recorded a tax liability of $26,000 for the employees and a corresponding tax liability for the Company of $2,000. In total,
the Company paid $28,000 for employee and employer taxes that resulted from the vesting of RSUs. In order to cover the employee tax liability,
the Company withheld 4,726 shares of Class A Common Stock owned by the Company’s employees upon vesting. The shares withheld are
available for reissuance pursuant to the 2021 Incentive Plan.
On June 22, 2022, a total of 27,854 RSUs were granted to the Company’s
former Chief Executive Officer, Geoff Green, in exchange for $170,000 of compensation agreed upon in connection with his separation.
On June 3, 2022, a total of 26,666 RSUs vested that previously had
been granted to our Chief Financial Officer and General Counsel. As a result, based on a closing price of $8.73 on June 3, 2022, the
Company recorded a tax liability of $55,000 for the employees and a corresponding tax liability for the Company of $2,000. In total,
the Company paid $57,000 for employee and employer taxes resulting from the vesting of RSUs. In order to cover the employee tax liability,
the Company withheld 6,254 shares of Class A Common Stock owned by the Company’s employees upon vesting. The shares withheld are
available for reissuance pursuant to the 2021 Incentive Plan.
On April 4, 2022, a total of 1,167 RSUs vested that previously had
been granted to our former Chief Medical Officer. Based on the closing price of $12.85 on April 3, 2022, the Company recorded a tax liability
of $5,000 for the employee and a corresponding tax liability for the Company of $1,000. In total, the Company paid $6,000 for employee
and employer taxes that resulted from the vesting of RSUs. In order to cover the employee tax liability, the Company withheld 357 shares
of Class A Common Stock shares owned by the Chief Medical Officer upon vesting. The shares withheld are available for reissuance pursuant
to the 2021 Incentive Plan.
On April 1, 2022, a total of 31,016 RSUs vested that previously had
been granted in connection with the Company’s IPO vested, of which 26,360 were held by Company employees. Based on the closing
price of $15.61 on April 1, 2022, the Company recorded a tax liability of $105,000 for the employees and a corresponding tax liability
for the Company of $14,000. In total, the Company paid $119,000 for employee and employer taxes that resulted from the vesting of RSUs.
In order to cover the employee tax liability, the Company withheld 6,222 shares of Class A Common Stock owned by the Company’s
employees upon vesting. The shares withheld are available for reissuance pursuant to the 2021 Incentive Plan.
On April 1, 2022, a total of 2,500 RSUs vested that were previously
granted to a member of the Company’s Board of Directors.
On February 12, 2022, a total of 8,750 RSUs vested that were previously
granted to members of the Company’s Board of Directors upon the completion of the IPO vested.
On January 3, 2022, a total of 35,246 RSUs vested that previously
had been granted in connection with the Company’s IPO vested, of which 29,614 were held by Company employees. Based on the closing
price of $12.09 on January 3, 2022, the Company recorded a tax liability of $92,000 for the employees and a corresponding tax liability
for the Company of $14,000. In total, the Company paid $106,000 for employee and employer taxes that resulted from the vesting of RSUs.
In order to cover the employee tax liability, the Company withheld 10,627 shares of Class A Common Stock owned by the Company’s
employees upon vesting. The shares withheld are available for reissuance pursuant to the 2021 Incentive Plan.
During the six months ended June 30, 2022, stock options were exercised
for Class A Common Stock shares at an average exercise price of $5.73 for proceeds of $2,143.
Class B Common Stock
In connection with the Corporate Conversion, 2,000,000 outstanding
Series A and B units were converted into 15,702,834 shares of our unregistered Class B Common Stock.
Holders of Class A Common Stock generally have rights identical to
holders of Class B Common Stock, except that holders of Class A Common Stock are entitled to one vote per share and holders of Class
B Common Stock are entitled to five (5) votes per share. The holders of Class B Common Stock may convert each share of Class B Common
Stock into one share of Class A Common Stock at any time at the holder’s option. Class B Common Stock is not publicly tradeable.
During the six months ended June 30, 2023, stockholders exchanged
35,546 shares of Class B Common Stock for 35,546 shares of Class A Common Stock. During the year ended December 31, 2022, stockholders
exchanged 811,749 shares of Class B Common Stock for 811,749 shares of Class A Common Stock.
Warrants
As part of the IPO, the underwriter received warrants to purchase
106,400 shares of Class A Common Stock. The warrants are exercisable at any time and from time to time, in whole or in part, during the
four and a half-year period commencing August 12, 2021, at a price of $12.00 per share and the fair value of the warrants as of December
31, 2021 was approximately $0.5 million. During 2021, the underwriters assigned 95,760 of the warrants to its employees. As of December
31, 2022, 51,061 warrants have been exercised for Class A Common Stock shares at an exercise price of $12.00 for $612,732.
As part of the 2021 PIPE Offering, the Company issued 1,169,288 warrants
to investors to purchase up to a number of shares of Class A Common Stock equal to the number of shares of Class A Common Stock purchased
by such investor in the offering, at an exercise price of $17.50 per share (the “Purchaser Warrants”). The Purchaser Warrants
are immediately exercisable, expire five years from the date of issuance and have certain downward pricing adjustment mechanisms, subject
to a floor, as set forth in greater detail therein. In addition, the Company granted the underwriters warrants, under similar terms,
to purchase 46,722 shares of Class A Common Stock, at an exercise price of $17.50 per share.
8. Equity Incentive Plan
As part of the Company’s IPO, the Company adopted and approved
the 2021 Incentive Award Plan, under which, the Company may grant equity incentive awards to eligible service providers in order to attract,
motivate and retain the talent for which the Company competes.
On March 1, 2023, the Company granted Mr. Hashad a signing bonus of
50,000 Restricted Stock Units, which shall vests in quarterly installments on each of April 1, 2023, July 1, 2023, September 1, 2023,
and December 31, 2023. Mr. Hashad will also be eligible to receive annual long-term equity incentive awards through 2026 consisting of
50,000 shares of time-based vesting stock options and up to 125,000 of performance share units “(PSUs”), in accordance with
the terms of the Longeveron 2021 Incentive Award Plan.
On November
16, 2022, the Company accounted for but had not issued 48,140 RSUs convertible to unregistered shares of Class A Common Stock,
with an aggregate value of $207,000 as payment for accrued expenses
under a consulting agreement with Dr. Hare. These shares were issued to Dr. Hare on May 24, 2023.
On June 22, 2022, the Company granted $170,000 of separation compensation
to Mr. Green (Mr. Green resigned as CEO effective June 1, 2022), which were converted into 27,854 RSUs. The RSUs were issued based on
the three-day average of the fair market value prior to the time of grant, June 22, 2022, of $6.10.
On June 3, 2022, the Company granted a bonus to Mr. Clavijo and Mr.
Lehr in the form of RSUs. Mr. Clavijo and Mr. Lehr were granted 40,000 RSUs each that vested one-third at the grant date, with the remaining
two-thirds vesting on each anniversary of the grant date. The RSUs were issued based on a fair market value at the time of grant, June
3, 2022, of $8.73. Mr. Clavijo forfeited 13,334 unvested RSUs on June 9, 2023.
On April 4, 2022, the Company appointed K. Chris Min, M.D., Ph.D.
as its Chief Medical Officer. Dr. Min’s employment agreement provides an annual base salary of $350,000, and he will be eligible
to receive a performance bonus equal to 30% of his base salary, prorated for his first year of employment. Dr. Min received a $60,000
signing bonus, with 50% of this amount paid in RSUs and 50% in stock options. Dr. Min also received two equity incentive awards; 150,000
RSUs and a stock option award exercisable for 50,000 shares. Each award will vest 25% upon the first-year anniversary of his first day
of employment with Longeveron, with 25% vesting thereafter on the second, third and fourth anniversaries of his employment. In each case,
the vesting of the equity awards was subject to Dr. Min’s continued service through the applicable vesting dates. RSUs were expensed
on a quarterly basis at the rate of $0.1 million for the quarterly vesting amount of 9,375 RSUs, with a price per share of $12.85 (the
closing price of the Company’s stock on April 4, 2022) through March 31, 2023. Stock options were expensed based upon a Black-Scholes
calculation, the price per share to be expensed was $11.34 and a total cost of $0.6 million was to be expensed ratably over 48 months.
Dr. Min forfeited these stock options in connection with his separation on March 31, 2023.
As of June 30, 2023 and December 31, 2022, the Company had 97,754
and 329,746, respectively RSUs outstanding (unvested).
RSU activity for the six months ended June 30,
2023 was as follows:
| | |
Number of
RSUs | |
| Outstanding (unvested) at December 31, 2022 | |
| 329,746 | |
| RSU granted | |
| 60,000 | |
| RSUs vested | |
| (182,223 | ) |
| RSU expired/forfeited | |
| (109,769 | ) |
| Outstanding (unvested) at June 30, 2023 | |
| 97,754 | |
Stock Options
Stock options may be granted under the 2021 Incentive Plan. The exercise
price of options is set to equal the fair market value of the Company’s Class A Common Stock as of the grant date. Options historically
granted have generally become exercisable over four years and expire ten years from the date of grant. The 2021 Incentive Plan provides
for equity grants to be granted up to 5% of the outstanding common stock shares.
The fair value of the options issued is estimated using the Black-Scholes
option-pricing model and using the following assumptions: a dividend yield of 0%; an expected life of 10 years; volatility
of 95%; and risk-free interest rate based on the grant date ranging from of 1.23% to 4.01%. Each option grant made during
2023 and 2022 will be expensed ratably over the option vesting periods, which approximates the service period.
As of June 30, 2023 and December 31, 2022, the Company has recorded
issued and outstanding options to purchase a total of 401,681, and 470,191 shares, respectively, of Class A Common Stock pursuant to
the 2021 Incentive Plan, at a weighted average exercise price of $6.07, and $7.07 per share, respectively.
For the six months ended June 30, 2023:
| | |
Number of
Stock Options | |
| Stock options vested (based on ratable vesting) | |
| 183,527 | |
| Stock options unvested | |
| 218,154 | |
| Total stock options outstanding at June 30, 2023 | |
| 401,681 | |
For the year ended December 31, 2022:
| | |
Number of
Stock Options | |
| Stock options vested (based on ratable vesting) | |
| 151,258 | |
| Stock options unvested | |
| 318,933 | |
| Total stock options outstanding at December 31, 2022 | |
| 470,191 | |
Stock Option activity for the six months ended
June 30, 2023 was as follows:
| | |
Number
of
Stock Options | | |
Weighted
Average
Exercise
Price | |
| Outstanding at December 31, 2022 | |
| 470,191 | | |
$ | 7.07 | |
| Options granted | |
| 50,000 | | |
$ | 3.62 | |
| Options exercised | |
| - | | |
| - | |
| Options expired/forfeited | |
| (118,510 | ) | |
$ | 9.01 | |
| Outstanding at June 30, 2023 | |
| 401,681 | | |
$ | 6.07 | |
On March 1, 2023, the Company granted an award of 50,000 Class A Common
Stock options to Mr. Hashad. The stock option award has a one-year vesting period, vesting on the first anniversary of the grant date,
and has an exercise price of $3.62. Based upon a Black-Scholes calculation, the price per share to be expensed was $3.23 and a total
cost of $0.2 million would be expensed ratably over 12 months.
On December 21, 2022, the Company granted an award of 5,000 Class
A Common Stock options to each of its directors (a total of 45,000). The stock option award has a four-year vesting period, vesting 25%
per year, and has an exercise price of $3.00. Based upon a Black-Scholes calculation, the price per share to be expensed was $2.67 and
a total cost of $135,000 that would be expensed ratably over 48 months.
On November 16, 2022, the Company granted an award of 22,843 Class
A Common Stock options to Mr. Lehr. The stock option award has a four-year vesting period, vesting 25% per year, and has an exercise
price of $4.30. Based upon a Black-Scholes calculation, the price per share to be expensed was $2.94 and a total cost of less than $0.1
million would be expensed ratably over 48 months.
On September 6, 2022, the Company granted an award of 10,000 Class
A Common Stock options to an employee. The stock option award has a four-year vesting period, vesting 25% per year, and has an exercise
price of $4.67. Based upon a Black-Scholes calculation, the price per share to be expensed was $4.15 and a total cost of less than
$0.1 million would be expensed ratably over 48 months.
On June 3, 2022, the Company granted an award of 5,000 Class A Common
Stock options to Mr. Lehr. The stock option award vested upon the grant date, and has an exercise price of $8.73. Based upon a Black-Scholes
calculation, the price per share to be expensed was $7.73 and a total cost of less than $0.1 million was expensed on the grant date.
On March 14, 2022, the Company granted an award of 22,000 Class
A Common Stock options to employees. The stock option award has a four-year vesting period, vesting 25% per year, and has an exercise
price of $5.94. Based upon a Black-Scholes calculation, the price per share to be expensed was $5.23 and a total cost of less than
$0.1 million would be expensed ratably over 48 months.
On January 6, 2022, the Company granted awards of 84,825 Class
A Common Stock options to employees. The stock option awards have four-year vesting periods, vesting 25% per year, and have an exercise
price of $10.00. Based upon a Black-Scholes calculation, the price per share to be expensed was $8.78 and a total cost of $0.7 million
would be expensed ratably over 48 months.
For the three months ended June 30, 2023 and 2022, the equity-based
compensation expense was $0.7 million and $.9 million, respectively, and for the six months ended June 30, 2023 and 2022, the equity-based
compensation expense amounted to approximately $1.1 million and $1.4 million, respectively. These amounts are included in the research
and development and general and administrative expenses in the condensed statements of operations for the three and six months ended
June 30, 2023 and 2022.
As of June 30, 2023, the remaining unrecognized equity-based compensation
(which includes RSUs, PSUs and stock options) of approximately $1.7 million will be recognized over approximately 3.5 years.
9. Commitments
and Contingencies
Master Services Agreements:
As of June 30, 2023, the Company had three active
master services agreements with third parties to conduct its clinical trials and manage clinical research programs and clinical development
services on behalf of the Company. The Company expects these agreements or amended current agreements to have total expenditures of approximately
$3.4 million over the next two years.
Consulting Services Agreements:
On November 20, 2014, the Company entered into a ten-year consulting
services agreement with Dr. Joshua Hare, its CSO. Under the agreement, the Company has agreed to pay the CSO $265,000 annually. The compensation
payments are for scientific knowledge, medical research, technical knowledge, skills, and abilities to be provided by the CSO to further
develop the intellectual property rights assigned by the CSO to the Company. This agreement requires the CSO to also assign to the Company
the exclusive right, title, and interest in any work product developed from his efforts during the term of this agreement. On November
16, 2022, the Company accounted for but had not issued 48,140 RSUs with an aggregate value of $0.2 million as payment for accrued expenses
under the consulting agreement with the CSO. These shares were issued on May 24, 2023. As of June 30, 2023 and December 31, 2022, the
Company had an accrued balance due to the CSO of less than $0.1 million.
Technology Services Agreement:
On March 27, 2015, the Company entered into a technology services
agreement with Optimal Networks, Inc. (a related company owned by Dr. Joshua Hare’s brother-in-law) for use of information technology
services. The Company agreed to issue the related party equity incentive units in the amount equal to 50% of the charges for invoiced
services, with such equity to be issued annually on or about the anniversary date of the agreement. During 2017, the Company issued 1,901
Series C Units, and on November 22, 2019 and January 29, 2021, the Company issued 820 and 410 Series C Units, respectively, as payment
for an aggregate of $0.2 million of accrued technology services. The Series C units were converted to 16,755 Class A common stock shares.
As of June 30, 2023, and December 31, 2022, the Company owed $0 and less than $0.1 million, respectively, pursuant to this agreement,
which is included in accounts payable in the accompanying June 30, 2023 and December 31, 2022 condensed balance sheets.
Exclusive Licensing Agreements:
UM Agreement
On November 20, 2014, the Company entered into an Exclusive License
Agreement with UM for the use of certain Aging-related Frailty MSC technology rights developed by our Chief Science Officer at UM. The
UM License is a worldwide, exclusive license, with right to sublicense, with respect to any and all know-how specifically related to
the development of the culture-expanded MSCs for Aging-related Frailty used at the IMSCs, all SOPs used to create the IMSCs, and all
data supporting isolation, culture, expansion, processing, cryopreservation and management of the IMSCs. The Company is required to pay
UM (i) a license issue fee of $5,000, (ii) a running royalty in an amount equal to three percent of annual net sales on products or services
developed from the technology, payable on a country-by-country basis beginning on the date of first commercial sale through termination
of the UM License Agreement, and which may be reduced to the extent we are required to pay royalties to a third party for the same product
or process, (iii) escalating annual cash payments of up to $50,000, subject to offset. The agreement extends for up to 20 years from
the last date a product or process is commercialized from the technology and was amended in 2017 to modify certain milestone completion
dates as detailed below In 2021 the license fee was increased by an additional $100,000, to defray patent costs. In addition, the Company
issued 110,387 unregistered shares of Class A Common Stock to UM.
The milestone payment amendments shifted the triggering payments to
three payments of $500,000, to be paid within six months of: (a) the completion of the first Phase 3 clinical trial of the products (based
upon the final data unblinding); (b) the receipt by the Company of approval for the first new drug application (“NDA”), biologics
application (“BLA”), or other marketing or licensing application for the product; and (c) the first sale following product
approval. “Approval” refers to Product approval, licensure, or other marketing authorization by the U.S. Food and Drug Administration,
or any successor agency. The amendments also provided for the Company’s license of additional technology, to the extent not previously
included in the UM License and granted the Company an exclusive option to obtain an exclusive license for (a) the HLHS investigational
new drug application (“IND”) with ckit+ cells; and (b) UMP-438 titled “Method of Determining Responsiveness to Cell
Therapy in Dilated Cardiomyopathy.”
The Company has the right to terminate the UM License upon 60 days’
prior written notice, and either party has the right to terminate upon a breach of the UM License. To date, the Company has made payments
totaling $190,000 to UM, and as of June 30, 2023 and December 31, 2022, we had accrued $40,000 and $50,000 in milestone fees payable
to UM, respectively and $70,000 for patent related reimbursements based on the estimated progress to date.
CD271
On December 22, 2016, the Company entered into an exclusive license
agreement with an affiliated entity of Dr. Joshua Hare, JMH MD Holdings, LLC (“JMHMD”), for the use of CD271 cellular therapy
technology. The Company recorded the value of the cash consideration and membership units issued to obtain this license agreement as
an intangible asset. The Company is required to pay as royalty, 1% of the annual net sales of the licensed product(s) used, leased, or
sold by or for licensee or its sub-licensees. If the Company sublicenses the technology, it is also required to pay an amount equal to
10% of the net sales of the sub-licensees. In addition, on December 23, 2016, as required by the license agreement, the Company paid
an initial fee of $250,000 to JMHMD, and issued to it 10,000 Series C Units, valued at $250,000. The $0.5 million of value provided to
JMHMD for the license agreement, along with professional fees of approximately $27,000, were recorded as an intangible asset that is
amortized over the life of the license agreement which was defined as 20 years. Further, expenses related to the furtherance of the CD271
technology is being capitalized and amortized as incurred over 20 years. There were no license fees due during the six months ended June
30, 2023 or year ended December 31, 2022 pertaining to this agreement.
Other Royalty
Under the grant award agreement with the Alzheimer’s Association,
the Company may be required to make revenue sharing or distribution of revenue payments for products or inventions generated or resulting
from this clinical trial program. The potential payments, although not currently defined, could result in a maximum payment of five times
(5x) the award amount.
Contingencies – Legal
On September 13, 2021, the Company and certain of its directors and
officers were named as defendants in a securities lawsuit filed in the U.S. District Court for the Southern District of Florida and brought
on behalf of a purported class. The suit alleges there were materially false and misleading statements made (or omissions of required
information) in the Company’s initial public offering materials and in other disclosures during the period from our initial public
offering on February 12, 2021, through August 12, 2021, in violation of the federal securities laws. The action sought damages on behalf
of a proposed class of purchasers of the Company’s Common Stock during said period. On July 12, 2022, all parties preliminarily
agreed to settle the action for approximately $1.4 million, which settlement was preliminarily approved by the Court on or about May
12, 2023, and which settlement amount was paid on May 24, 2023. Legal expenses incurred in ordinary business activities are reported
within general and administrative expenses.
10.
Employee Benefits Plan
The Company sponsors a defined contribution employee benefit plan
(the “Plan”) under the provisions of Section 401(k) of the Internal Revenue Code. The Plan covers substantially all full-time
employees of the Company who have completed one year of service. Contributions to the Plan by the Company are at the discretion of the
Board of Directors.
The Company contributed approximately $0.1 million to the Plan during
both of the six months ended June 30, 2023 and 2022, and less than $0.1 million for both of the three months ended June 30, 2023 and
2022.
11.
Loss Per Share
Basic and diluted net loss per share have been computed using the
weighted-average number of shares of common stock outstanding during the period. We have outstanding stock-based awards that are not
used in the calculation of diluted net loss per share because to do so would be anti-dilutive.
The following instruments (in thousands) were excluded from the calculation
of diluted net loss per share because their effects would be antidilutive:
| | |
Six months ended
June 30, | |
| | |
2023 | | |
2022 | |
| RSUs | |
| 98 | | |
| 302 | |
| PSUs | |
| 125 | | |
| - | |
| Stock options | |
| 401 | | |
| 416 | |
| Warrants | |
| 1,271 | | |
| 1,271 | |
| Total | |
| 1,895 | | |
| 1,989 | |
Item 2. Management’s
Discussion and Analysis of Financial Condition and Results of Operations.
In this document, the terms “Longeveron,”
“Company,” “we,” “us,” and “our” refer to Longeveron Inc. We have no subsidiaries.
This Quarterly Report on Form 10-Q (this “10-Q”) contains
forward-looking statements, within the meaning of the Private Securities Litigation Reform Act of 1995, that reflect our current expectations
about our future results, performance, prospects and opportunities. This 10-Q contains forward-looking statements that can involve
substantial risks and uncertainties. All statements other than statements of historical facts contained in this 10-Q, including statements
regarding our future results of operations and financial position, business strategy, prospective products, product approvals, research
and development costs, future revenue, timing and likelihood of success, plans and objectives of management for future operations, future
capital raising, future results of anticipated products and prospects, plans and objectives of management are forward-looking statements.
These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance
or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking
statements.
In some cases, you can identify forward-looking statements by terms
such as “anticipate,” “believe,” “contemplate,” “continue,” “could,” “estimate,”
“expect,” “intend,” “may,” “plan,” “potential,” “predict,” “project,”
“should,” “target,” “will,” or “would” or the negative of these terms or other similar
expressions, although not all forward-looking statements contain these words. Factors that could cause actual results to differ materially
from those expressed or implied in any forward-looking statements contained in this report include, but are not limited to, statements
about:
| |
● |
the ability of our clinical trials to demonstrate safety
and efficacy of our product candidates, and other positive results; |
| |
|
|
| |
● |
the timing and focus of our ongoing and future preclinical
studies and clinical trials, and the reporting of data from those studies and trials; |
| |
|
|
| |
● |
the size of the market opportunity for our product
candidates, including our estimates of the number of patients who suffer from the diseases we are targeting; |
| |
|
|
| |
● |
the success of competing therapies that are or may
become available; |
| |
|
|
| |
● |
the beneficial characteristics, safety, efficacy and
therapeutic effects of our product candidates; |
| |
|
|
| |
● |
our ability to obtain and maintain regulatory approval
of our product candidates; |
| |
|
|
| |
● |
our plans relating to the further development of our
product candidates, including additional disease states or indications we may pursue; |
| |
|
|
| |
● |
our plans and ability to obtain or protect intellectual
property rights, including extensions of existing patent terms where available and our ability to avoid infringing the intellectual
property rights of others; |
| |
|
|
| |
● |
the need to hire additional personnel and our ability
to attract and retain such personnel; |
| |
|
|
| |
● |
our estimates regarding expenses, future revenue, capital
requirements and needs for additional financing; |
| |
|
|
| |
● |
our need to raise additional capital, the difficulties
we may face in obtaining access to capital, and the dilutive impact it may have on our investors; |
| |
|
|
| |
● |
our financial performance and ability to continue as
a going concern; and |
| |
|
|
| |
● |
the period over which we estimate our existing cash
and cash equivalents will be sufficient to fund our future operating expenses and capital expenditure requirements. |
The forward-looking statements contained in this 10-Q are made on
the basis of the views and assumptions of management regarding future events and business performance as of the date this 10-Q is filed
with the Securities and Exchange Commission (the “SEC”). In addition, we operate in a highly competitive and rapidly
changing environment; therefore, new risk factors can arise, and it is not possible for management to predict all such risk factors,
nor to assess the impact of all such risk factors on our business or the extent to which any individual risk factor, or combination of
risk factors, may cause results to differ materially from those contained in any forward-looking statement. We do not undertake
any obligation to update these statements to reflect events or circumstances occurring after the date this 10-Q is filed. In addition,
this discussion and analysis should be read in conjunction with our unaudited condensed financial statements and notes thereto included
in this 10-Q and the audited condensed financial statements and notes thereto included in our Annual Report on Form 10-K for the year
ended December 31, 2022, filed with the SEC on March 14, 2023 (“2022 10-K”). Operating results are not necessarily indicative
of results that may occur in future periods.
Overview and Recent Developments
Overview
We are a clinical stage biotechnology company developing regenerative
medicines to address unmet medical needs. The Company’s lead investigational product is Lomecel-B™, an allogeneic medicinal
signaling cell (MSC) formulation sourced from bone marrow of young, healthy adult donors. Lomecel-B™ has multiple potential mechanisms
of action that promote tissue repair and healing with broad potential applications across a spectrum of disease areas. The underlying
mechanism(s) of action that lead to the tissue repair programs include the stimulation of new blood vessel formation, modulation of the
immune system, reduction in tissue fibrosis, and the stimulation of endogenous cells to divide and increase the numbers of certain specialized
cells in the body.
We are currently pursuing three pipeline indications: Hypoplastic
Left Heart Syndrome (HLHS), Aging-related Frailty, and Alzheimer’s disease (AD). Our mission is to advance Lomecel-B™ and
other cell-based candidates into pivotal Phase 3 trials, with the goal of achieving regulatory approvals, subsequent commercialization,
and broad use by the healthcare community.
Our HLHS program is focused on the potential clinical benefits of
Lomecel-B™ as an adjunct therapeutic to standard-of-care HLHS surgery. HLHS is a rare and devastating congenital heart defect in
which the left ventricle is severely underdeveloped. As such, babies born with this condition die shortly after birth without undergoing
a complex series of reconstructive heart surgeries. Despite the life-saving surgical interventions, clinical studies show that only 50
to 60 percent of affected individuals survive to adolescence. We have early clinical state evidence supporting pro-vascular, pro-regenerative,
and anti-inflammatory properties of Lomecel-B™ to improve heart function in HLHS patients. We have completed a Phase 1 open-label
study (ELPIS)1 that supported the safety and tolerability of Lomecel-B™ for HLHS, when directly injected into the
functional right ventricle during the second-stage standard-of-care surgery (adding minimal additional time to the surgical procedure).
Preliminary data also suggested potential benefits on heart function. In addition, our early clinical stage data is favorable as compared
to historical controls for survival and reduced need for heart transplants. Longeveron is currently conducting a controlled Phase 2a
(ELPIS II)study which, if positive, could add to the clinical data suggesting the functional and clinical benefits of Lomecel-B™
in these patients.
Our other clinical programs focus on aging populations. Life-expectancy
has substantially increased over the past century due to medical and public health advancements. However, this longevity increase has
not been paralleled by healthspan – the period of time one can expect to live in relatively good health and independence. For many
developed and developing countries, healthspan lags life-expectancy by over a decade. This has placed tremendous strain on healthcare
systems in the management of aging-related ailments, and presents additional socioeconomic consequences due to patient decreased independence
and quality-of-life. Since these strains continue to increase with demographic shifts towards an increasingly older population, improving
healthspan has become a priority for health agencies, such as the National Institute on Aging (NIA) of the NIH, the Japanese Pharmaceuticals
and Medical Devices Agency (PMDA), and the European Medicines Agency (EMA). As we age, we experience a decline in our own stem cells,
a decrease in immune system function (known as “immunosenescence”), diminished blood vessel functioning, chronic inflammation
(known as “inflammaging”), and other aging-related alterations that affect biological functioning. Our preliminary clinical
data suggest that Lomecel-B™ can potentially address these problems through multiple mechanisms of action (MOAs) that simultaneously
target key aging-related processes. Longeveron is currently engaged in clinical programs studying Lomecel-B™ for Alzheimer’s
disease and Aging-related Frailty under INDs with the US FDA and under the PMDA in Japan, as well as using Lomecel-B™ in registry
trials in The Bahamas.
Summary of Clinical Development Strategy
Our core mission is to become a world-leading regenerative medicine
company through the development, approval, and commercialization of novel cell therapy products for unmet medical needs, with a focus
on HLHS. Key elements of our current business strategy are as follows.
| ● | Execution of ELPIS II, a Phase 2 randomized controlled trial
set forth in greater detail below, to measure the efficacy of Lomecel-B™ in HLHS. This
trial is ongoing and is being conducted in collaboration with the National Heart, Lung, and
Blood Institute (NHLBI) through grants from the NIH. |
| ● | Continue developing our international programs. Japan is our
first non-U.S. territory in which we are conducting a randomized, double-blinded, placebo-controlled
clinical trial to evaluate Lomecel-B™ for Aging-related Frailty. With successful completion
of this trial and demonstration of safety, we intend to seek marketing approval under the
Act on the Safety of Regenerative Medicine (ASRM). We also intend to explore conditional
or full approval in Japan of Lomecel-B™ under the Pharmaceuticals and Medical Devices
(PMD) Act for the treatment of Aging-related Frailty in the future, which will be guided
by results from this trial and potentially others in our Frailty program. We may also explore
other indications in Japan, and potentially pursue Aging-related Frailty and other indications
in additional international locations for further development and commercialization. We also
continue to successfully enroll in our Frailty and Cognitive Impairment registry trials in
The Bahamas, and are launching an Osteoarthritis registry trial. |
| ● | Continue to pursue the therapeutic potential of Lomecel-B™ in
Alzheimer’s disease (AD). We completed a Phase 1b study, which indicated the potential of Lomecel-B™ to preserve cognition
in patients with mild AD through multiple potential mechanisms of action. Based on the outcomes of this trial, we are now conducting a
Phase 2a randomized placebo-controlled trial (CLEAR MIND) to determine the safety and effects of up to four doses of Lomecel-B™
infused every 4 weeks in mild AD patients. This study entails a heavy focus on target engagement through multiple endpoints that include
evaluation of fluid-based biomarkers, brain imaging, vascular function, and neurocognitive measures. |
| 1 | Sunjay Kaushal, MD, PhD, Joshua M Hare, MD, Jessica R Hoffman,
PhD, Riley M Boyd, BA, Kevin N Ramdas, MD, MPH, Nicholas Pietris, MD, Shelby Kutty, MD, PhD, MS, James S Tweddell, MD, S Adil Husain,
MD, Shaji C Menon, MBBS, MD, MS, Linda M Lambert, MSN-cFNP, David A Danford, MD, Seth J Kligerman, MD, Narutoshi Hibino, MD, PhD, Laxminarayana
Korutla, PhD, Prashanth Vallabhajosyula, MD, MS, Michael J Campbell, MD, Aisha Khan, PhD, Eric Naioti, MSPH, Keyvan Yousefi, PharmD,
PhD, Danial Mehranfard, PharmD, MBA, Lisa McClain-Moss, Anthony A Oliva, PhD, Michael E Davis, PhD. “Intramyocardial cell-based
therapy with Lomecel-B™ during bidirectional cavopulmonary anastomosis for hypoplastic left heart syndrome: The ELPIS phase I trial”
(2023) European Heart Journal Open, 2023. |
| ● | Expand our manufacturing capabilities to commercial-scale production.
We operate a current good manufacturing practice (cGMP)-compliant manufacturing facility
and produce our own product candidates for testing. We continue to improve and expand our
capabilities with the goal of achieving cost-effective manufacturing that may potentially
satisfy future commercial demand for potential Lomecel-B™ commercialization. |
| ● | Collaborative arrangements and out-licensing opportunities.
We will be opportunistic and consider entering into co-development, out-licensing, or other
collaboration agreements for the purpose of eventually commercializing Lomecel-B™ and
other products domestically and internationally if appropriate approvals are obtained. |
| ● | Product candidate development pipeline through internal research
and development, and in-licensing. Through our research and development program, and through
strategic in-licensing agreements, or other business development arrangements, we intend
to actively explore promising potential additions to our pipeline. |
| ● | Continue to expand our intellectual property portfolio. Our
intellectual property is vitally important to our business strategy, and we take significant
steps to develop this property and protect its value. Results from our ongoing research and
development efforts are intended to add to our existing intellectual property portfolio. |
Clinical Development Pipeline in 2023
We are currently in clinical development of a
single product, Lomecel-B™ for three potential indications (See Figure 1).
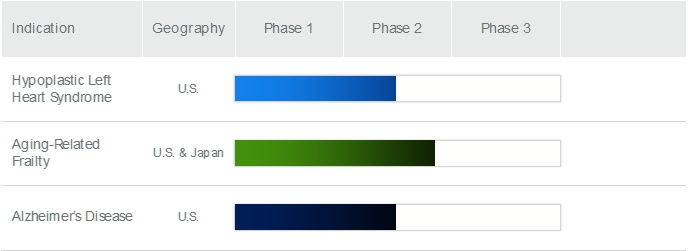
Figure 1: Lomecel-B™ clinical development
pipeline
| |
|
Hypoplastic Left Heart Syndrome (HLHS). The FDA granted
Lomecel-B™ for the treatment of HLHS a Rare Pediatric Disease (RPD) Designation (on November 8, 2021), Orphan Drug Designation
(ODD) (on December 2, 2021), and Fast Track Designation (on August 24, 2022). HLHS is a rare congenital heart condition that affects
roughly 1,000 to 2,000 newborns annually in the US. HLHS babies are born with a severely underdeveloped left ventricle. To prevent
certain death shortly after birth, these babies undergo a series of three heart surgeries that converts the normally 4-chamber heart
into a 3-chamber one with a single ventricle (the right ventricle) supporting systemic circulation. Despite these life-saving surgeries,
HLHS patients nevertheless still have high early mortality and morbidity rates due primarily to heart failure.
We are currently conducting an ongoing Phase 2 clinical trial
(ELPIS II) under FDA IND 017677. ELPIS II is a multi-center, randomized, double-blind, controlled clinical trial designed to evaluate
Lomecel-B™ as an adjunct therapy to the standard-of-care second-stage HLHS heart reconstructive surgery. The primary endpoint
is to evaluate improvement in heart function after Lomecel-B™ treatment versus standard-of-care surgery alone (38 subjects
total: 19 per arm). This trial is over 50% enrolled, and is funded in part by the NHLBI/NIH. While we cannot predict a specific time
when the trial will be fully enrolled, the plan is to complete enrollment around mid-2024. |
| |
|
ELPIS II is a next-step trial to our completed 10-patient open-label
Phase 1 trial (ELPIS) under the same IND. This Phase 1 trial was designed to evaluate the safety and tolerability of Lomecel-B™
as an adjunct to the second-stage HLHS surgery, and to obtain preliminary evidence of Lomecel-B™ effect to support a next-phase
trial. The primary safety endpoint was met: no major adverse cardiac events (MACE) or treatment-related infections during the first
month post-treatment, and no triggering of stopping rules. Furthermore, fluid-based and imaging biomarker data supported multiple
potential relevant mechanisms-of-action of Lomecel-B™, and the potential to improve post-surgical heart function. In addition
to the 12-month follow-up evaluation on ELPIS, we continue to follow these patients on an annual basis. All 10 patients remain alive
without the need for a heart transplant for 3.5 to 5.0 years since treatment with Lomecel-B™ (updated as of May 9, 2023), and
five have already successfully undergone the third-stage surgery. Based on historical data, over 15% of patients would be expected
to have received a heart transplant or have died within 3-years after the second-stage surgery, rising to nearly 20% by 5 years.
We are prosecuting a number of patent applications relating to
the administration of mesenchymal stem cells for treating juvenile HLHS in Taiwan and the Bahamas.
Aging-related Frailty. Aging-related Frailty is a life-threatening
geriatric condition that disproportionately increases risks for poor clinical outcomes from disease and injury. While the definition
of Aging-related Frailty lacks consensus and would be a new indication from a regulatory standpoint, and while Aging-related Frailty
has no approved pharmaceutical or biologic treatments, there are a number of companies now working to develop potential therapeutics
for this unmet medical need. We will work with regulatory agencies, such as the U.S. FDA and Japan’s PMDA, to advance Lomecel-B™
as potentially the first approved drug for Aging-related Frailty. |
| |
○ |
We have previously completed two U.S. clinical trials
under FDA IND 016644. One is a multicenter, randomized, placebo-controlled Phase 2b trial which showed that a single infusion of
Lomecel-B™ significantly improved 6-Minute Walk Test (6MWT) distance 9 months after infusion, and also showed a dose-dependent
increase in 6MWT distance 6 months after infusion. The second is a multicenter, randomized, placebo-controlled Phase 1/2 trial (“HERA
Trial”) that showed that Lomecel-B™ was generally safe and well tolerated in patient with Aging-related Frailty, and
showed that hemagglutinin inhibition (HAI) assay results in the Lomecel-B™ and placebo groups to influenza were not statistically
different, indicating Lomecel-B™ does not suppress the immune system. |
| |
|
|
| |
○ |
Japan Clinical Trial: The Japanese PMDA has approved a
Clinical Trial Notification (CTN), which is equivalent to a U.S. IND, allowing an Investigator-sponsored Phase 2 clinical study for
Aging-related Frailty patients in Japan. This study is a 45-patient randomized placebo-controlled study with a primary objective
of evaluating the safety of Lomecel-B™ in Japanese patients with Aging-related Frailty. Patient screening began in the 4th
quarter of 2022, and the first patient was randomized in the first quarter of 2023. The goal of this study is to enable ASRM
approval when combined with previous clinical results in non-Japanese patients.
We are prosecuting a number of patent applications relating to
the administration of mesenchymal stem cells for Aging-related Frailty in Taiwan, the Bahamas and United States. |
| |
Alzheimer’s disease. Alzheimer’s disease, a devastating
neurologic disease leading to cognitive decline, has very limited therapeutic options. An estimated 6.7 million Americans age 65 and older
have Alzheimer’s disease, and this number is projected to more than double by 2060. We have been testing Lomecel-B™ as a therapeutic
with the possibility to slow the decline in cognitive function in patients with mild (i.e., early-stage) Alzheimer’s disease. Our
completed and published Phase 1 clinical trial results suggest that Lomecel-B™ is safe in this population and does not cause Alzheimer
Related Imaging Abnormality (ARIA), a serious complication of certain treatment strategies. Our early study also suggested the possibility
of a therapeutic effect, and we are testing this possibility in a new Phase 2 clinical trial.
In the Phase 1 multicenter randomized placebo-controlled trial, patients
received a single dose of Lomecel-B™ or placebo, and were evaluated for one year thereafter. The trial met its primary endpoint
of safety (incidence of serious adverse events – SAEs – within one month of treatment), and supported the safety and tolerability
of Lomecel-B™ through additional endpoint measures. Furthermore, through evaluation of blood-based and imaging biomarkers, neurocognitive
assessments and quality-of-life measures, the trial provided insights into mechanisms of action of Lomecel-B™ for Alzheimer’s
disease, as well as indications of potential to improve clinical outcomes2. |
| |
|
| 2 | Mark Brody, Marc Agronin, Brad J. Herskowitz, Susan Y. Bookheimer,
Gary W. Small, Benjamin Hitchinson, Kevin Ramdas, Tyler Wishard, Katalina Fernández McInerney, Bruno Vellas, Felipe Sierra, Zhijie
Jiang, Lisa McClain-Moss, Carmen Perez, Ana Fuquay, Savannah Rodriguez, Joshua M. Hare, Anthony A. Oliva Jr., Bernard Baumel. “Results
and insights from a phase I clinical trial of Lomecel-B™ for Alzheimer’s disease” (2023) Alzheimer’s & Dementia:
The Journal of the Alzheimer’s Association 19:261-273. |
| |
The company is now conducting a next-step Phase 2a trial, CLEAR MIND,
to evaluate multiple doses of Lomecel-B™ in patients with mild Alzheimer disease (ClinicalTrials.gov #NCT05233774). Each patient
receives 4 treatments with Lomecel-B™, 4 treatments with placebo, or 1 treatment with Lomecel-B™ followed by 3 treatments
with placebo, with each treatment delivered by intravenous (IV) infusion separated by 4 weeks. Patients are then followed for 9 months
from the start of the first treatment. The primary objective is safety of multiple treatments with Lomecel-B™. This study also entails
a rigorous evaluation of target engagement through endpoints that include evaluation of fluid-based biomarkers, brain imaging via magnetic
resonance imaging (MRI), vascular function, and neurocognitive measures. Forty-nine (49) subjects have been enrolled and have received
Lomacel-B™. The last patient visit is projected near the end of the third quarter of 2023, and topline CLEAR MIND results are expected
around early October of 2023.
We are prosecuting a number of patent applications relating to
the administration of allogenic mesenchymal stem cells for treating Alzheimer’s Disease in Hong Kong, South Africa, Singapore,
Korea, New Zealand, Japan, Israel, Canada, Australia, the Bahamas, and the United States. |
| |
|
|
| |
○ |
The Bahamas Registry Trials: We sponsor and
operate Registry Trials in Nassau and Lyford Cay in The Bahamas, where participants may receive Lomecel-B™ for Aging-related
Frailty and other indications, at the participant’s own expense. Lomecel-B™ is designated as an investigational product
in The Bahamas. |
Impact of Macroeconomic Conditions
We have not to date been directly adversely impacted by the current
banking sector volatility, and specifically the volatility being experienced within the regional banking sector volatility. However,
we previously maintained deposits and marketable securities with a regional bank, and have made the determination, in light of such volatility
and uncertainty, to transfer our deposits and marketable securities to a larger bank, so as to mitigate the likelihood of our experiencing
a direct adverse impact from any ongoing or future volatility within the regional banking sector.
In addition, although we have experienced some supply constraints
and marginal price increases, to date current macroeconomic conditions have not materially impacted our programs or our operations. We
continue to monitor economic conditions in the U.S. and globally and expect to act proactively where possible to minimize the impact
of continued inflation or supply constraints on materials and inventory needed for operations.
Components of Our Results of Operations
Revenue
We have generated revenue from three sources:
| ● | Grant awards. Extramural grant award funding, which is non-dilutive,
has been a core strategy for supporting our ongoing clinical research. Since 2016 our clinical
programs have received over $16.0 million in competitive extramural grant awards ($11.5 million
which has been directly awarded to us and which are recognized as revenue when the performance
obligations are met) from the NIH, Alzheimer’s Association, and Maryland Stem Cell
Research Fund (MSCRF). |
| ● | The Bahamas Registry Trial. Participants in The Bahamas Registry
Trial pay us a fee to receive Lomecel-B™, imported into The Bahamas, and administered
at one of two private medical clinics in Nassau. While Lomecel-B™ is considered an
investigational product in The Bahamas, under the approval terms received from the National
Stem Cell Ethics Committee, we are permitted to charge a fee for participation in the Registry
Trial. The fee is recognized as revenue, and is used to pay for the costs associated with
manufacturing and testing of Lomecel-B™, administration, shipping and importation fees,
data collection and management, biological sample collection and sample processing for biomarkers
and other data, and overall management of the Registry, including personnel costs. Lomecel-B™
is considered an investigational treatment in The Bahamas and is not licensed for commercial
sale. |
| ● | Contract development and manufacturing services. From time to
time, we enter into fee-for-service agreements with third parties for our product development
and manufacturing capabilities. |
Cost of Revenues
We record cost of revenues based on expenses directly related to revenue.
For grants we record allocated expenses for research and development costs to a grant as a cost of revenues. For the clinical trial revenue,
directly related expenses for that program are allocated and accrued as incurred. These expenses are similar to those described under
“Research and Development Expenses” below.
Selling and Marketing Expenses
Selling and marketing expenses consist primarily of royalty and license
fees associated with our agreements with the University of Miami as well as attending and sponsoring industry, investment, organization
and medical conferences and events.
Research and Development Expenses
Research and development costs are charged to expense when incurred
in accordance with Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 730
Research and Development. ASC 730 addresses the proper accounting and reporting for research and development costs. It identifies: first,
those activities that should be identified as research and development; second, the elements of costs that should be identified with
research and development activities, and the accounting for these costs; and third, the financial statement disclosures related to them.
Research and development include costs such as clinical trial expenses, contracted research and license agreement fees with no alternative
future use, supplies and materials, salaries, share-based compensation, employee benefits, property and equipment depreciation and allocation
of various corporate costs. We accrue for costs incurred by external service providers, including contract research organizations (CROs)
and clinical investigators, based on estimates of service performed and costs incurred. These estimates include the level of services
performed by the third parties, subject enrollment in clinical trials, administrative costs incurred by the third parties, and other
indicators of the services completed. Based on the timing of amounts invoiced by service providers, we may also record payments made
to those providers as prepaid expenses that will be recognized as expense in future periods as the related services are rendered.
We currently do not carry any inventory for our product candidates,
as we have yet to receive regulatory approval and launch a product for commercial distribution. Historically our operations have focused
on conducting clinical trials, product research and development efforts, and improving and refining our manufacturing processes, and
accordingly, manufactured clinical doses of product candidates were expensed as incurred, consistent with the accounting for all other
research and development costs. Once we begin commercial distribution, all newly manufactured approved products will be allocated either
for use in commercial distribution, which will be carried as inventory and not expensed, or for research and development efforts, which
will continue to be expensed as incurred.
We expect that our research and development expenses will increase
in the future as we increase our headcount to support increased research and development activities relating to our clinical programs,
as well as incur additional expenses related to our clinical trials.
General and Administrative Expenses
General and administrative expenses consist primarily of salaries
and other related costs, including stock-based compensation, for personnel in our executive, finance, business development and administrative
functions. General and administrative expenses also include public company related expenses; legal fees relating to corporate matters;
insurance costs; professional fees for accounting, auditing, tax and consulting services; travel expenses; and facility-related expenses,
which include direct depreciation costs and allocated expenses for rent and maintenance of facilities and other operating costs.
We expect that our general and administrative expenses will increase
in the future as we increase our headcount to support the administrative activities related to being a public company, including costs
of accounting, audit, legal, regulatory and tax-related services associated with maintaining compliance with Nasdaq and SEC requirements,
director and officer insurance costs, and investor and public relations costs.
Other Income and Expenses
Interest income consists of interest earned on cash equivalents and
marketable securities. We expect our interest income to fluctuate due to changes in the current cash and marketable securities balances.
Other income consists of funds earned that are not part of our normal operations. In past years they have been primarily a result of
tax refunds received for social security taxes as part of a research and development tax credit program.
Income Taxes
As of February 12, 2021, we are treated as a C corporation for federal
and state income tax purposes. Prior to February 12, 2021, we were treated as a partnership for federal and state income tax purposes,
whereby we passed our earnings and losses through to our members based on the terms of our Operating Agreement. No provision for income
taxes has been recorded for the years ended December 31, 2022 and 2021. We may incur income taxes in the future if we have earnings.
At this time the Company has not evaluated the impact of any future profits.
RESULTS OF OPERATIONS
COMPARISON OF THE THREE MONTHS ENDED JUNE
30, 2023 AND 2022
The following table summarizes our results of operations for the three
months ended June 30, 2023 and 2022, together with the changes in those items in dollars (in thousands):
| | |
Three Months Ended
June 30, | | |
Increase | |
| | |
2023 | | |
2022 | | |
(Decrease) | |
| Revenues | |
$ | 217 | | |
$ | 466 | | |
$ | (249 | ) |
| Cost of revenues | |
| 124 | | |
| 306 | | |
| (182 | ) |
| Gross profit | |
| 93 | | |
| 160 | | |
| (67 | ) |
| Expenses | |
| | | |
| | | |
| | |
| General and administrative | |
| 3,375 | | |
| 2,427 | | |
| 948 | |
| Research and development | |
| 2,287 | | |
| 1,720 | | |
| 567 | |
| Selling and marketing | |
| 143 | | |
| 234 | | |
| (91 | ) |
| Total operating expenses | |
| 5,805 | | |
| 4,381 | | |
| 1,424 | |
| | |
| | | |
| | | |
| | |
| Loss from operations | |
| (5,712 | ) | |
| (4,221 | ) | |
| (1,491 | ) |
| Non-operating lawsuit expense | |
| - | | |
| (1,398 | ) | |
| 1,398 | |
| Other income (expense) | |
| 80 | | |
| (5 | ) | |
| 85 | |
| Net loss | |
$ | (5,632 | ) | |
$ | (5,624 | ) | |
$ | (8 | ) |
Revenues, Cost of Revenues and Gross Profit: Revenues for each
of the three months ended June 30, 2023 and 2022 were approximately $0.2 million and $0.5 million, respectively. Grant revenue for the
three months ended June 30, 2023 and 2022 was $0 and $0.1 million, respectively. The decrease of $0.1 million, or 100%, was primarily
due to a reduction in grant funds available due in part to the completion of the grant-funded clinical trials. Clinical trial revenue,
which is derived from the Bahamas Registry Trial, for the three months ended June 30, 2023 and 2022 was $0.2 million and $0.3 million,
respectively. Clinical trial revenue for the three months ended June 30, 2023 was approximately $0.1 million, or 36%, lower when compared
to the same period in 2022 as a result of a decrease in participant demand.
Related cost of revenues was approximately $0.1 million and $0.3 million
for the three months ended June 30, 2023 and 2022, respectively. The decrease of $0.2 million, or 59%, was primarily due to the decrease
in the revenues earned from the Bahamas Registry Trial. This resulted in a gross profit of approximately $0.1 million and $0.2 million
for the three months ended June 30, 2023 and 2022, respectively.
General and Administrative Expense: General and administrative
expenses for the three months ended June 30, 2023 increased to approximately $3.4 million, compared to $2.4 million for the same period
in 2022. The increase of approximately $1.0 million, or 39%, was primarily related to an increase of $0.7 million in compensation and
benefit expenses during the current year period and expenses related to professional fees.
Research and Development Expenses: Research and development
expenses for the three months ended June 30, 2023 increased to approximately $2.3 million, from approximately $1.7 million for the same
period in 2022. The increase of $0.6 million, or 33%, was primarily due to an increase of $0.5 million in research and development expenses
that were not reimbursable by grants. Research and development expenses consisted primarily of the following items (less those expenses
allocated to the cost of revenues for the grants) (in thousands):
| | |
Three Months Ended
June 30, | |
| | |
2023 | | |
2022 | |
| Clinical trial expenses-statistics, monitoring, labs,
sites, etc. | |
$ | 1,145 | | |
$ | 678 | |
| Supplies and costs to manufacture Lomecel-B™ | |
| 285 | | |
| 159 | |
| Employee compensation and benefits | |
| 559 | | |
| 501 | |
| Equity-based compensation | |
| 4 | | |
| 98 | |
| Depreciation | |
| 184 | | |
| 173 | |
| Amortization | |
| 55 | | |
| 55 | |
| Travel | |
| 55 | | |
| 34 | |
| Other activities | |
| - | | |
| 22 | |
| | |
$ | 2,287 | | |
$ | 1,720 | |
Selling and Marketing Expenses: Selling and marketing expenses
for the three months ended June 30, 2023 and 2022 were approximately $0.1 million and $0.2 million, respectively. Selling and marketing
expenses consist primarily of investor and public relations expenses.
Non-operating Lawsuit Expense: Non-operating lawsuit expense
for the three months ended June 30, 2022 was approximately $1.4 million. This expense was deemed probable and therefore the amount was
accrued in this period. Additional detail can be found in Part II, Item 1 “Legal Proceedings” of this Quarterly Report on
Form 10-Q. Legal expenses incurred in ordinary business activities are reported within general and administrative expenses. There was
no non-operating lawsuit expense for the current year period.
Other Income (Expense): Other income for the three months
ended June 30, 2023 was $0.1 million, which consisted of interest income. Other expense for the three months ended June 30, 2022 was
less than $0.1 million.
Net Loss: Net loss was approximately $5.6 million for the three
month periods ended June 30, 2023 and 2022.
COMPARISON OF THE SIX MONTHS ENDED JUNE 30,
2023 AND 2022
The following table summarizes our results of operations for the six
months ended June 30, 2023 and 2022, together with the changes in those items in dollars (in thousands):
| | |
Six Months Ended
June 30, | | |
Increase | |
| | |
2023 | | |
2022 | | |
(Decrease) | |
| Revenues | |
$ | 496 | | |
$ | 836 | | |
$ | (340 | ) |
| Cost of revenues | |
| 327 | | |
| 376 | | |
| (49 | ) |
| Gross profit | |
| 169 | | |
| 460 | | |
| (291 | ) |
| Expenses | |
| | | |
| | | |
| | |
| General and administrative | |
| 5,230 | | |
| 4,407 | | |
| 823 | |
| Research and development | |
| 5,067 | | |
| 3,147 | | |
| 1,920 | |
| Selling and marketing | |
| 300 | | |
| 521 | | |
| (221 | ) |
| Total operating expenses | |
| 10,597 | | |
| 8,075 | | |
| 2,522 | |
| | |
| | | |
| | | |
| | |
| Loss from operations | |
| (10,428 | ) | |
| (7,615 | ) | |
| (2,813 | ) |
| Non-operating Lawsuit expense | |
| - | | |
| (1,398 | ) | |
| 1,398 | |
| Other income (expense) | |
| 149 | | |
| (121 | ) | |
| 270 | |
| Net loss | |
$ | (10,279 | ) | |
$ | (9,134 | ) | |
$ | (1,145 | ) |
Revenues, Cost of Revenues and Gross Profit: Revenues for each
of the six months ended June 30, 2023 and 2022 were approximately $0.5 million and $0.8 million, respectively. Revenues for the six months
ended June 30, 2023 were approximately $0.3 million, or 41% lower when compared to the same period in 2022. Grant revenue for the six
months ended June 30, 2023 and 2022 was less than $0.1 million and $0.2 million, respectively. Grant revenue for the six months ended
June 30, 2023 was approximately $0.1 million, or 78% lower when compared to the same period in 2022, primarily due to a reduction in
grant funds available due in part to the completion of the grant-funded clinical trials. Clinical trial revenue, which is derived from
the Bahamas Registry Trial, for the six months ended June 30, 2023 and 2022 was $0.5 million and $0.6 million, respectively. Clinical
trial revenue for the six months ended June 30, 2023 was approximately $0.1 million, or 30%, lower when compared to the same period in
2022. During the first half of 2023, clinical trial revenue decreased as a result of a decrease in participant demand.
Related cost of revenues was approximately $0.3 million and $0.4 million
for the six months ended June 30, 2023 and 2022, respectively. Cost of revenues for the six months ended June 30, 2023 was $0.1 million,
or 13%, less when compared to the same period in 2022, primarily due to the corresponding decrease in the revenues earned from the Bahamas
Registry Trial. This resulted in a gross profit of approximately $0.2 million and $0.5 million for the six months ended June 30, 2023
and 2022, respectively.
General and Administrative Expense: General and administrative
expenses for the six months ended June 30, 2023 increased to approximately $5.2 million, compared to $4.4 million for the same period
in 2022. The increase of approximately $0.8 million, or 19%, was primarily related to an increase of $0.8 million in compensation and
benefit expenses.
Research and Development Expenses: Research and development
expenses for the six months ended June 30, 2023, increased to approximately $5.1 million, from approximately $3.1 million for the same
period in 2022. The increase of $1.9 million, or 61%, was primarily due to an increase of $1.4 million in research and development expenses
that were not reimbursable by grants, an increase of $0.3 million in supplies to manufacture Lomecel-B™, an increase in equity-based
compensation allocated to research and development expenses, of $0.2 million. Research and development expenses consisted primarily of
the following items (less those expenses allocated to the cost of revenues for the grants) (in thousands):
| | |
Six Months Ended June 30, | |
| | |
2023 | | |
2022 | |
| Clinical trial expenses-statistics, monitoring, labs, sites, etc. | |
$ | 2,495 | | |
$ | 1,119 | |
| Supplies and costs to manufacture Lomecel-B™ | |
| 571 | | |
| 246 | |
| Employee compensation and benefits | |
| 1,102 | | |
| 1,010 | |
| Equity-based compensation | |
| 277 | | |
| 197 | |
| Depreciation | |
| 366 | | |
| 317 | |
| Amortization | |
| 112 | | |
| 100 | |
| Travel | |
| 138 | | |
| 41 | |
| Other activities | |
| 6 | | |
| 117 | |
| | |
$ | 5,067 | | |
$ | 3,147 | |
Selling and Marketing Expenses: Selling and marketing expenses
for the six months ended June 30, 2023 and 2022 were approximately $0.3 million and $0.5 million, respectively. Selling and marketing
expenses consist primarily of investor and public relations expenses.
Non-operating Lawsuit Expense: Non-operating lawsuit expense
for the six months ended June 30, 2022 was approximately $1.4 million. This expense was deemed probable and therefore the amount was
accrued in this period. Additional detail can be found in Part II, Item 1 “Legal Proceedings” of this Quarterly Report on
Form 10-Q. Legal expenses incurred in ordinary business activities are reported within general and administrative expenses. There was
no non-operating lawsuit expense in the current year period.
Other Income (Expense): Other income for the six months ended
June 30, 2023 was $0.1 million. Other income consisted of interest income. Other expense for the six months ended June 30, 2022 was $0.1
million.
Net Loss: Net loss increased to approximately $10.3 million
for the six months ended June 30, 2022, from a net loss of $9.1 million for the same period in 2022. The increase in the net loss of
$1.2 million, or 13%, was a result of the items outlined above.
Cash Flows
The following table summarizes our sources and
uses of cash for the period presented (in thousands):
| | |
Six Months Ended June 30, | |
| | |
2023 | | |
2022 | |
| Net cash used in operating activities | |
$ | (10,449 | ) | |
$ | (7,427 | ) |
| Net cash provided by investing activities | |
| 2,796 | | |
| 2,282 | |
| Net cash used in financing activities | |
| (103 | ) | |
| (289 | ) |
| Change in cash and cash equivalents | |
$ | (7,756 | ) | |
$ | (5,434 | ) |
Operating Activities. We have incurred losses since
inception. Net cash used in operating activities for the six months ended June 30, 2023 was $10.4 million, consisting primarily of our
net loss of $10.3 million and payments for prepaid and other assets of $1.1 million and the non-operating lawsuit of $1.4 million. This
was partially offset by non-cash expenses of $1.1 million in equity-based compensation expenses, $0.5 million in depreciation and amortization,
and an increase in accrued expenses of $0.7 million. Net cash used in operating activities for the six months ended June 30, 2022 was
$7.4 million, consisting primarily of our net loss of $9.1 million and payments for prepaid expenses and other assets of $0.8 million
and accrued expenses of $0.5 million. This was partially offset by non-cash expenses of $3.3 million, primarily due to $1.4 million in
non-operating lawsuit expenses, equity-based compensation expenses of $1.2 million, and depreciation and amortization of $0.4 million.
Investing Activities. Net cash provided by investing
activities for the six months ended June 30, 2023 was $2.8 million consisting primarily of proceeds from the sale of marketable securities.
Net cash provided by investing activities for the six months ended June 30, 2022 was $2.3 million, consisting primarily of proceeds from
the sale of marketable securities.
Financing Activities. Net cash used in financing
activities for the six months ended June 30, 2023 was $0.1 million for the payment of taxes upon vesting of RSUs. Net cash provided by
financing activities for the six months ended June 30, 2022 was $0.3 million for the payment of taxes upon vesting of RSUs.
LIQUIDITY AND CAPITAL RESOURCES
Since our inception, we have incurred significant operating losses.
We expect to incur significant expenses and operating losses as we advance the preclinical and clinical development of our programs.
We expect that our sales, research and development and general and administrative costs will increase in connection with conducting additional
preclinical studies and clinical trials for our current and future programs and product candidates, contracting with CROs to support
preclinical studies and clinical trials, expanding our intellectual property portfolio, and providing general and administrative support
for our operations. As a result, we will need additional capital to fund our operations, which we may obtain from additional equity or
debt financings, collaborations, licensing arrangements, or other sources.
To date, we have financed our operations primarily through our IPO,
private placement equity financings, grant awards, and fees generated from the Bahamas Registry Trial and contract manufacturing services.
Since we were formed, we have raised approximately $77.2 million in gross proceeds from the issuance of equity. As of June 30, 2023, the
Company had cash, and cash equivalents of $2.7 million, marketable securities of $5.9 million and working capital of approximately $6.2
million.
Capital Raising Efforts
In our IPO, we sold 2,910,000 shares of Class A Common Stock at a
public offering price of $10.00 per share for aggregate gross proceeds of $29.1 million, inclusive of the underwriter’s partial
exercise of its over-allotment option, prior to deducting underwriting discounts, commissions, and other offering expenses.
The underwriter received warrants to purchase 106,400 Class A Common
Stock shares. The warrants are exercisable at any time and from time to time, in whole or in part, during the four and a half-year period
commencing August 12, 2021, at a price of $12.00 per Class A Common Stock share. During 2021, the underwriters assigned 95,760 of the
warrants to its employees. As of December 31, 2022, 51,061 warrants have been exercised, which provided net proceeds to the Company of
$0.6 million.
On December 3, 2021, we closed a private purchase and sale to certain
accredited investors of an aggregate of 1,169,288 shares of our Class A Common Stock and warrants to purchase 1,169,288 shares of Class
A Common Stock at an initial exercise price of $17.50 per share, which are subject to pricing reset under certain conditions, resulting
in aggregate gross proceeds of $20.5 million prior to deducting fees and offering expenses (the 2021 PIPE Offering”). We also
issued warrants exercisable for 46,772 shares of Class A Common Stock to affiliates of the Placement Agent, with an initial exercise
price of $17.50 per share, and are subject to the same pricing reset under certain conditions.
On June 27, 2023 we announced that we have filed a registration statement
with the SEC to conduct a tradeable subscription rights offering for up to $30.0 million of shares of Class A common stock to our stockholders
and holders of warrants to purchase common stock as of a future record date to be determined. We filed an amendment to the registration
statement with the SEC on July 28, 2023 with additional details regarding the proposed offering We anticipate closing this transaction
as outlined in the registration statement, as amended; however there can be no assurances that we can consummate this transaction or
any subsequent transaction at favorable pricing or at all.
Grant Awards
Through June 30, 2023, we have been awarded approximately $11.9 million
in governmental and non-profit association grants, which have been used to fund our clinical trials, research and development, production
and overhead. Grant awards are recognized as revenue, and depending on the funding mechanism, are deposited directly in our accounts
as lump sums, which are staggered over a predetermined period, or drawn down from a federal payment management system account for reimbursement
of expenses incurred. Revenue recognition occurs when the grant related expenses are incurred, or supplies and materials are received.
As of June 30, 2023, and December 31, 2022, the amount of unused grant funds that were available for us to draw was approximately $0.1 million
and $0.8 million, respectively.
Terms and Conditions of Grant Awards
Grant projects are typically divided into periods (e.g., a three-year
grant may have three one-year periods), and the total amount awarded is divided according to the number of periods. At pre-specified
time points, which are detailed in the grant award notifications, we are required to submit interim financial and scientific reports
to the granting agency totaling funds spent, and in some cases, detailing use of proceeds and progress made during the reporting period.
After funding the initial period, receipt of additional grant funds is contingent upon satisfactory submission of our interim reports
to the granting agency.
Grant awards arise from submitting detailed research proposals to
granting agencies, and winning a highly competitive and rigorous application review and process that is judged on the merits of the proposal.
There are typically multiple applicants applying and competing for a finite amount of funds. As such we cannot be sure that we will be
awarded grant funds in the future despite our past success in receiving such awards.
Funding Requirements
Our operating costs will continue to increase substantially for the
foreseeable future in connection with our ongoing activities. In past years we have been able to fund a large portion of our clinical
programs and our administrative overhead with the use of grant funding.
Specifically, our expenses will increase as we:
| |
● |
advance the clinical development of Lomecel-B™
for the treatment of several disease states and indications; |
| |
● |
pursue the preclinical and clinical development of
other current and future research programs and product candidates; |
| |
● |
in-license or acquire the rights to other products,
product candidates or technologies; |
| |
● |
maintain, expand and protect our intellectual property
portfolio; |
| |
● |
hire additional personnel in research, manufacturing
and regulatory and clinical development as well as management personnel; |
| |
● |
seek regulatory approval for any product candidates
that successfully complete clinical development; and |
| |
● |
expand our operational, financial
and management systems and increase personnel, including personnel to support our operations as a public company. |
We believe that our existing cash and cash equivalents will enable
us to fund our operating expenses and capital expenditure requirements into the first quarter of 2024.We have based these estimates on
assumptions that may prove to be imprecise, and we could utilize our available capital resources sooner than we expect.
Because of the numerous risks and uncertainties
associated with research, development and commercialization of our product candidates, it is difficult to estimate with certainty the
amount of our working capital requirements. Our future funding requirements will depend on many factors, including:
| ● | the progress, costs and results
of our clinical trials for our programs for our cell-based therapies, and additional research
and preclinical studies in other research programs we initiate in the future; |
| | | |
| ● | the costs and timing of process development and manufacturing scale-up activities
associated with our product candidates and other programs we advance through preclinical and clinical development; |
| | | |
| ● | our ability to establish and maintain strategic collaborations, licensing or
other agreements and the financial terms of such agreements; |
| | | |
| ● | the extent to which we in-license or acquire rights to other products, product
candidates or technologies; and |
| | | |
| ● | the costs and timing of preparing, filing and prosecuting patent applications,
maintaining and protecting our intellectual property rights and defending against any intellectual property-related
claims. |
Further, our operating results may change in the future, and we may
need additional funds to meet operational needs and capital requirements associated with such operating plans. Until such time,
if ever, that we can generate product revenue sufficient to achieve profitability, we expect to finance our cash needs through a combination
of equity offerings, debt financings, grant awards, collaboration agreements, other third-party funding, strategic alliances, licensing
arrangements and marketing and distribution arrangements.
We currently have no credit facility or committed sources of capital.
Debt financing and equity financing, if available, may involve agreements that include covenants limiting or restricting our ability
to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. If we raise additional
funds through other third-party funding, collaboration agreements, strategic alliances, licensing arrangements or marketing and distribution
arrangements, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates
or grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds through equity or debt financings
when needed, we may be required to delay, limit, reduce or terminate our biologic drug development or future commercialization efforts
or grant rights to develop and market products or product candidates that we would otherwise prefer to develop and market ourselves.
In order to meet our operational goals, we will need to obtain additional
capital, which we will likely obtain through a variety of means, including through public or private equity, debt financings or other
sources, including up-front payments and milestone payments from strategic collaborations. To the extent that we raise additional capital
through the sale of convertible debt or equity securities, current stockholder ownership interest will be diluted, and the terms may
include liquidation or other preferences that adversely affect stockholder rights. Such financing will likely result in dilution to stockholders,
and may result in imposition of debt covenants, increased fixed payment obligations or other restrictions that may affect our business.
If we raise additional funds through up-front payments or milestone payments pursuant to strategic collaborations with third parties,
we may have to relinquish valuable rights to our product candidates, or grant licenses on terms that are not favorable to us. In addition,
we may seek additional capital due to favorable market conditions or strategic considerations even if we believe we have sufficient funds
for our current or future operating plans.
Contractual Obligations and Commitments
As of June 30, 2023, we have $2.3 million in operating lease obligations
and $1.2 million in CRO payment obligations. We enter into contracts in the normal course of business with third-party contract organizations
for clinical trials, preclinical studies, manufacturing and other services and products for operating purposes. These contracts generally
provide for termination following a certain period after notice and therefore we believe that our non-cancelable obligations under these
agreements are not material.
We have not included milestone or royalty payments or other contractual
payment obligations if the timing and amount of such obligations are unknown or uncertain.
Critical Accounting Policies and Use of Estimates
Our management’s discussion and analysis of financial condition,
results of operations and liquidity are based on our condensed financial statements, which have been prepared in accordance with generally
accepted accounting principles in the U.S. (“U.S. GAAP”). The preparation of our condensed financial statements and related
disclosures requires us to make estimates, judgements and assumptions that affect the reported amounts of assets and liabilities and
the disclosure of contingent assets and liabilities at the date of the condensed financial statements and the reported amounts of revenues
and expenses during the reporting period. We base our estimates on historical experience, known trends and events and various other factors
that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values
of assets and liabilities that are not readily apparent from other sources. We evaluate our estimates and assumptions on an ongoing basis.
Our actual results may materially differ from these estimates under different assumptions or conditions. On an on-going basis, we review
our estimates to ensure that they appropriately reflect changes in our business or new information as it becomes available.
While our significant accounting policies are described in more detail
in the notes to our financial statements included in the 2022 10-K, we believe that the following accounting policies are those most
critical due to the judgments and estimates used in the preparation of our condensed financial statements.
Impairment of Long-Lived Assets. We evaluate long-lived
assets for impairment, including property and equipment and intangible assets, when events or changes in circumstances indicate that
the carrying value of such assets may not be recoverable. Upon the occurrence of a triggering event, the asset is reviewed to assess
whether the estimated undiscounted cash flows expected from the use of the asset plus the residual value from the ultimate disposal exceeds
the carrying value of the asset. If the carrying value exceeds the estimated recoverable amounts, the asset is written down to the estimated
fair value. Any resulting impairment loss is reflected on the condensed statements of operations. Management determined that there was
no impairment of long-lived assets during the three months ended June 30, 2023 and 2022.
Revenue recognition. Effective January 1, 2018, we adopted
ASC Topic 606, Revenue from Contracts with Customers, which establishes a single and comprehensive framework on how much revenue is to
be recognized, and when. The core principle is that a vendor should recognize revenue to depict the transfer of promised goods or services
to customers in an amount that reflects the consideration to which the vendor expects to be entitled in exchange for those goods or services.
Revenue will be recognized by a vendor when control over the goods or services is transferred to the customer.
We recognize revenue when performance obligations related to respective
revenue streams are met. For grant revenue, we consider the performance obligation met when the grant related expenses are incurred,
or supplies and materials are received. For clinical trial revenue, we consider the performance obligation met when the participant has
received the therapy. For contract manufacturing revenue, we consider the performance obligation met when the contractual obligation
and/or statement of work has been satisfied.
Research and development expense. Research and development
costs are charged to expense when incurred in accordance with FASB ASC 730, Research and Development. Research and development include
costs such as clinical trial expenses, contracted research and license agreement fees with no alternative future use, supplies and materials,
salaries, share-based compensation, employee benefits, property and equipment depreciation and allocation of various corporate costs.
We accrue for costs incurred by external service providers, including contract research organizations and clinical investigators, based
on estimates of service performed and costs incurred. These estimates include the level of services performed by the third parties, subject
enrollment in clinical trials, administrative costs incurred by the third parties, and other indicators of the services completed. Based
on the timing of amounts invoiced by service providers, we may also record payments made to those providers as prepaid expenses that
will be recognized as expense in future periods as the related services are rendered.
Emerging Growth Company Status
We are an “emerging growth company,” as defined in the
Jumpstart Our Business Startups Act, or JOBS Act, which is a law intended to encourage funding of small businesses in the U.S. by easing
many of the country’s securities regulations, and we may take advantage of reduced reporting requirements that are otherwise applicable
to public companies. Section 107 of the JOBS Act exempts emerging growth companies from being required to comply with new or revised
financial accounting standards until private companies are required to comply with those standards. We have elected to take advantage
of the extended transition period for complying with new or revised accounting standards, and as a result of this election, our financial
statements may not be comparable to companies that comply with public company effective dates. The JOBS Act also exempts us from having
to provide an auditor attestation of internal control over financial reporting under Sarbanes-Oxley Act Section 404(b).
We will remain an “emerging growth company” until the
earliest of (1) the last day of the fiscal year in which we have total annual gross revenues of $1.07 billion or more, (2) the last day
of the fiscal year following the fifth anniversary of the completion of our IPO, (3) the date on which we have issued more than $1.0
billion in nonconvertible debt during the previous three years or (4) the date on which we are deemed to be a large accelerated filer
under the rules of the SEC, which generally is when a company has more than $700 million in market value of its reported class of stock
held by non-affiliates and has been a public company for at least 12 months and has filed at least one Annual Report on Form 10-K.
Recent Accounting Pronouncements
A description of recent accounting pronouncements that may potentially
impact our financial position, results of operations or cash flows is disclosed in Note 2 to our unaudited condensed financial statements
included in Item 1 of this 10-Q.
Item 3. Quantitative and Qualitative
Disclosures About Market Risk.
There were no material changes in our exposure to market risk since
the disclosure included in Management’s Discussion and Analysis of Financial Condition and Results of Operations in our 2022 10-K.
Item 4. Controls and
Procedures.
Disclosure controls and procedures
Our management, under the supervision of and with the participation
of our Chief Executive Officer and our Chief Financial Officer, has evaluated the effectiveness of our disclosure controls and procedures,
as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as of the end of the period covered by this Quarterly
Report on Form 10-Q. Based upon this evaluation, our Chief Executive Officer and Chief Financial Officer have concluded that, as of the
end of the period covered by this Quarterly Report on Form 10-Q, our disclosure controls and procedures were effective.
Changes in internal control over financial reporting
There were no changes in our internal control over financial reporting
that occurred during the fiscal quarter ended June 30, 2023 that have materially affected, or are reasonably likely to materially
affect, our internal control over financial reporting.
PART II. OTHER INFORMATION
Item 1. Legal Proceedings
From time to time, the Company could become involved in disputes and
various litigation matters that arise in the normal course of business. These may include disputes and lawsuits related to intellectual
property, licensing, contract law and employee relations matters.
As previously disclosed in our 10-Q filed with the SEC on November
12, 2021, a securities class action lawsuit was filed on or about September 13, 2021 against the Company and certain of our directors
and officers in the United States District Court for the Southern District of Florida. On or about April 26, 2022, plaintiff filed an
amended complaint with related allegations. The complaint, as amended, alleged that there were materially false and misleading statements
made (or omissions of material information) in the Company’s initial public offering documents and in other disclosures during
the period from our initial public offering on February 12, 2021, through August 12, 2021, in violation of federal securities laws. The
complaint sought unspecified damages on behalf of a purported class of purchasers of our common stock during said period. On July 12,
2022, all parties preliminarily agreed to settle the action for approximately $1.4 million, which amount was accrued as of June 30, 2022
and was paid during the quarter ended June 30, 2023.
Item 1A. Risk Factors.
Adverse developments affecting the
financial services industry, such as actual events or concerns involving liquidity, defaults, or non-performance by financial institutions
or transactional counterparties, could adversely affect the Company’s financial condition and results of operations.
Actual events involving limited liquidity,
defaults, non-performance or other adverse developments that affect financial institutions, transactional counterparties or other companies
in the financial services industry or the financial services industry generally, or concerns or rumors about any events of these kinds
or other similar risks, have in the past and may in the future lead to market-wide liquidity problems. For example, on March 10, 2023,
Silicon Valley Bank (“SVB”) was closed by the California Department of Financial Protection and Innovation, which appointed
the Federal Deposit Insurance Corporation (“FDIC”) as receiver. Further, on Monday, May 1, 2023, First Republic Bank (“FRB”)
was closed by the California Department of Financial Protection and Innovation, the FDIC was appointed as receiver and JPMorgan Chase
Bank, National Association (N.A.) acquired all of FRB’s deposit accounts and substantially all of its assets. Although we are not
a borrower or party to any such instruments with SVB, FRB or any other financial institution currently in receivership, if any of our
banking entities with which we do business were to be placed into receivership, we may be unable to access such funds. In addition, if
any of our suppliers or other parties with whom we conduct business are unable to access funds pursuant to such instruments or lending
arrangements with such a financial institution, such parties’ ability to satisfy their obligations to us or to enter into new commercial
arrangements with us could be adversely affected. Uncertainty remains over liquidity concerns in the broader financial services industry,
including financial institutions with which we do business, borrow money or have funds on deposit. The results of or concerns with events
like this could include a variety of material and adverse impacts on the Company. In addition, investor concerns regarding the U.S. or
international financial systems could result in less favorable commercial financing terms, including higher interest rates or costs and
tighter financial and operating covenants, or systemic limitations on access to credit and liquidity sources, thereby making it more
difficult for us to acquire financing on acceptable terms or at all. Any decline in available funding generally could, among other risks,
adversely impact our ability to meet our operating demands or continue to develop our product candidates, or result in breaches of our
financial and/or contractual obligations. Any of these impacts, or any other impacts resulting from the factors described above or other
related or similar factors not described above, could have material adverse impacts on our liquidity and our current and/or projected
business operations and financial condition and results of operations.
Item 2. Unregistered Sales of Equity Securities
and Use of Proceeds.
ISSUER PURCHASES OF EQUITY SECURITIES
| Period | | |
Total
Number of
Shares
Purchased (a) | | |
Average Price Paid per
Share (or Unit) | | |
Total
Number of Shares
Purchased as
Part of Publicly
Announced Plans or Programs | | |
Dollar Value of Shares that May
Yet
Be Purchased Under the
Plans or Programs | |
| April
1-30, 2023 | | |
| 16,687 | | |
$ | 2.71 | | |
| - | | |
| - | |
| May
1-31, 2023 | | |
| - | | |
| - | | |
| - | | |
| - | |
| June
1-30, 2023 | | |
| 7,246 | | |
$ | 3.28 | | |
| | | |
| | |
| Total | | |
| 23,933 | | |
$ | 2.88 | | |
| - | | |
| - | |
| (a) | Includes shares withheld from
employees to satisfy minimum tax withholding obligations associated with the vesting of restricted stock units during the period. |
Item 3. Defaults Upon Senior Securities
None.
Item 4. Mine Safety Disclosures
Not applicable.
Item 5. Other Information
None.
Item 6. Exhibits.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934,
the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| |
LONGEVERON
INC. |
| |
|
| Date:
August 11, 2023 |
/s/
Mohamed Wa’el Ahmed Hashad |
| |
Mohamed
Wa’el Ahmed Hashad |
| |
Chief
Executive Officer |
| |
(Principal
Executive Officer) |
| Date:
August 11, 2023 |
/s/
Lisa A. Locklear |
| |
Lisa
A. Locklear |
| |
Executive Vice President
and Chief Financial Officer |
| |
(Principal Financial and
Accounting Officer) |
0.27
0.27
0.44
0.49
20927640
20943897
21069714
21105420
false
--12-31
Q2
0001721484
0001721484
2023-01-01
2023-06-30
0001721484
us-gaap:CommonClassAMember
2023-08-09
0001721484
us-gaap:CommonClassBMember
2023-08-09
0001721484
2023-06-30
0001721484
2022-12-31
0001721484
us-gaap:CommonClassAMember
2023-06-30
0001721484
us-gaap:CommonClassAMember
2022-12-31
0001721484
us-gaap:CommonClassBMember
2023-06-30
0001721484
us-gaap:CommonClassBMember
2022-12-31
0001721484
2023-04-01
2023-06-30
0001721484
2022-04-01
2022-06-30
0001721484
2022-01-01
2022-06-30
0001721484
us-gaap:CommonClassAMember
us-gaap:CommonStockMember
2022-12-31
0001721484
us-gaap:CommonClassBMember
us-gaap:CommonStockMember
2022-12-31
0001721484
us-gaap:ReceivablesFromStockholderMember
2022-12-31
0001721484
us-gaap:AdditionalPaidInCapitalMember
2022-12-31
0001721484
us-gaap:RetainedEarningsMember
2022-12-31
0001721484
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2022-12-31
0001721484
us-gaap:CommonClassAMember
us-gaap:CommonStockMember
2023-01-01
2023-06-30
0001721484
us-gaap:CommonClassBMember
us-gaap:CommonStockMember
2023-01-01
2023-06-30
0001721484
us-gaap:ReceivablesFromStockholderMember
2023-01-01
2023-06-30
0001721484
us-gaap:AdditionalPaidInCapitalMember
2023-01-01
2023-06-30
0001721484
us-gaap:RetainedEarningsMember
2023-01-01
2023-06-30
0001721484
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2023-01-01
2023-06-30
0001721484
us-gaap:CommonClassAMember
us-gaap:CommonStockMember
2023-06-30
0001721484
us-gaap:CommonClassBMember
us-gaap:CommonStockMember
2023-06-30
0001721484
us-gaap:ReceivablesFromStockholderMember
2023-06-30
0001721484
us-gaap:AdditionalPaidInCapitalMember
2023-06-30
0001721484
us-gaap:RetainedEarningsMember
2023-06-30
0001721484
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2023-06-30
0001721484
us-gaap:CommonClassAMember
us-gaap:CommonStockMember
2021-12-31
0001721484
us-gaap:CommonClassBMember
us-gaap:CommonStockMember
2021-12-31
0001721484
us-gaap:ReceivablesFromStockholderMember
2021-12-31
0001721484
us-gaap:AdditionalPaidInCapitalMember
2021-12-31
0001721484
us-gaap:RetainedEarningsMember
2021-12-31
0001721484
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2021-12-31
0001721484
2021-12-31
0001721484
us-gaap:CommonClassAMember
us-gaap:CommonStockMember
2022-01-01
2022-06-30
0001721484
us-gaap:CommonClassBMember
us-gaap:CommonStockMember
2022-01-01
2022-06-30
0001721484
us-gaap:ReceivablesFromStockholderMember
2022-01-01
2022-06-30
0001721484
us-gaap:AdditionalPaidInCapitalMember
2022-01-01
2022-06-30
0001721484
us-gaap:RetainedEarningsMember
2022-01-01
2022-06-30
0001721484
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2022-01-01
2022-06-30
0001721484
us-gaap:CommonClassAMember
us-gaap:CommonStockMember
2022-06-30
0001721484
us-gaap:CommonClassBMember
us-gaap:CommonStockMember
2022-06-30
0001721484
us-gaap:ReceivablesFromStockholderMember
2022-06-30
0001721484
us-gaap:AdditionalPaidInCapitalMember
2022-06-30
0001721484
us-gaap:RetainedEarningsMember
2022-06-30
0001721484
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2022-06-30
0001721484
2022-06-30
0001721484
us-gaap:CommonClassAMember
us-gaap:CommonStockMember
2023-03-31
0001721484
us-gaap:CommonClassBMember
us-gaap:CommonStockMember
2023-03-31
0001721484
us-gaap:ReceivablesFromStockholderMember
2023-03-31
0001721484
us-gaap:AdditionalPaidInCapitalMember
2023-03-31
0001721484
us-gaap:RetainedEarningsMember
2023-03-31
0001721484
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2023-03-31
0001721484
2023-03-31
0001721484
us-gaap:CommonClassAMember
us-gaap:CommonStockMember
2023-04-01
2023-06-30
0001721484
us-gaap:CommonClassBMember
us-gaap:CommonStockMember
2023-04-01
2023-06-30
0001721484
us-gaap:ReceivablesFromStockholderMember
2023-04-01
2023-06-30
0001721484
us-gaap:AdditionalPaidInCapitalMember
2023-04-01
2023-06-30
0001721484
us-gaap:RetainedEarningsMember
2023-04-01
2023-06-30
0001721484
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2023-04-01
2023-06-30
0001721484
us-gaap:CommonClassAMember
us-gaap:CommonStockMember
2022-03-31
0001721484
us-gaap:CommonClassBMember
us-gaap:CommonStockMember
2022-03-31
0001721484
us-gaap:ReceivablesFromStockholderMember
2022-03-31
0001721484
us-gaap:AdditionalPaidInCapitalMember
2022-03-31
0001721484
us-gaap:RetainedEarningsMember
2022-03-31
0001721484
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2022-03-31
0001721484
2022-03-31
0001721484
us-gaap:CommonClassAMember
us-gaap:CommonStockMember
2022-04-01
2022-06-30
0001721484
us-gaap:CommonClassBMember
us-gaap:CommonStockMember
2022-04-01
2022-06-30
0001721484
us-gaap:ReceivablesFromStockholderMember
2022-04-01
2022-06-30
0001721484
us-gaap:AdditionalPaidInCapitalMember
2022-04-01
2022-06-30
0001721484
us-gaap:RetainedEarningsMember
2022-04-01
2022-06-30
0001721484
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2022-04-01
2022-06-30
0001721484
2023-06-01
2023-06-27
0001721484
us-gaap:CommonClassAMember
us-gaap:OverAllotmentOptionMember
2023-01-01
2023-06-30
0001721484
srt:MinimumMember
us-gaap:FiniteLivedIntangibleAssetsMember
2023-06-30
0001721484
srt:MaximumMember
us-gaap:FiniteLivedIntangibleAssetsMember
2023-06-30
0001721484
lgvn:ClinicalTrialRevenueMember
2023-04-01
2023-06-30
0001721484
lgvn:NationalInstitutesOfHealthGrantMember
2023-06-30
0001721484
lgvn:NationalInstitutesOfHealthGrantMember
2022-12-31
0001721484
lgvn:NationalInstituteOfHealthGrantMember
2023-04-01
2023-06-30
0001721484
lgvn:NationalInstituteOfHealthGrantMember
2022-04-01
2022-06-30
0001721484
lgvn:NationalInstituteOfHealthGrantMember
2023-01-01
2023-06-30
0001721484
lgvn:NationalInstituteOfHealthGrantMember
2022-01-01
2022-06-30
0001721484
lgvn:ClinicalTrialRevenueMember
2023-04-01
2023-06-30
0001721484
lgvn:ClinicalTrialRevenueMember
2022-04-01
2022-06-30
0001721484
lgvn:ClinicalTrialRevenueMember
2023-01-01
2023-06-30
0001721484
lgvn:ClinicalTrialRevenueMember
2022-01-01
2022-06-30
0001721484
lgvn:MSCRFTEDCOGrantsMember
2023-04-01
2023-06-30
0001721484
lgvn:MSCRFTEDCOGrantsMember
2022-04-01
2022-06-30
0001721484
lgvn:MSCRFTEDCOGrantsMember
2023-01-01
2023-06-30
0001721484
lgvn:MSCRFTEDCOGrantsMember
2022-01-01
2022-06-30
0001721484
us-gaap:FairValueInputsLevel1Member
2023-06-30
0001721484
us-gaap:FairValueInputsLevel2Member
2023-06-30
0001721484
us-gaap:FairValueInputsLevel3Member
2023-06-30
0001721484
us-gaap:FairValueInputsLevel1Member
2023-01-01
2023-06-30
0001721484
us-gaap:FairValueInputsLevel2Member
2023-01-01
2023-06-30
0001721484
us-gaap:FairValueInputsLevel3Member
2023-01-01
2023-06-30
0001721484
us-gaap:FairValueInputsLevel1Member
2022-12-31
0001721484
us-gaap:FairValueInputsLevel2Member
2022-12-31
0001721484
us-gaap:FairValueInputsLevel3Member
2022-12-31
0001721484
us-gaap:FairValueInputsLevel1Member
2022-01-01
2022-12-31
0001721484
us-gaap:FairValueInputsLevel2Member
2022-01-01
2022-12-31
0001721484
us-gaap:FairValueInputsLevel3Member
2022-01-01
2022-12-31
0001721484
2022-01-01
2022-12-31
0001721484
us-gaap:LeaseholdImprovementsMember
2023-01-01
2023-06-30
0001721484
us-gaap:LeaseholdImprovementsMember
2023-06-30
0001721484
us-gaap:LeaseholdImprovementsMember
2022-12-31
0001721484
us-gaap:FurnitureAndFixturesMember
2023-01-01
2023-06-30
0001721484
us-gaap:FurnitureAndFixturesMember
2023-06-30
0001721484
us-gaap:FurnitureAndFixturesMember
2022-12-31
0001721484
us-gaap:ComputerEquipmentMember
2023-01-01
2023-06-30
0001721484
us-gaap:ComputerEquipmentMember
2023-06-30
0001721484
us-gaap:ComputerEquipmentMember
2022-12-31
0001721484
us-gaap:SoftwareDevelopmentMember
2023-01-01
2023-06-30
0001721484
us-gaap:SoftwareDevelopmentMember
2023-06-30
0001721484
us-gaap:SoftwareDevelopmentMember
2022-12-31
0001721484
srt:MinimumMember
us-gaap:LicensingAgreementsMember
2023-06-30
0001721484
us-gaap:LicensingAgreementsMember
2023-06-30
0001721484
us-gaap:PatentsMember
2023-06-30
0001721484
us-gaap:TrademarksMember
2023-06-30
0001721484
srt:MinimumMember
us-gaap:LicensingAgreementsMember
2022-12-31
0001721484
us-gaap:LicensingAgreementsMember
2022-12-31
0001721484
us-gaap:PatentsMember
2022-12-31
0001721484
us-gaap:TrademarksMember
2022-12-31
0001721484
2023-04-19
2023-04-19
0001721484
2023-04-18
2023-04-18
0001721484
2023-04-03
2023-04-03
0001721484
us-gaap:LiabilityMember
2023-04-03
2023-04-03
0001721484
lgvn:RSUMember
2023-04-03
0001721484
2023-04-03
0001721484
srt:MinimumMember
2023-04-03
2023-04-03
0001721484
srt:MaximumMember
2023-04-03
2023-04-03
0001721484
us-gaap:LiabilityMember
2023-04-03
0001721484
us-gaap:CommonClassAMember
2023-04-03
0001721484
2023-01-03
2023-01-03
0001721484
us-gaap:CommonClassAMember
2023-01-03
0001721484
lgvn:RSUMember
2023-01-03
0001721484
2023-01-03
0001721484
us-gaap:SeriesCPreferredStockMember
2022-11-16
2022-11-16
0001721484
lgvn:ConsultingAgreementsMember
us-gaap:SeriesCPreferredStockMember
2022-11-16
2022-11-16
0001721484
us-gaap:IPOMember
2022-10-03
2022-10-03
0001721484
2022-10-03
2022-10-03
0001721484
2022-10-03
0001721484
2022-07-01
2022-07-01
0001721484
us-gaap:IPOMember
2022-07-01
0001721484
2022-07-01
0001721484
us-gaap:CommonClassAMember
2022-07-01
0001721484
2022-06-22
2022-06-22
0001721484
2022-06-03
2022-06-03
0001721484
us-gaap:LiabilityMember
2022-06-03
2022-06-03
0001721484
2022-06-03
0001721484
us-gaap:CommonClassAMember
2022-06-03
2022-06-03
0001721484
2022-04-04
2022-04-04
0001721484
2022-04-04
0001721484
2022-04-01
0001721484
srt:BoardOfDirectorsChairmanMember
2022-04-01
2022-04-01
0001721484
2022-02-01
2022-02-12
0001721484
2022-01-01
2022-01-03
0001721484
2022-01-03
0001721484
us-gaap:CommonClassAMember
2022-01-03
0001721484
us-gaap:CommonClassAMember
2023-01-01
2023-06-30
0001721484
lgvn:OutstandingSeriesAAndBMember
2023-01-01
2023-06-30
0001721484
us-gaap:CommonClassBMember
2023-01-01
2023-06-30
0001721484
us-gaap:IPOMember
2023-06-30
0001721484
us-gaap:IPOMember
2023-01-01
2023-06-30
0001721484
us-gaap:CommonClassAMember
us-gaap:WarrantMember
2021-12-31
0001721484
2021-01-01
2021-12-31
0001721484
us-gaap:CommonClassAMember
2021-01-01
2021-12-31
0001721484
us-gaap:CommonClassAMember
2021-12-31
0001721484
us-gaap:RestrictedStockUnitsRSUMember
2023-03-01
0001721484
us-gaap:CommonClassAMember
2022-11-16
0001721484
2022-11-01
2022-11-16
0001721484
lgvn:MrGreenMember
2022-06-22
2022-06-22
0001721484
lgvn:MrGreenMember
2022-06-22
0001721484
lgvn:MrClavijoMember
2022-06-03
0001721484
2023-06-01
2023-06-09
0001721484
us-gaap:RestrictedStockUnitsRSUMember
2022-04-04
2022-04-04
0001721484
us-gaap:RestrictedStockUnitsRSUMember
2022-04-04
0001721484
lgvn:SecondThirdAndFourthAnniversariesMember
2022-04-04
2022-04-04
0001721484
lgvn:MrBaileyMember
2022-04-04
2022-04-04
0001721484
lgvn:MrBaileyMember
2022-04-04
0001721484
srt:MinimumMember
2023-01-01
2023-06-30
0001721484
srt:MaximumMember
2023-01-01
2023-06-30
0001721484
2023-03-01
2023-03-01
0001721484
2023-03-01
0001721484
us-gaap:CommonClassAMember
2022-12-01
2022-12-21
0001721484
2022-12-01
2022-12-21
0001721484
2022-12-21
0001721484
2022-11-16
0001721484
2022-09-01
2022-09-06
0001721484
2022-09-06
0001721484
lgvn:MrLehrMember
us-gaap:CommonClassAMember
2022-06-03
2022-06-03
0001721484
lgvn:MrLehrMember
us-gaap:CommonClassAMember
2022-06-03
0001721484
2022-03-01
2022-03-14
0001721484
2022-03-14
0001721484
2022-01-01
2022-01-06
0001721484
2022-01-06
0001721484
lgvn:StockOptionsVestedMember
2023-06-30
0001721484
lgvn:StockOptionsUnvestedMember
2023-06-30
0001721484
lgvn:StockOptionsVestedMember
2022-12-31
0001721484
lgvn:StockOptionsUnvestedMember
2022-12-31
0001721484
lgvn:StockOptionActivityMember
2022-12-31
0001721484
lgvn:StockOptionActivityMember
2023-01-01
2023-06-30
0001721484
lgvn:StockOptionActivityMember
2023-06-30
0001721484
lgvn:ConsultingServicesAgreementMember
2014-11-20
2014-11-20
0001721484
lgvn:MasterServicesAgreementsMember
2022-11-01
2022-11-16
0001721484
lgvn:ConsultingServicesAgreementMember
2023-06-30
0001721484
lgvn:SeriesCUnitsMember
2017-12-31
0001721484
lgvn:SeriesCUnitsMember
2019-11-22
0001721484
lgvn:SeriesCUnitsMember
2021-01-29
0001721484
2019-11-22
0001721484
2021-01-29
0001721484
us-gaap:CommonClassAMember
2022-01-01
2022-12-31
0001721484
2022-01-01
2023-12-31
0001721484
2014-11-20
0001721484
2022-07-12
0001721484
us-gaap:RestrictedStockUnitsRSUMember
2023-01-01
2023-06-30
0001721484
us-gaap:RestrictedStockUnitsRSUMember
2022-01-01
2022-06-30
0001721484
us-gaap:PhantomShareUnitsPSUsMember
2023-01-01
2023-06-30
0001721484
us-gaap:PhantomShareUnitsPSUsMember
2022-01-01
2022-06-30
0001721484
us-gaap:EmployeeStockOptionMember
2023-01-01
2023-06-30
0001721484
us-gaap:EmployeeStockOptionMember
2022-01-01
2022-06-30
0001721484
us-gaap:WarrantMember
2023-01-01
2023-06-30
0001721484
us-gaap:WarrantMember
2022-01-01
2022-06-30
xbrli:shares
iso4217:USD
iso4217:USD
xbrli:shares
xbrli:pure
I, Lisa A. Locklear, certify that:
Pursuant to the requirement set forth in Rule
13a-14(b) or Rule 15d-14(b) of the Securities Exchange Act of 1934, as amended, and Section 1350 of Chapter 63 of Title 18 of the United
States Code (18 U.S.C. § 1350), as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, Mohamed Wa’el Ahmed Hashad,
Chief Executive Officer (principal executive officer) of Longeveron Inc. (the “Company”), and Lisa A. Locklear, the Chief
Financial Officer (principal financial officer) of the Company, each hereby certifies that, to his knowledge on the date hereof:
This certification shall not be deemed to be filed
with the Securities and Exchange Commission and shall not be incorporated by reference into any filing of the Company under the Securities
Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended (whether made before or after the date of the Quarterly Report),
irrespective of any general incorporation language contained in such filing. A signed original of this written statement required by Section
906 has been provided to the Company and will be retained and furnished to the Securities and Exchange Commission or its staff upon request.