0001498382trueTuHURA Biosciences, Inc./NV00014983822024-10-182024-10-18
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 8-K/A
(Amendment No. 1)
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): October 18, 2024
TuHURA Biosciences, Inc.
(Exact Name of Registrant as Specified in its Charter)
|
|
|
|
|
|
|
|
|
|
Nevada |
|
001-37823 |
|
99-0360497 |
(State or other jurisdiction
of incorporation) |
|
(Commission File Number) |
|
(I.R.S. Employer
Identification No.) |
|
|
|
|
|
|
10500 University Dr., Suite 110 Tampa, Florida |
|
33612 |
(Address of Principal Executive Offices) |
|
(Zip Code) |
Registrant’s telephone number, including area code: (813) 875-6600
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
|
|
☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
|
|
☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
|
|
☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
|
|
☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
|
|
|
|
|
Title of each class |
|
Trading
Symbol(s) |
|
Name of each exchange on which registered |
Common Stock, par value $0.001 per share |
|
HURA |
|
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (Sec.230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (Sec.240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
INTRODUCTORY NOTE
This Amendment No. 1 on Form 8-K/A (this “Amendment No. 1”) amends the Current Report on Form 8-K filed by TuHURA Biosciences, Inc. (f/k/a Kintara Therapeutics, Inc.) (the “Company”) on October 21, 2024 (the “Original Report”), in which the Company reported, among other events, the completion of the Merger, pursuant to which Kayak Mergeco, Inc. merged with and into TuHURA Biosciences, Inc., a Delaware corporation (“Private TuHURA”). This Amendment No. 1 is filed to (1) update the Item 2.01 information to reflect Management’s Discussion and Analysis of Financial Condition and Results of Operations of Private TuHURA for the three and nine months ended September 30, 2024 and September 30, 2023; and (2) amend the historical financial statements provided under Items 9.01(a) in the Original Report to include the unaudited interim financial statements of Private TuHURA as of September 30, 2024 and for the three and nine months ended September 30, 2024 and September 30, 2023. This Amendment No. 1 does not amend any other item of the Original Report or purport to provide an update or a discussion of any developments at the Company subsequent to the filing date of the Original Report.
Capitalized terms used but not defined herein have the meanings given in the Original Report.
Item 2.01. Completion of Acquisition or Disposition of Assets.
Management’s Disclosure and Analysis of Financial Condition and Results of Operations
The Form 10 information in Item 2.01 of the Original Report is hereby amended and supplemented by adding the following: “Reference is made to the disclosure contained in the section titled “MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULT OF OPERATIONS OF TUHURA BIOSCIENCES, INC. (A DELAWARE CORPORATION)” filed as Exhibit 99.2 to this Amendment No. 1, which is incorporated herein by reference.”
Item 9.01. Financial Statements and Exhibits.
(a) Financial Statements of Business Acquired.
Private TuHURA’s audited balance sheets as of December 31, 2023 and 2022, the related statements of operations, statements of changes in convertible preferred stock and stockholders’ deficit, and cash flows for each of the two years in the period ended December 31, 2023, and the related notes are incorporated herein by reference to such financial statements appearing on pages F-2 to F-17 of the Proxy Statement/Prospectus.
The unaudited condensed interim financial statements of Private TuHURA as of September 30, 2024 and for the periods ended September 30, 2024 and September 30, 2023 are filed as Exhibit 99.3 to this Amendment No. 1 and incorporated herein by reference.
Also included herewith as Exhibit 99.2 and incorporated herein by reference is the Management’s Discussion and Analysis of Financial Condition and Results of Operations for Private TuHURA for the three and nine months ended September 30, 2024 and 2023.
(d) Exhibits
SIGNATURE
Pursuant to the requirements of the Securities and Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
TuHURA Biosciences, Inc. |
|
|
|
November 14, 2024 |
|
By: |
|
/s/ Dan Dearborn |
|
|
|
|
Name: |
|
Dan Dearborn |
|
|
|
|
Title: |
|
Chief Financial Officer |
Exhibit 99.2
Management’s Discussion and Analysis of Financial Condition and Results of Operations of TuHURA Biosciences, Inc. (a Delaware corporation)
The following discussion and analysis of the financial condition and results of operations of TuHURA Biosciences, Inc., a Delaware corporation (“TuHURA”), should be read in conjunction with its audited financial statements and interim unaudited financial statements and the notes related thereto which are attached as Exhibit 99.2 to the Current Report on Form 8-K of which this exhibit is a part. Certain information contained in the discussion and analysis set forth below includes forward-looking statements. TuHURA’s actual results may differ materially from those anticipated in these forward-looking statements as a result of many factors, including those set forth under “Cautionary Note Regarding Forward-Looking Statements” contained in the Current Report on Form 8-K filed by TuHURA Biosciences, Inc., a Nevada corporation formerly known as Kintara Therapeutics, Inc. (“Kintara”), with the Securities and Exchange Commission (the “SEC”) on October 21, 2024.
Overview
TuHURA is a clinical stage immuno-oncology company developing novel personalized cancer vaccine product candidates designed to overcome primary resistance to immunotherapies like checkpoint inhibitors. TuHURA has entered into a Special Protocol Assessment agreement with the FDA for a single Phase 3 randomized placebo and injection controlled trial for IFx-2.0, the company’s lead personalized cancer vaccine product candidate, as adjunctive therapy to pembrolizumab (Keytruda®) in the first line treatment of patients with advanced or metastatic Merkel cell carcinoma who are checkpoint inhibitor naïve utilizing the FDA’s accelerated approval pathway. TuHURA is also developing novel bi-functional antibody drug conjugates, or ADCs, targeting myeloid derived suppressor cells, or MDSCs, to modulate their immunosuppressive effects on the tumor microenvironment to overcome acquired resistance to immunotherapies.
To date, TuHURA has devoted substantially all of its resources to organizing and staffing TuHURA, business planning, raising capital, identifying and developing product candidates, enhancing its intellectual property portfolio, undertaking research, conducting preclinical studies and clinical trials, and securing manufacturing for its development programs. TuHURA does not have any products approved for sale and has not generated any revenue from product sales. TuHURA has funded its operations primarily through the private placement of common and preferred stock and convertible notes.
TuHURA has incurred significant operating losses since its inception, which are mainly attributed to research and development costs associated with TuHURA’s portfolio and general and administrative expenses. TuHURA’s net loss was $29.3 million for the year ended December 31, 2023 (which includes the expensing of the entire $16.2 million purchase price for the assets of TuHURA Biopharma, Inc., of which $15.0 million was paid in the form of TuHURA Common Stock) and $15.7 million for the nine months ended September 30, 2024. As of September 30, 2024, TuHURA had an accumulated deficit of $105.1 million. TuHURA’s operating losses may fluctuate significantly from quarter-to-quarter and year-to-year as a result of several factors, including the timing of its preclinical studies and clinical trials and the expenditures related to other research and development activities. TuHURA expects to continue to incur operating losses. TuHURA anticipates these losses will increase substantially as it advances its product candidates through preclinical and clinical development, develops additional product candidates and seeks regulatory approvals for its product candidates. TuHURA does not expect to generate any revenues from product sales unless and until it successfully completes development and obtains regulatory approval for one or more product candidates. In addition, if TuHURA obtains marketing approval for any product candidate, TuHURA expects to incur pre-commercialization expenses and significant commercialization expenses related to marketing, sales, manufacturing and distribution. TuHURA may also incur expenses in connection with the in-licensing of additional product candidates. Furthermore, TuHURA expects to incur additional costs associated with operating as a public company, including significant legal, accounting, investor relations, compliance and other expenses that TuHURA did not incur as a private company.
As a result, TuHURA will need substantial additional funding to support its continuing operations and pursue its growth strategy. Until such time as TuHURA can generate significant revenue from sales of its product
candidates, if ever, TuHURA expects to finance its cash needs through public or private equity offerings, debt financings, collaborations and licensing arrangements or other capital sources. However, TuHURA may be unable to raise additional funds or enter into such other arrangements when needed on favorable terms or at all. TuHURA’s failure to raise capital or enter into such other arrangements as and when needed would have a negative impact on its financial condition and could force it to delay, limit, reduce or terminate its product development or future commercialization efforts or grant rights to develop and market its product candidates that it would otherwise prefer to develop and market itself.
Because of the numerous risks and uncertainties associated with pharmaceutical product development, TuHURA is unable to accurately predict the timing or amount of increased expenses or when or if it will be able to achieve or maintain profitability. Even if TuHURA is able to generate product sales, it may not become profitable. If TuHURA fails to become profitable or is unable to sustain profitability on a continuing basis, TuHURA may be unable to continue its operations at planned levels and be forced to reduce or terminate its operations.
As of September 30, 2024, TuHURA had cash and cash equivalents of $19.6 million. See “— Liquidity and Capital Resources” below.
Recent Developments
Merger with Kintara Therapeutics
On October 18, 2024, TuHURA completed the transactions contemplated by its previously disclosed Agreement and Plan of Merger, dated April 2, 2024 (the “Merger Agreement”), with Kintara Therapeutics, Inc., a Nevada corporation that has been renamed TuHURA Biosciences, Inc. (“Kintara”), and Kayak Mergeco, Inc., a Delaware corporation wholly owned subsidiary Kintara (“Merger Sub”). Pursuant to the Merger Agreement, Merger Sub merged with and into TuHURA with TuHURA surviving the merger (the “Merger”) and becoming Kintara’s direct, wholly-owned subsidiary. In connection with the completion of the Merger, effective at 12:01 a.m. Eastern Time on October 18, 2024, Kintara effected a 1-for-35 reverse stock split of its common stock (the “Reverse Stock Split”). Effective at 12:03 a.m. Eastern Time on October 18, 2024, TuHURA completed the Merger, and effective at 12:04 a.m. Eastern Time on October 18, 2024, Kintara changed its name to “TuHURA Biosciences, Inc.”
The Merger is being accounted for as a reverse recapitalization in accordance with GAAP, with Kintara treated as the acquired company for financial reporting purposes TuHURA treated as the accounting acquirer. The Merger is intended to qualify for U.S. federal income tax purposes as a tax-free reorganization under the provisions of Section 368(a) of the Code.
Subject to the terms and conditions of the Merger Agreement, at the closing of the Merger, (a) each then-outstanding share of TuHURA Common Stock (other than shares held in treasury and excluding dissenting shares), including shares of TuHURA Common Stock issued upon conversion of TuHURA preferred stock and conversion of all TuHURA convertible promissory notes issued in the TuHURA Note Financing, were converted into the right to receive a number of shares of Kintara Common Stock (after giving effect to the Reverse Stock Split) based on an exchange ratio of 0.1789 shares of Kintara Common Stock for each outstanding shares of TuHURA Common Stock per the Merger Agreement (the “Exchange Ratio”), and (b) each then-outstanding TuHURA stock option and warrant that has not previously been exercised immediately prior to the effective time of the Merger was assumed by Kintara with the number of underlying shares and exercise price being adjusted in accordance with the Exchange Ratio.
Also at the closing of the Merger, Kintara entered into a Contingent Value Rights Agreement with the Rights Agent (as defined in the Merger Agreement), pursuant to which holders of Kintara Common Stock and Kintara Common Stock warrants, in each case, as of the close of business on the business day immediately prior to the effective time of the Merger, received one CVR for each outstanding share of Kintara Common Stock held by such stockholder (or, in the case of the warrants, each share of Kintara Common Stock for which such warrant is exercisable). Each CVR shall entitle the holder thereof to receive its portion of 1,539,918 shares of Kintara common stock if Kintara achieves the following milestone: (i) Kintara enrolls a minimum of ten cutaneous metastatic breast cancer patients in a study to determine whether a dose of Kintara’s REM-001 lower than 1.2 mg/kg elicits a treatment effect similar to that seen in prior studies of REM-001 at the 1.2 mg/kg dose and (ii) such patients enrolled in the study complete eight weeks of follow-up, in each case, on or before December 31, 2025.
Kineta Exclusivity Agreement and July 2024 Private Placement
On July 8, 2024, TuHURA issued a press release announcing that it has entered into an Exclusivity and Right of First Offer Agreement (the “Exclusivity Agreement”) with Kineta, Inc. (“Kineta”) for the potential acquisition of Kineta’s KVA12123 anti-VISTA antibody and related rights and assets associated with and derived from the asset.
KVA12123 is a rationally targeted, anti-VISTA antibody checkpoint inhibitor designed to reverse VISTA immune suppression and remodel the tumor microenvironment (TME) to overcome acquired resistance to immunotherapies.
Pursuant to the Exclusivity Agreement, among other things, Kineta has granted TuHURA an exclusive right to acquire Kineta’s worldwide patents, patent rights, patent applications, product and development program assets, technical and business information, and other rights and assets associated with and derived from its development program related to KVA12123 during the period commencing as of July 3, 2024 (the “Effective Date”) and continuing through the first to occur of (a) the execution of any Definitive Agreement (as defined therein) with respect to a Potential Transaction (as defined therein) by TuHURA or one or more of its affiliates and (b) 11:59 PM Eastern Time on October 1, 2024, subject to extension as noted in the following sentence (the “Exclusivity Period”). In the event that the parties are engaged in good faith discussions regarding a Potential Transaction on the date on which the Exclusivity Period (or any renewal thereof) is scheduled to expire and TuHURA has not yet closed the transactions contemplated by the Merger Agreement, then on such date, the Exclusivity Period shall automatically renew for an additional ten (10) day period (a “Renewal Period”) (up to a total of two (2) Renewal Periods for an aggregate of twenty (20) days).
Under the terms of the Exclusivity Agreement, TuHURA paid Kineta a fee in the amount of $5,000,000, with $2,500,000 paid at signing and an additional $2,500,000 paid on July 15, 2024. The fee is nonrefundable other than in the case of an uncured breach of the Exclusivity Agreement by Kineta. No later than two (2) business days after a Renewal Period has started (to be confirmed in writing by both parties), TuHURA shall pay an additional $150,000 as an additional Exclusivity Payment, in an amount not to exceed $300,000 for the two (2) available Renewal Periods. The Exclusivity Payment will be credited against the initial cash consideration that may be payable to Kineta pursuant to any Definitive Agreement (if any) between Kineta and TuHURA and/or its affiliates with respect to a Potential Transaction. TuHURA has exercised the two (2) available Renewal Periods, which have since expired, but TuHURA continues to collaborate with Kineta on the ongoing KVA12123 VISTA clinical trial program.
In conjunction with the Exclusivity Agreement, TuHURA sold 4,009,623 shares of its common stock in a private offering with a purchase price of $5,000,000 (the “July Private Placement”) to an existing TuHURA shareholder (the “Investor”). In connection with the July Private Placement, the Investor is entitled to a 1.5% royalty on certain sales by TuHURA of products based on KVA12123 as set forth in the Investor’s subscription agreement. Due to no definitive transaction agreement with Kineta for the purchase of KVA12123 and the inherent uncertainties surrounding the regulatory approval of KVA12123 and future monetization, TuHURA has not allocated any of the $5,000,000 purchase price consideration to the royalty agreement.
Special Protocol Assessment Agreement
On January 25, 2024 TuHURA successfully completed its negotiations with FDA and entered into a Special Protocol Assessment Agreement for a single registration directed, randomized, placebo controlled Phase 3 trial for IFx-Hu2.0 as adjunctive therapy to pembrolizumab (Keytruda®) in first line treatment for patients with advanced or metastatic Merkel Cell carcinoma who are checkpoint inhibitor naive. The trial utilizes a novel design recommended by the FDA which incorporates Overall Response Rate (ORR) as the primary endpoint for accelerated approval. The trial also includes Progression Free Survival (PFS) as a key secondary endpoint which, if achieved, without demonstrating a detriment to Overall Survival, could allow conversion from accelerated approval to full approval satisfying the requirement for a post marketing trial. Before initiating this Phase 3 trial TuHURA is required to complete certain manufacturing activities as noted in a partial clinical hold correspondence from FDA. Based on correspondence following a type C meeting with the FDA, TuHURA has ongoing development and validation of several testing and mixing studies which TuHURA believes will be adequate to address the CMC requirements to initiate the Phase 3 clinical trial. TuHURA believes, it will be in position to initiate the phase 3 study in the first
quarter of 2025 and anticipates enrollment to take approximately 12 months with topline data 6 to 7 months following the last patient enrolled.
Components of TuHURA’s Results of Operations
Revenue
TuHURA did not generate any revenue and does not expect to generate any revenue from the sale of products in the near future.
Research and Development Expenses
To date, TuHURA’s research and development expenses have related primarily to development of IFx-Hu2.0, manufacturing, clinical studies, and other early pre-clinical activities related to TuHURA’s portfolio. Research and development expenses are recognized as incurred and payments made prior to the receipt of goods or services to be used in research and development are capitalized until the goods or services are received.
Research and development expenses include:
• salaries, payroll taxes, employee benefits
• external research and development expenses incurred under agreements with contract research organizations (“CROs”), and consultants to conduct TuHURA’s clinical studies;
• laboratory supplies;
• costs related to manufacturing product candidates, including fees paid to third-party manufacturers and raw material suppliers;
• stock-based compensation charges for those individuals involved in research and development efforts; and
• facilities, depreciation, and other allocated expenses, which include direct and allocated expenses for rent.
Clinical trial costs are a significant component of research and development expenses and include costs associated with third-party contractors. TuHURA outsources a substantial portion of its clinical trial activities, utilizing external entities such as CROs, independent clinical investigators and other third-party service providers to assist it with the execution of its clinical trials.
TuHURA plans to substantially increase its research and development expenses for the foreseeable future as it continues the development of its product candidates and seeks to discover and develop new product candidates. Due to the inherently unpredictable nature of preclinical and clinical development, TuHURA cannot determine with certainty the timing of the initiation, duration or costs of future clinical trials and preclinical studies of product candidates. Clinical and preclinical development timelines, the probability of success and the amount of development costs can differ materially from expectations. TuHURA anticipates that it will make determinations as to which product candidates and development programs to pursue and how much funding to direct to each product candidate or program on an ongoing basis in response to the results of ongoing and future preclinical studies and clinical trials, regulatory developments and TuHURA’s ongoing assessments as to each product candidate’s commercial potential. In addition, TuHURA cannot forecast which product candidates may be subject to future collaborations, when such arrangements will be secured, if at all, and to what degree such arrangements would affect TuHURA’s development plans and capital requirements.
TuHURA’s future clinical development costs may vary significantly based on factors such as:
• per-patient trial costs;
• the number of trials required for regulatory approval;
• the number of sites included in the trials;
• the countries in which the trials are conducted;
• the length of time required to enroll eligible patients;
• the number of patients that participate in the trials;
• the number of doses that patients receive;
• the drop-out or discontinuation rates of patients;
• potential additional safety monitoring requested by regulatory agencies;
• the phase of development of the product candidate; and
• the efficacy and safety profile of the product candidate.
Acquired In-Process Research and Development (“IPR&D”)
Acquired in-process research and development expenses consist of existing research and development projects at the time of the acquisition. Projects that qualify as IPR&D assets represent those that have not yet reached technological feasibility and have no alternative future use. TuHURA acquisitions of assets have included IPR&D assets that had not yet reached technological feasibility and had no alternative future use, which resulted in a write-off of these IPR&D assets to acquired in-process research and development expenses in our consolidated statement of operations.
General and Administrative Expenses
General and administrative expenses consist primarily of salaries and employee-related costs, including stock-based compensation, for personnel in TuHURA’s executive, finance, and other administrative functions. Other significant costs include facility related costs, legal fees relating to intellectual property and corporate matters, professional fees for accounting and consulting services and insurance costs. TuHURA anticipates that its general and administrative expenses will increase in the future to support TuHURA’ continued research and development activities, and, if any product candidates receive marketing approval, commercialization activities. TuHURA also anticipates increased expenses related to audit, legal, regulatory, and tax-related services associated with maintaining compliance with exchange listing and SEC requirements, director and officer insurance premiums and investor relations costs associated with operating as a public company.
Other Income (Expense)
Other income (expense) consists of interest income on our cash and cash equivalents, interest expense on borrowings under our convertible note agreements, and non-cash changes in the fair value of our derivative liability associated with the make-whole premium on our convertible notes. Other income (expense) also included grant income from our NIH-funded research grants completed in May 2023, employee retention tax credit for companies with employees affected during the COVID-19 pandemic, and forgiveness of a paycheck protection program loan in April 2022.
Results of Operations
Comparisons for the Three Months Ended September 30, 2024, and September 30, 2023
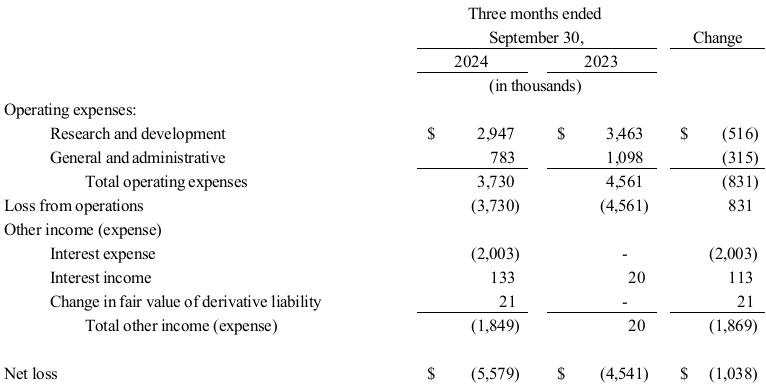
Research and Development Expenses. The following table summarizes TuHURA research and development expenses by program for the periods presented.
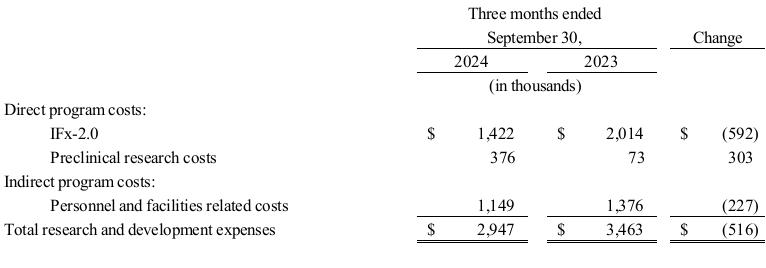
Research and development expenses were $3.0 million and $3.5 million for the three months ended September 30, 2024, and 2023, respectively. The decrease of $0.5 million related to the following.
•a decrease of approximately $0.6 million due to ongoing clinical development of IFx-2.0;
•an increase of $0.3 million due to preclinical research of IFx-3.0 and MDSCs; and
•a decrease of $0.2 million in facilities, salary and personnel related costs.
General and Administrative Expenses. General and administrative expenses were $0.8 million and $1.1 million for the three months ended September 30, 2024, and 2023, respectively. The decrease of $0.3 million was primarily due to legal fees associated with the TuHURA Biopharma Inc. asset acquisition and the terminated CohBar merger all that were incurred in the previous year.
Interest Expense. During various dates from December 2023 to September 2024, as part of the TuHURA Note Financing, TuHURA issued convertible notes totaling $31,253,000. The convertible notes included interest at 20% per annum, accretion to maturity date, and amortization of debt discount.
Interest Income. For the three months ended September 30, 2024 and 2023, interest income was earned on deposits at various banks.
Change in fair value of derivative liability. For the three months ended September 30, 2024, there was a gain of less than $0.1 million associated with the bifurcated embedded derivative liability related to the make-whole premium on the convertible notes.
Comparisons for the Nine Months Ended September 30, 2024, and September 30, 2023
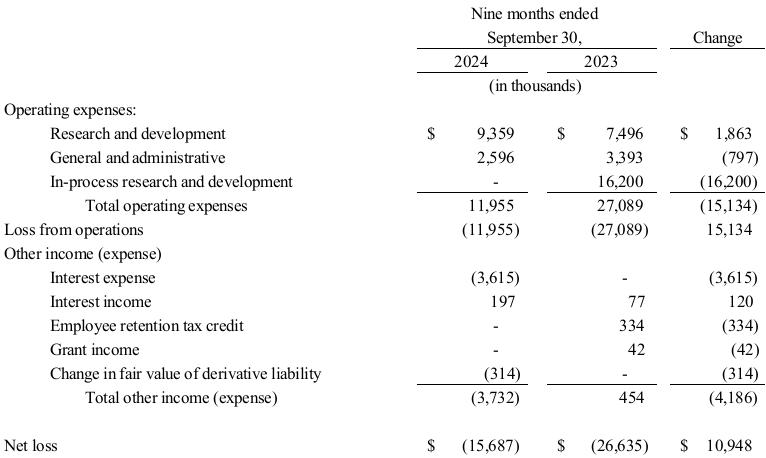
Research and Development Expenses. The following table summarizes TuHURA research and development expenses by program for the periods presented.
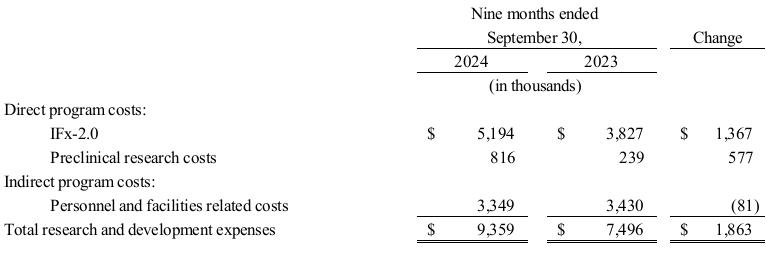
Research and development expenses were $9.4 million and $7.5 million for the nine months ended September 30, 2024, and 2023, respectively. The increase of $1.9 million related to the following.
•an increase of approximately $1.4 million due to ongoing clinical development of IFx-2.0;
•an increase of $0.6 million due to preclinical research of IFx-3.0 and MDSCs; and
•a decrease of $0.1 million in facilities, salary and personnel related costs.
Acquired in process research and development (“IPR&D”). On January 26, 2023, TuHURA acquired certain assets of TuHURA Biopharma, Inc., for $1.2 million in cash and 22.7 million common shares. The common shares issued to TuHURA have an estimated fair market value of $15.0 million. TuHURA performed the “screen test” and determined that substantially all of the fair value of the gross assets acquired in the TuHURA Biopharma acquisition is concentrated in a single identifiable asset or group of similar identifiable assets. As such, the TuHURA Biopharma acquisition has been accounted for as an asset acquisition. As the underlying asset is in-process research and development, TuHURA immediately expensed the entire $16.2 million purchase price for the nine months ended September 30, 2023, in accordance with FASB ASC Topic 730.
General and Administrative Expenses. General and administrative expenses were $2.6 million and $3.4 million for the nine months ended September 30, 2024, and 2023, respectively. The decrease of $0.8 million was primarily due to legal fees associated with the TuHURA Biopharma Inc. asset acquisition and proposed merger with CohBar, Inc. which was terminated in accordance with its terms in November 2023.
Employee Retention Tax Credit. The IRS provides a refundable tax credit for businesses that had employees and were affected during the COVID-19 pandemic. In October 2022, TuHURA applied for a credit under this program through ADP Totalsource, which manages the TuHURA payroll and benefits. In May 2023, TuHURA received a letter from ADP Totalsource that the credit will be $0.3 million.
Grant Income. Grant income was $0.0 million and less than $0.1 million for the nine months ended September 30, 2024 and 2023, respectively. In April 2021, TuHURA received approval from the Department of Health and Human Services for a $0.4 million grant to study cervical cancer and received reimbursements for related expenses associated with the grant in these years. TuHURA received the final payment under this grant in May 2023.
Interest Expense. In December 2023 to September 2024, as part of the TuHURA Note Financing, TuHURA issued convertible notes totaling $31,253,000. The convertible notes included interest at 20% per annum, accretion to maturity date, and amortization of debt discount.
Interest Income. For the nine months ended September 30, 2024 and 2023, respectively, interest income was earned on deposits at various banks.
Change in fair value of derivative liability. For the nine months ended September 30, 2024, there was a loss of $0.3 million associated with the bifurcated embedded derivative liability related to the make-whole premium on the convertible notes.
Liquidity and Capital Resources
TuHURA has incurred net losses and negative cash flows from operations since TuHURA’s inception and anticipates it will continue to incur net losses for the foreseeable future. TuHURA incurred net losses of $15.7 million and $26.6 million for the nine months ended September 30, 2024, and 2023, respectively, and used $17.3 million and $8.9 million of cash from TuHURA’s operating activities for the nine months ended September 30, 2024, and 2023, respectively. As of September 30, 2024, TuHURA had an accumulated deficit of $105.1 million. The $26.6 million loss for the nine months ended September 30, 2023, included the expensing of the entire $16.2 million purchase price for the assets of TuHURA Biopharma, Inc., of which $15.0 million was paid in the form of TuHURA Common Stock.
As of September 30, 2024, TuHURA had cash and cash equivalents of $19.6 million.
Sources of Liquidity
To date, TuHURA has financed its operations principally through private placements of TuHURA’s common and preferred stock and issuance of convertible notes.
Series A Preferred Stock Financing
In August 2017 through April 2018, TuHURA issued an aggregate of 33,186,952 shares of its Series A Preferred Stock at a purchase price of $0.52 per share for aggregate net proceeds of $15.6 million. There were 15,976,413 common stock warrants associated with these preferred shares.
Series A-1 Preferred Stock Financing
From October 2020 to October 2021, TuHURA issued an aggregate of 14,288,076 shares of its Series A-1 Preferred Stock at a purchase price of $0.66 per share for aggregate consideration of $9,430,000. There were 6,468,026 common stock warrants associated with these preferred shares.
Series B Preferred Stock Financing
From June through August 2022, TuHURA issued Series B preferred shares and received $16.6 million for 25,153,030 Series B shares at a purchase price of $0.66 along with 18,864,773 warrants that are exercisable at a fixed price of $0.66.
Prior Convertible Note Financing
From May 2019 through December 2020, TuHURA issued $4,995,000 aggregate principal amount of convertible notes, which bear interest at the rate of 10% per annum.
On February 24, 2021, a majority of note holders elected to voluntarily convert their notes under the terms of a non-qualified financing in the Note. This forced a conversion of all Notes into preferred shares. The conversion price was set by the same terms offered in the non-qualified financing. As a result, the $4,995,000 Note principal plus $277,000 accrued interest was converted into 7,988,169 Series A-1 preferred shares at $0.66 a share. There were 3,765,851 common stock warrants associated with this conversion.
TuHURA Note Financing
On April 2, 2024, TuHURA completed a private placement under which it offered and sold convertible promissory notes (the “TuHURA Notes”) to approximately 40 accredited investors during the period from December 2023 through April 2, 2024 (the “TuHURA Note Financing”). In the transaction, TuHURA received subscriptions for an aggregate principal amount of $31,253,000 of TuHURA Notes, of which the entire amount was funded as of September 30, 2024.
The TuHURA Notes are general unsecured obligations of TuHURA that have various maturity dates through 2026, and that bear interest at a rate of 20% per annum, simple interest. The TuHURA Notes contain a make-whole provision under which, upon payment or conversion of the TuHURA Notes, the holders of the notes will receive additional interest equal to the amount of interest that would have accrued through the first anniversary of the initial closing of the TuHURA Note Financing (if the notes are paid or converted prior to such first anniversary), through the 18-month anniversary of the initial closing (if the notes are paid or converted on or after the first anniversary and before the 18-month anniversary), or though the maturity date (if the notes are paid or converted after the 18-month anniversary of the initial closing).
As provided in the TuHURA Notes, upon the completion of the Merger, all principal and accrued and unpaid interest and make-whole amounts under the TuHURA Notes automatically converted into shares of TuHURA Common Stock at a conversion price $0.68 per share of TuHURA Common Stock.
In the TuHURA Note Financing, the investors that purchased at least $4.0 million in principal amount of TuHURA Notes, together with their affiliates, were issued warrants to purchase an aggregate of 18,797,794 additional shares of TuHURA Common Stock (the “TuHURA Common Warrants”). The TuHURA Common Warrants have an
exercise price of $1.02 per share of TuHURA Common Stock and have an expiration date of 3 years following the respective issue dates of the warrants. The TuHURA Common Warrants are exercisable at any time prior to the expiration date of the warrants, and the warrants are exercisable for cash and, at such time as there is no registration statement covering the resale of the shares issuable upon the exercise of the warrants, on a cashless basis. The TuHURA Common Warrants contain customary adjustments to the exercise price and number of underlying warrant shares by reason of stock splits, stock dividends, reverse stock split, and the like.
In connection with the TuHURA Note Financing, TuHURA issued an aggregate of 343,560 shares of TuHURA Common Stock to a placement agent for the private placement of the TuHURA note financing.
Private Placement of Common Stock
In July 2024, TuHURA sold 4,009,623 shares of its common stock in a private offering with a purchase price of $5,000,000 to an existing TuHURA shareholder.
Cash Flows
The following table sets forth a summary of the net cash flow activity for the nine months ended September 30, 2024 and 2023, respectively:
|
|
|
|
|
|
|
|
|
|
|
Nine Months Ended
September 30, |
|
|
2024 |
|
2023 |
|
|
(in thousands) |
Net cash provided by (used in): |
|
|
|
|
|
|
|
|
Operating activities |
|
$ |
(12,122 |
) |
|
$ |
(8,920 |
) |
Investing activities |
|
|
(5,229) |
|
|
|
(1,257 |
) |
Financing activities |
|
|
33,288 |
|
|
|
(25) |
|
Net increase (decrease) in cash |
|
$ |
15,931 |
|
|
$ |
(10,202 |
) |
Operating Activities
For the nine months ended September 30, 2024, net cash used in operating activities was $12.1 million, which primarily consisted of a net loss of $15.7 million, a change in net operating assets and liabilities of $1.2 million, partially offset by non-cash charges of $2.4 million. The net non-cash charges were primarily related to a $0.3 change in fair value of derivative liability, amortization of debt discount of $1.1 million, and stock-based compensation of $0.9 million. The change in net operating assets and liabilities is due to a decrease in accounts payable and accrued expenses of $1.2 million due to timing of invoices and vendor payments offset by increases in other current and non-current assets of $0.1 million.
For the nine months ended September 30, 2023, net cash used in operating activities was $8.9 million, which primarily consisted of a net loss of $26.6 million and a change in net operating assets and liabilities of $1.0 million, partially offset by non-cash charges of $16.7 million. The net non-cash charges were primarily related to a $16.2 write-off of in-process research and development expense on the asset acquisition of TuHURA Biopharma, Inc., depreciation and amortization expense of $0.1 million, and stock-based compensation of $0.3 million. The change in net operating assets and liabilities is due to a decrease in accounts payable and accrued expenses of $0.8 million due to timing of invoices and vendor payments.
Investing Activities
For the nine months ended September 30, 2024, net cash used in investing activities was $5.2 million, which consisted of property and equipment purchases and an exclusivity deposit payment to Kineta.
For the nine months ended September 30, 2023, net cash used in investing activities was $1.3 million. On January 26, 2023, TuHURA acquired certain assets of TuHURA Biopharma, Inc. for $1.2 million in cash and 22.7 million common shares. The cash component of the transaction is considered an investing activity. The entire transaction was valued at $16.2 million.
Financing Activities
For the nine months ended September 30, 2024, net cash provided by financing activities was $33.3 million, which consisted of $27.5 net proceeds from convertible notes issued as part of the TuHURA Note Financing, $4.7 million net proceeds from the common stock private offering, $2.0 million proceeds from stock options and warrants exercises, and $1.1 million in deferred offering costs paid in connection with the proposed merger with Kintara.
For the nine months ended September 30, 2023, net cash used in used in financing activities was less than $0.1 million, which consisted of repurchased shares from an investor.
Funding Requirements
TuHURA expects to incur additional costs associated with operating as a public company. In addition, TuHURA anticipates that it will need substantial additional funding in connection with its continuing operations. TuHURA believes that its existing cash and cash equivalents, together with the estimated net proceeds from the TuHURA Note Financing, will be sufficient to meet its anticipated cash requirements through the end of 2025.
However, TuHURA’s forecast of the period through which its financial resources will be adequate to support its operations is a forward-looking statement that involves risks and uncertainties, and actual results could vary materially. Management based projections of operating capital requirements on TuHURA’s current operating plan, which includes several assumptions that may prove to be incorrect, and TuHURA may deplete its available capital resources sooner than management expects. TuHURA’s future capital requirements will depend on many factors, including:
• the initiation, progress, timing, costs and results of drug discovery, preclinical studies and clinical trials of IFx-Hu2.0, IFx-Hu3.0 and any other future product candidates;
• the costs associated with hiring additional personnel and consultants as TuHURA’s preclinical and clinical activities increase;
• the outcome, timing and costs of seeking regulatory approvals;
• the cost of manufacturing IFx-Hu2.0 and IFx-Hu3.0 and future product candidates for clinical trials in preparation for marketing approval and in preparation for commercialization;
• the emergence of competing therapies and other adverse market developments;
• the ability to establish and maintain strategic licensing or other arrangements and the financial terms of such agreements; and
• the costs of operating as a public company.
Until such time, if ever, as TuHURA can generate substantial product revenues to support its capital requirements, TuHURA expects to finance its cash needs through a combination of public or private equity offerings, debt financings, collaborations and licensing arrangements or other capital sources. To the extent that TuHURA raises additional capital through the sale of equity or convertible debt securities, the ownership interest of TuHURA’s stockholders will be or could be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect the rights of holders of TuHURA’s Common Stock. Debt financing and equity financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures, or declaring dividends. If TuHURA raises funds through collaborations, or other similar arrangements with third parties, TuHURA may need to relinquish valuable rights to its product candidates, future revenue streams or research programs or may have to grant licenses on terms that may not be favorable to it and/or may reduce the value of TuHURA’s Common Stock. If TuHURA is unable to raise additional funds through equity or debt financings as and when needed, TuHURA may be required to delay, limit, reduce or terminate its product development or future commercialization efforts or grant
rights to develop and market its product candidates even if TuHURA would otherwise prefer to develop and market such product candidates themselves.
Critical Accounting Policies and Significant Judgments and Estimates
TuHURA’s management’s discussion and analysis of its financial condition and results of operations is based on its financial statements, which have been prepared in accordance with U.S. generally accepted accounting principles. The preparation of these financial statements requires TuHURA to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses and the disclosure of contingent assets and liabilities in TuHURA’s financial statements. On an ongoing basis, TuHURA evaluates its estimates and judgments, including those related to accrued expenses and stock-based compensation. TuHURA bases its estimates on historical experience, known trends and events, and various other factors that TuHURA believes to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. TuHURA’s actual results may differ from these estimates under different assumptions or conditions. While TuHURA’s significant accounting policies are described in more detail in Note 2 of its financial statements appearing elsewhere in this Current Report on Form 8-K, TuHURA believes the following accounting policies and estimates to be most critical to the preparation of its financial statements.
Accrued Research and Development Expenses
As part of the process of preparing TuHURA’s financial statements, TuHURA is required to estimate its accrued expenses as of each balance sheet date. This process involves reviewing open contracts and purchase orders, communicating with TuHURA’s personnel to identify services that have been performed on TuHURA’s behalf and estimating the level of service performed and the associated cost incurred for the service when TuHURA has not yet been invoiced or otherwise notified of the actual cost. TuHURA makes estimates of its accrued expenses as of each balance sheet date based on facts and circumstances known to it at that time. TuHURA periodically confirms the accuracy of its estimates with the service providers and adjusts, if necessary. The significant estimates in TuHURA’s accrued research and development expenses include the costs incurred for services performed by its vendors in connection with research and development activities for which TuHURA has not yet been invoiced.
TuHURA bases its expenses related to research and development activities on its estimates of the services received and efforts expended pursuant to quotes and contracts with vendors that conduct research and development on TuHURA’s behalf. The financial terms of these agreements are subject to negotiation, vary from contract-to-contract and may result in uneven payment flows. There may be instances in which payments made to TuHURA’s vendors will exceed the level of services provided and result in a prepayment of the research and development expense. In accruing service fees, TuHURA estimates the time period over which services will be performed and the level of effort to be expended in each period. If the actual timing of the performance of services or the level of effort varies from TuHURA’s estimate, TuHURA adjusts the accrual or prepaid expense accordingly. Advance payments for goods and services that will be used in future research and development activities are expensed when the activity has been performed or when the goods have been received rather than when the payment is made.
Although TuHURA does not expect its estimates to be materially different from amounts actually incurred, if TuHURA’s estimates of the status and timing of services performed differ from the actual status and timing of services performed, it could result in TuHURA reporting amounts that are too high or too low in any particular period. To date, there have been no material differences between TuHURA’s estimates of such expenses and the amounts actually incurred.
Stock-Based Compensation Expense
Stock-based compensation expense represents the cost of the grant date fair value of equity awards recognized over the requisite service period of the awards (usually the vesting period) on a straight-line basis. TuHURA estimates the fair value of equity awards using the Black-Scholes option pricing model and recognizes forfeitures as they occur. Estimating the fair value of equity awards as of the grant date using valuation models, such as the Black-Scholes option pricing model, is affected by assumptions regarding a number of variables, including the risk-free interest rate, the expected stock price volatility, the expected term of stock options, the expected dividend yield and the fair value of the underlying common stock on the date of grant. Changes in the assumptions can materially affect
the fair value and ultimately how much stock-based compensation expense is recognized. These inputs are subjective and generally require significant analysis and judgment to develop. See Note 2 of TuHURA’s financial statements for information concerning certain of the specific assumptions TuHURA used in applying the Black-Scholes option pricing model to determine the estimated fair value of TuHURA’s stock options granted.
Common stock valuations
TuHURA is required to estimate the fair value of the common stock underlying its equity awards when performing fair value calculations. The fair value of the common stock underlying its equity awards was determined on each grant taking into account input from management and taking into account the pricing offered in TuHURA’s equity raises. All options to purchase shares of TuHURA’s Common Stock are intended to be granted with an exercise price per share no less than the fair value per share of TuHURA’s Common Stock underlying those options on the date of grant, based on the information known to TuHURA on the date of grant. In the absence of a public trading market for TuHURA’s Common Stock, on each grant date TuHURA develops an estimate of the fair value of its common stock in order to determine an exercise price for the option grants. TuHURA’s determinations of the fair value of its common stock were made by considering the prices of preferred stock sold to investors in arm’s length transactions and the rights, preferences and privileges of TuHURA’s preferred stock relative to those of its common stock.
In determining the fair value of TuHURA’s Common Stock underlying stock option grants for the nine months ended September 30, 2024 and 2023, TuHURA used the market approach by reference to the closest round of equity financing, preceding the date of valuation and analysis of the trading values of publicly traded companies deemed comparable to TuHURA.
Recently Issued and Adopted Accounting Pronouncements
A description of recently issued and adopted accounting pronouncements that may potentially impact TuHURA’s financial position and results of operations is disclosed in Note 2 to TuHURA’s financial statements.
Off-Balance Sheet Arrangements
During the periods presented, TuHURA did not have, nor does it currently have, any off-balance sheet arrangements as defined under SEC rules.
Quantitative and Qualitative Disclosures about Market Risk
TuHURA is exposed to market risks in the ordinary course of its business. These risks primarily include interest rate risks and inflation risks. Periodically, TuHURA maintains deposits in accredited financial institutions in excess of federally insured limits. TuHURA deposits its cash in financial institutions that it believes has high credit quality and have not experienced any losses on such accounts and does not believe it is exposed to any unusual credit risk beyond the normal credit risk associated with commercial banking relationships.
Interest Rate Risk
TuHURA’s cash consists of cash in readily-available checking accounts. TuHURA may also invest in short-term money market fund investments. Such interest-earning instruments carry a degree of interest rate risk; however, historical fluctuations in interest income have not been significant.
Inflation Risk
Inflation generally affects TuHURA by increasing its cost of labor and research and development contract costs. TuHURA does not believe inflation has had a material effect on its results of operations during the periods presented.
Exhibit 99.3

TUHURA BIOSCIENCES, INC. AND SUBSIDIARY
CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
As of September 30, 2024 and December 31, 2023 and for the three and nine months ended September 30 2024 and 2023.
TUHURA BIOSCIENCES, INC AND SUBSIDIARY
Condensed consolidated balance sheets
As of September 30, 2024 (Unaudited), and December 31, 2023
|
|
|
|
Unaudited |
|
|
September 30, |
December 31, |
|
2024 |
2023 |
Assets |
|
|
Current Assets: |
|
|
Cash and cash equivalents |
$ 19,596,022 |
$ 3,665,032 |
Exclusivity rights deposit |
5,192,371 |
- |
Deferred offering costs |
1,387,685 |
- |
Other current assets |
547,450 |
493,769 |
Total Current Assets |
26,723,528 |
4,158,801 |
|
|
|
Property and equipment, net |
119,593 |
182,170 |
Operating right-of-use assets |
236,069 |
20,820 |
Other noncurrent assets |
33,769 |
- |
Total Assets |
$ 27,112,959 |
$ 4,361,791 |
|
|
|
Liabilities and Stockholders' Deficit |
|
|
Current Liabilities: |
|
|
Accounts payable and accrued expenses |
$ 2,420,833 |
$ 3,438,559 |
Derivative Liability |
2,853,000 |
137,000 |
Lease liabilities, current |
155,211 |
20,820 |
Total Current Liabilities |
5,429,044 |
3,596,379 |
|
|
|
Long-term Liabilities: |
|
|
Convertible notes payable, net |
24,366,814 |
2,324,158 |
Lease liability, long term |
84,346 |
- |
Total Liabilities |
29,880,204 |
5,920,537 |
|
|
|
Stockholders' Deficit: |
|
|
Preferred stock |
8,056 |
8,056 |
Common stock |
7,637 |
6,801 |
Additional paid in capital |
102,343,730 |
86,901,394 |
Accumulated deficit |
(105,126,668) |
(88,474,997) |
Total Stockholders' Deficit |
(2,767,245) |
(1,558,746) |
Total Liabilities and Stockholders' Deficit |
$ 27,112,959 |
$ 4,361,791 |
The accompanying notes to the condensed consolidated financial statements are an integral part of this statement. 2
TUHURA BIOSCIENCES, INC AND SUBSIDIARY
Condensed consolidated statements of operations
For the three and nine months ended September 30, 2024, and 2023
(Unaudited)
|
|
|
|
|
|
|
|
|
Three Months Ended September 30, |
|
Nine Months Ended September 30, |
|
2024 |
|
2023 |
|
2024 |
|
2023 |
|
|
|
|
|
|
|
|
Research and development expenses |
$ 2,946,769 |
|
$ 3,463,198 |
|
$ 9,358,846 |
|
$ 7,496,153 |
Acquired in-process research and development ("IPR&D") |
- |
|
- |
|
- |
|
16,200,000 |
General and administrative expenses |
783,459 |
|
1,098,079 |
|
2,595,860 |
|
3,392,569 |
Operating Loss |
(3,730,228) |
|
(4,561,277) |
|
(11,954,706) |
|
(27,088,722) |
|
|
|
|
|
|
|
|
Other (Expense) Income: |
|
|
|
|
|
|
|
Employee Retention Tax Credit |
- |
|
- |
|
- |
|
334,443 |
Interest expense |
(2,002,886) |
|
- |
|
(3,615,466) |
|
- |
Interest income |
132,767 |
|
20,294 |
|
197,449 |
|
77,097 |
Grant income |
- |
|
- |
|
- |
|
42,466 |
Change in fair value of derivative liability |
(21,229) |
|
- |
|
(313,772) |
|
- |
Total Other (Expense) Income |
(1,848,890) |
|
20,294 |
|
(3,731,789) |
|
454,006 |
Net Loss |
$ (5,579,118) |
|
$ (4,540,983) |
|
$ (15,686,495) |
|
$ (26,634,716) |
Deemed dividend on warrant modifications |
(965,177) |
|
- |
|
(965,177) |
|
- |
Net Loss attributable to common stockholders |
$ (6,544,295) |
|
$ (4,540,983) |
|
$ (16,651,672) |
|
$ (26,634,716) |
The accompanying notes to the condensed consolidated financial statements are an integral part of this statement. 3
TUHURA BIOSCIENCES, INC AND SUBSIDIARY
Condensed consolidated statements of stockholders’ equity (deficit)
For the three and nine months ended September 30, 2024, and 2023
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total |
|
|
Preferred Stock |
|
Common Stock |
|
Additional |
|
Accumulated |
|
Stockholders' |
|
|
Shares |
|
Dollars |
|
Shares |
|
Dollars |
|
Paid in Capital |
|
Deficit |
|
(Deficit) Equity |
Balances at July 1, 2024 |
|
80,561,229 |
|
$ 8,056 |
|
68,074,466 |
|
$ 6,807 |
|
$ 91,608,677 |
|
$ (98,582,374) |
|
$ (6,958,834) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of common shares, net of offering costs |
|
- |
|
- |
|
4,009,623 |
|
401 |
|
4,599,599 |
|
- |
|
4,600,000 |
Issuance of common shares for equity issuance placement agent fees |
|
- |
|
- |
|
98,040 |
|
10 |
|
99,990 |
|
- |
|
100,000 |
Issuance of common shares for convertible note placement agent fees |
|
- |
|
- |
|
336,824 |
|
34 |
|
343,526 |
|
- |
|
343,560 |
Issuance of common shares for warrants exercised |
|
- |
|
- |
|
3,587,760 |
|
359 |
|
1,930,645 |
|
- |
|
1,931,004 |
Stock options exercised, cash and cashless |
|
- |
|
- |
|
260,000 |
|
26 |
|
103,974 |
|
- |
|
104,000 |
Stock compensation expense |
|
- |
|
- |
|
- |
|
- |
|
269,277 |
|
- |
|
269,277 |
Fair value of warrants associated with convertible notes payable |
|
- |
|
- |
|
- |
|
- |
|
2,422,865 |
|
- |
|
2,422,865 |
Deemed dividend on warrant modifications |
|
- |
|
- |
|
- |
|
- |
|
965,177 |
|
(965,177) |
|
- |
Net loss |
|
- |
|
- |
|
- |
|
- |
|
- |
|
(5,579,117) |
|
(5,579,117) |
Balances at September 30, 2024 |
|
80,561,229 |
|
$ 8,056 |
|
76,366,713 |
|
$ 7,637 |
|
$ 102,343,730 |
|
$ (105,126,668) |
|
$ (2,767,245) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balances at July 1, 2023 |
|
80,561,229 |
|
$ 8,062 |
|
68,013,861 |
|
$ 6,801 |
|
$ 86,692,340 |
|
$ (81,251,904) |
|
$ 5,455,293 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock compensation expense |
|
- |
|
- |
|
- |
|
- |
|
118,153 |
|
- |
|
118,153 |
Net loss |
|
- |
|
- |
|
- |
|
- |
|
- |
|
(4,540,983) |
|
(4,540,983) |
Balances at September 30, 2023 |
|
80,561,229 |
|
$ 8,056 |
|
68,013,861 |
|
$ 6,801 |
|
$ 86,692,340 |
|
$ (85,792,887) |
|
$ 1,032,463 |
The accompanying notes to the condensed consolidated financial statements are an integral part of this statement. 4
TUHURA BIOSCIENCES, INC AND SUBSIDIARY
Condensed consolidated statements of stockholders’ equity (deficit)
For the three and nine months ended September 30, 2024, and 2023
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total |
|
|
Preferred Stock |
|
Common Stock |
|
Additional |
|
Accumulated |
|
Stockholders' |
|
|
Shares |
|
Dollars |
|
Shares |
|
Dollars |
|
Paid in Capital |
|
Deficit |
|
(Deficit) Equity |
Balances at January 1, 2024 |
|
80,561,229 |
|
$ 8,056 |
|
68,013,861 |
|
$ 6,801 |
|
$ 86,901,394 |
|
$ (88,474,997) |
|
$ (1,558,746) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of common shares, net of offering costs |
|
- |
|
- |
|
4,009,623 |
|
401 |
|
4,599,599 |
|
- |
|
4,600,000 |
Issuance of common shares for equity issuance placement agent fees |
|
- |
|
- |
|
98,040 |
|
10 |
|
99,990 |
|
- |
|
100,000 |
Issuance of common shares for convertible note placement agent fees |
|
- |
|
- |
|
336,824 |
|
34 |
|
343,526 |
|
- |
|
343,560 |
Issuance of common shares for warrants exercised |
|
- |
|
- |
|
3,587,760 |
|
359 |
|
1,930,645 |
|
- |
|
1,931,004 |
Stock options exercised, cash and cashless |
|
- |
|
- |
|
260,000 |
|
26 |
|
103,974 |
|
- |
|
104,000 |
Stock compensation expense |
|
- |
|
- |
|
- |
|
- |
|
879,375 |
|
- |
|
879,375 |
Fair value of warrants associated with convertible notes payable |
|
- |
|
- |
|
- |
|
- |
|
6,520,056 |
|
- |
|
6,520,056 |
Deemed dividend on warrant modifications |
|
- |
|
- |
|
- |
|
- |
|
965,177 |
|
(965,177) |
|
- |
Net loss |
|
- |
|
- |
|
- |
|
- |
|
- |
|
(15,686,494) |
|
(15,686,494) |
Balances at September 30, 2024 |
|
80,561,229 |
|
$ 8,056 |
|
76,366,713 |
|
$ 7,637 |
|
$ 102,343,730 |
|
$ (105,126,668) |
|
$ (2,767,245) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balances at January 1, 2023 |
|
80,616,229 |
|
$ 8,062 |
|
45,286,589 |
|
$ 4,529 |
|
$ 71,449,521 |
|
$ (59,158,171) |
|
$12,303,941 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of common shares for asset acquisition |
|
- |
|
- |
|
22,727,272 |
|
2,272 |
|
14,997,728 |
|
- |
|
15,000,000 |
Shares repurchased |
|
(55,000) |
|
(6) |
|
- |
|
- |
|
(24,745) |
|
- |
|
(24,751) |
Stock compensation expense |
|
- |
|
- |
|
- |
|
- |
|
387,989 |
|
- |
|
387,989 |
Net loss |
|
- |
|
- |
|
- |
|
- |
|
- |
|
(26,634,716) |
|
(26,634,716) |
|
|
80,561,229 |
|
$ 8,056 |
|
68,013,861 |
|
$ 6,801 |
|
$ 86,810,493 |
|
$ (85,792,887) |
|
$ 1,032,463 |
The accompanying notes to the condensed consolidated financial statements are an integral part of this statement. 5
TUHURA BIOSCIENCES, INC AND SUBSIDIARY
Condensed consolidated statements of cash flows
For the nine months ended September 30, 2024, and 2023
(Unaudited)
|
|
|
|
Nine months ended |
|
September 30, |
September 30, |
|
2024 |
2023 |
Cash flows from Operating activities: |
|
|
Net loss |
$ (15,686,494) |
$ (26,634,716) |
Adjustments to reconcile net loss to cash used in operating activities: |
|
|
Stock compensation expense |
879,375 |
387,989 |
Depreciation and amortization |
99,075 |
141,333 |
Change in fair value of derivative liability |
313,772 |
- |
Amortization of debt discount |
1,107,009 |
- |
Changes in operating assets and liabilities: |
|
|
Other current assets |
69,704 |
(17,816) |
Other noncurrent assets |
(153,283) |
120,697 |
Accounts payable and accrued expenses |
1,242,321 |
882,135 |
Write-off of in-process R&D |
- |
16,200,000 |
Net cash flows from operating activities |
(12,128,521) |
(8,920,378) |
|
|
|
Cash flows from investing activities: |
|
|
Cash paid for asset acquisition |
- |
(1,200,000) |
Exclusivity rights deposit |
(5,192,371) |
- |
Purchase of property and equipment |
(36,498) |
(57,224) |
Net cash flows from investing activities |
(5,228,869) |
(1,257,224) |
|
|
|
Cash flows from financing activities: |
|
|
Shares repurchased |
- |
(24,751) |
Proceeds from convertible notes payable |
28,568,000 |
- |
Proceeds from issuance of common stock |
5,000,000 |
- |
Proceeds from stock options exercised |
104,000 |
- |
Proceeds from warrants exercised |
1,931,004 |
- |
Payment of offering costs associated with issuance of common stock |
(300,000) |
- |
Payment of deferred offering costs |
(902,262) |
- |
Payment of debt issuance costs |
(1,112,362) |
- |
Net cash flows from financing activities |
33,288,380 |
(24,751) |
|
|
|
Net change in cash and cash equivalents |
15,930,990 |
(10,202,353) |
Cash and cash equivalents at the beginning of the period |
3,665,032 |
14,252,518 |
Cash and cash equivalents at the end of the period |
$ 19,596,022 |
$ 4,050,165 |
|
|
|
Supplemental non-cash activity |
|
|
Shares issued and reserved for asset acquisition |
$ - |
$ 15,000,000 |
Right-of-use asset recognized in exchange for operating lease obligations |
318,722 |
- |
Debt issuance costs not yet paid |
5,135 |
- |
Deferred offering costs not yet paid |
385,820 |
- |
Derivative liability associated with make-whole premium |
2,402,228 |
- |
Fair value of warrants associated with convertible notes payable |
6,520,056 |
- |
Deemed dividend on warrant modifications |
965,177 |
- |
Issuance of common stock for placement agent fees |
443,560 |
- |
The accompanying notes to the condensed consolidated financial statements are an integral part of this statement. 6
TUHURA BIOSCIENSES, INC AND SUBSIDIARY
Notes to the condensed consolidated financial statements
For the nine months ended September 30, 2024, and 2023
(Unaudited)
Note 1—Description of business
TuHURA Biosciences, Inc., a Delaware corporation (the “Company”), is a clinical stage immuno-oncology company developing novel personalized cancer vaccine product candidates designed to overcome primary resistance to immunotherapies like checkpoint inhibitors. The Company has entered into a Special Protocol Assessment agreement with the FDA for a single Phase 3 randomized placebo and injection-controlled trial for IFx-2.0, the Company’s lead personalized cancer vaccine product candidate, as adjunctive therapy to pembrolizumab (Keytruda®) in the first line treatment of patients with advanced or metastatic Merkel cell carcinoma who are checkpoint inhibitor naïve utilizing the FDA’s accelerated approval pathway. The Company is also developing novel bi-functional antibody drug conjugates, or ADCs, targeting myeloid derived suppressor cells, or MDSCs, to modulate their immunosuppressive effects on the tumor microenvironment to overcome acquired resistance to immunotherapies.
Merger with Kintara – On April 2, 2024, the Company entered into a definitive Agreement and Plan of Merger with Kintara Therapeutics, Inc., a publicly traded Nevada corporation listed on the Nasdaq Capital Market (“Kintara”), and Kayak Mergeco, Inc., a Delaware corporation wholly owned subsidiary Kintara (“Merger Sub”), for an all-stock merger transaction (the “Merger”) forming a company with expertise and resources to advance a risk diversified late-stage oncology pipeline (the “Merger Agreement”). The Merger Agreement provided that, upon completion of the Merger, the former Company shareholders would own the majority of the shares of the public company. The Merger and other transactions contemplated by the Merger Agreement closed on October 18, 2024 (see note 11).
Exclusivity and Right of First Offer Agreement with Kineta – On July 3, 2024, the Company entered into an Exclusivity and Right of First Offer Agreement (the “Exclusivity Agreement”) with Kineta, Inc., a publicly traded Delaware corporation (“Kineta”). Under this agreement, Kineta granted to the Company an exclusive right to acquire Kineta’s worldwide patent rights, other intellectual property rights, and other rights and assets related to KVA12123, which is Kineta’s VISTA blocking immunotherapy. Such exclusive right commenced as of July 3, 2024 and generally continued through October 1, 2024, subject to extension at the option of the Company for up to 20 days. Under the terms of the Exclusivity Agreement, the Company paid Kineta a $5.0 million payment, and additional payments of up to $0.3 million in the aggregate will become due if the Company exercises its extension rights (collectively, the “Exclusivity Payment”). The Exclusivity Payment will be credited against the initial cash consideration that may be payable to Kineta pursuant to any definitive agreement, if any, that the Company and Kineta enter into relating to the KVA12123 assets. In August 2024, Kineta, in collaboration with the Company, announced that it reopened enrollment in the VISTA-101 clinical trial, in which Kineta and the Company continue to collaborate on the ongoing Phase 1 clinical trial program in patients with advanced solid tumor cancer. Payments made to Kineta toward the VISTA-101 clinical trial program will be credited one-half towards any upfront cash consideration and one half towards non-cash consideration.
July 2024 Private Placement – In connection with the Company’s entrance into the Exclusivity Agreement, on July 3, 2024, the Company completed a private placement of its common stock to an existing investor, under which the investor paid $5.0 million in exchange for 4,009,623 shares of the Company’s common stock and a 1.5% royalty right on certain future sales by the Company of products based on KVA12123. The proceeds received from the Company’s July 2024 private placement were used to fund the Exclusivity Payment due to Kineta pursuant to the Exclusivity Agreement.
TUHURA BIOSCIENSES, INC AND SUBSIDIARY
Notes to the condensed consolidated financial statements
For the nine months ended September 30, 2024, and 2023
(Unaudited)
Note 2—Summary of significant accounting policies
Basis for Consolidation – The consolidated financial statements are comprised of all of the accounts of TuHURA Biosciences, Inc., a Delaware corporation, and Veterinary Oncology Services, a wholly owned subsidiary (collectively the “Company”). All intercompany accounts and transactions have been eliminated in consolidation.
Accounting Estimates – The preparation of consolidated financial statements in conformity with generally accepted accounting principles in the United States of America (“U.S. GAAP”) requires management to make estimates and assumptions that affect various amounts reported in consolidated financial statements and accompanying notes. Actual results could differ from those estimates.
Deferred Offering Costs – Deferred offering costs consist of direct legal, accounting, and other fees and costs directly related to the Merger with Kintara (See note 1 and note 11). The Company capitalized deferred offering costs prior to the close of the Merger.
Property and Equipment – Property and equipment are carried at cost, less accumulated depreciation and amortization. Depreciation is computed using the straight-line method over the estimated useful lives of the assets (generally five to seven years). Leasehold improvements are amortized straight-line over the shorter of the lease term or the estimated useful life of the asset. Property and equipment are reviewed for impairment when events or changes in circumstances indicate that the carrying amount may not be recoverable. No impairment was recorded for the period ended September 30, 2024, nor the year ended December 31, 2023.
Lease Accounting – The Company recognizes right-of-use lease assets and corresponding liabilities arising from leasing activities over the requisite lease period.
Income Taxes – Income taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are recognized for future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carryforwards. In December 2023, the FASB issued ASU No. 2023-09, Income Taxes (Topic 740), which enhances the income tax disclosure requirements for public entities on an annual basis. Under ASU 2023-09, public entities will be required to disclose in their rate reconciliation, on an annual basis, both percentages and amounts in their reporting currency for certain categories in a tabular format, with accompanying qualitative disclosures. The amendments in ASU 2023-09 are effective fiscal years beginning after December 31, 2024, and early adoption is permitted. The Company does not believe that the adoption of ASU 2023-09 will have a material impact on its condensed consolidated financial statements.
Research and Development Expenses – Research and development consists of expenses incurred in connection with the discovery and development of product candidates. The Company expenses research and development costs as incurred.
Acquired In-Process Research and Development - Acquired in-process research and development expenses consist of existing research and development projects at the time of the acquisition. Projects that qualify as IPR&D assets represent those that have not yet reached technological feasibility and had no alternative future use, which resulted in a write-off of these IPR&D assets to acquired in-process research and development expenses in our consolidated statements of operations.
Concentration of Credit Risk – The Company maintains cash balances in domestic financial institutions. These balances are insured by the Federal Deposit Insurance Corporation up to $250,000. As of September 30, 2024, the uninsured portion of cash held by the Company was approximately $18,813,000.
TUHURA BIOSCIENSES, INC AND SUBSIDIARY
Notes to the condensed consolidated financial statements
For the nine months ended September 30, 2024, and 2023
(Unaudited)
2—Summary of significant accounting policies (continued)
Fair Value of Financial Instruments - ASC 820, Fair Value Measurement, establishes a fair value hierarchy for instruments measured at fair value that distinguishes between assumptions based on market data (observable inputs) and the Company’s own assumptions (unobservable inputs). Observable inputs are inputs that market participants would use in pricing the asset or liability based on market data obtained from sources independent of the Company. Unobservable inputs are inputs that reflect the Company’s assumptions about the inputs that market participants would use in pricing the asset or liability, and are developed based on the best information available in the circumstances.
ASC 820 identifies fair value as the exchange price, or exit price, representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. As a basis for considering market participant assumptions in fair value measurements, ASC 820 establishes a three-tier fair value hierarchy that distinguishes between the following:
|
|
|
|
|
• |
|
Level 1 inputs are quoted prices (unadjusted) in active markets for identical assets or liabilities. |
|
|
|
|
|
• |
|
Level 2 inputs are inputs other than quoted prices included within Level 1 that are observable for the asset or liability, either directly or indirectly. |
|
|
|
|
|
• |
|
Level 3 inputs are unobservable inputs that reflect the Company’s own assumptions about the assumptions market participants would use in pricing the asset or liability. Financial assets and liabilities are classified in their entirety based on the lowest level of input that is significant to the fair value measurement. |
To the extent that the valuation is based on models or inputs that are less observable or unobservable in the market, the determination of fair value requires more judgment. Accordingly, the degree of judgment exercised by the Company in determining fair value is greatest for instruments categorized in Level 3. A financial instrument’s level within the fair value hierarchy is based on the lowest level of any input that is significant to the fair value measurement. See Note 7 for more information related to the Company’s Level 3 fair value measurement.
The carrying values reported in the Company’s balance sheets for cash and cash equivalents, other current assets, accounts payable, and accrued expenses are reasonable estimates of their fair values due to the short-term nature of these items.
Derivative Financial Instruments – The Company evaluates all of its agreements to determine if such instruments have derivatives or contain features that qualify as embedded derivatives. The Company accounts for certain make-whole features that are associated with convertible notes as derivative liabilities at fair value and adjusts the instruments to their fair value at the end of each reporting period. Derivative financial liabilities are initially recorded at fair value, with gains and losses arising from changes in the fair value recognized in other income (expense) in the accompanying consolidated statements of operations for each reporting period while such instruments are outstanding. The embedded derivative liabilities are valued using a probability-weighted expected return method (“PWERM”). The critical inputs used to value the PWERM are a discount rate of 19.68%, the estimated make-whole interest payments for various settlement scenarios and the probability of each settlement scenario. If the Company repays the noteholders or if, during the next round of financing, the noteholders convert the debt into equity, the derivative financial liabilities will be de-recognized and reclassified to the condensed consolidated statements of stockholders’ (deficit) equity on that date. Derivative instrument liabilities are classified in the balance sheet as current or non-current based on whether or not net-cash settlement of the derivative instrument could be required within 12 months of the balance sheet date.
TUHURA BIOSCIENSES, INC AND SUBSIDIARY
Notes to the condensed consolidated financial statements
For the nine months ended September 30, 2024, and 2023
(Unaudited)
Note 2—Summary of significant accounting policies (continued)
Debt Discount and Debt Issuance Costs- Debt issuance costs are deferred and presented as a reduction to the convertible note payable. The initial fair value of the derivative liability on the make-whole premium is treated as a debt discount. Debt discount and debt issuance costs are amortized using the effective interest rate method over the term of the convertible promissory note. Amortization of debt discount and debt issuance costs are included within interest expense in the condensed consolidated statements of operations.
Stock Compensation Expense – The Company accounts for stock-based awards to employees and nonemployees using the fair value-based method to determine compensation for all arrangements where shares of stock or equity instruments are issued for compensation. Fair value of each common stock option is estimated on the date of grant using the Black-Scholes valuation model. The Black-Scholes model uses assumptions for expected volatility, expected dividends, expected term, and the risk-free interest rate. Expected volatility is based on historical volatility of a peer group’s common stock and other factors estimated over the expected term of the options. The expected term of the options granted is derived using the “simplified method” which computes expected term as the average of the sum of the average vesting term plus the contract term. The risk-free rate is based on the U.S. Treasury yield.
Common Stock Valuation – We are required to estimate the fair value of the common stock underlying our equity awards when performing fair value calculations. The fair value of the common stock underlying our equity awards was determined on each grant taking into account input from management and taking into account the pricing offered in our equity raises. All options to purchase shares of our common stock are intended to be granted with an exercise price per share no less than the fair value per share of our common stock underlying those options on the date of grant, based on the information known to us on the date of grant. In the absence of a public trading market for our common stock, on each grant date we develop an estimate of the fair value of our common stock in order to determine an exercise price for the option grants. Our determinations of the fair value of our common stock were made by considering the prices of preferred stock sold to investors in arm’s length transactions and the rights, preferences and privileges of our preferred stock relative to those of our common stock.
Business Combinations and Asset Acquisitions – We account for acquired businesses using the acquisition method of accounting, which requires that the assets acquired, and liabilities assumed be recorded at the date of acquisition at their respective fair values if the acquisition meets the definition of a business combination. If the acquisition does not meet the definition of a business combination, then it is accounted for as an asset acquisition and the purchase consideration is allocated to the acquired assets.
ASC 805, Business Combinations, provides a model for determining whether an acquisition represents a business combination. In order to be a business, the integrated set of activities of the acquired entity needs to have an input and a substantive process that together significantly contribute to the ability to create outputs. The acquired entity must also pass the “Screen Test” which involves determining whether the acquisition represents an in-substance asset acquisition based on whether the fair value of the gross assets acquired is “substantially all” concentrated in a single asset or group of similar assets. This evaluation excludes certain acquired assets such as cash, deferred taxes, and goodwill associated with deferred taxes, but includes all other gross assets, including any consideration transferred in excess of the identified assets.
TUHURA BIOSCIENSES, INC AND SUBSIDIARY
Notes to the condensed consolidated financial statements
For the nine months ended September 30, 2024, and 2023
(Unaudited)
Note 3—Liquidity and management’s plans
The Company has been engaged in research and development activities related to ImmuneFx, the Company’s patented product, which will require additional investment until revenue-generating activities can begin.
The Company has historically incurred negative cash flows from operations.
For the nine months ended September 30, 2024, the Company incurred $12.1 million of negative cash flows from operations. The Company has approximately $19.6 million of cash and cash equivalents on hand at September 30, 2024. The Company expects that this will be able to fund future operations, including the expanded clinical trials into the second half of 2025.
The Company expects to raise cash through the sale of common shares, issuance of convertible notes, obtaining grants, or commercial partnerships. However, there can be no assurance that any fundraising will be achieved or on commercially reasonable terms, if at all. As such, there is substantial doubt about the Company’s ability to continue as a going concern for the next 12 months from date that the financial statements were available to be issued.
Note 4—Other current assets
Other current assets consist of the following as of September 30, 2024, and December 31, 2023:
|
|
|
|
Unaudited |
|
|
September 30, |
December 31, |
|
2024 |
2023 |
Employee Retention Tax Credit |
$ 214,699 |
$ 334,443 |
Clinical trial refund |
144,634 |
|
Other current assets |
188,117 |
159,326 |
|
$ 547,450 |
$ 493,769 |
Note 5—Property and equipment, net
Property and equipment, net consists of the following as of September 30, 2024, and December 31, 2023:
|
|
|
|
Unaudited |
|
|
September 30, |
December 31, |
|
2024 |
2023 |
Furniture and fixtures |
$ 170,607 |
$ 170,607 |
Leasehold improvements |
544,629 |
544,628 |
Machinery and office equipment |
1,401,775 |
1,365,277 |
Software |
72,394 |
72,394 |
|
2,189,405 |
2,152,906 |
Less accumulated depreciation and amortization |
(2,069,812) |
(1,970,736) |
|
$ 119,593 |
$ 182,170 |
TUHURA BIOSCIENSES, INC AND SUBSIDIARY
Notes to the condensed consolidated financial statements
For the nine months ended September 30, 2024, and 2023
(Unaudited)
Note 5—Property and equipment, net (continued)
Depreciation and amortization of property and equipment totaled approximately $29,000 and $39,000 for the three months ending September 30, 2024, and 2023, respectively, and totaled approximately $99,000 and $141,000 for the nine months ending September 30, 2024, and 2023, respectively.
Note 6—Accounts payable and accrued expenses
Accounts payable and accrued expenses consist of the following as of September 30, 2024, and December 31, 2023:
|
|
|
|
Unaudited |
|
|
September 30, |
December 31, |
|
2024 |
2023 |
Trade accounts payable |
$ 1,332,680 |
$ 1,866,762 |
Accrued compensation |
948,403 |
1,415,397 |
Other accrued expenses |
139,750 |
156,400 |
|
$ 2,420,833 |
$ 3,438,559 |
Note 7—Convertible promissory notes
On various dates beginning on December 11, 2023 through September 18, 2024, the Company completed a private placement in which the Company issued Convertible Promissory Notes (the “Notes”) with various entities at various amounts for an aggregate of $31,253,000. The Notes bear interest at a rate of twenty percent (20%) per annum and mature on the second anniversary of the issuance date. In addition, the investors in the private placement also received common stock purchase warrants (the “2024 Warrants”) in the event they subscribe to purchase Notes in the aggregate principal amount of more than $4.0 million or more, with such number of 2024 Warrants being equal to 50% of the aggregate principal amount of the Note purchased divided by $0.68 (see note 8). The 2024 Warrants related to these Notes have an exercise price of $1.02 per share and expire three years from the date of issuance.
The Notes are convertible into New Securities (as defined in the Notes) upon the following: (i) automatic conversion upon an initial public offering (“Mandatory Conversion 1”), (ii) automatic conversion upon the occurrence of a de-SPAC transaction (“Mandatory Conversion 2”), (iii) automatic conversion upon the occurrence of a reverse public merger transaction (”Specified Merger Transaction”) at a conversion price equal to (a) the outstanding principal and interest of the Notes prior to conversion divided by (b) $0.68 (“Mandatory Conversion 3”), or (iv) optional new securities conversion upon a qualified equity financing, transaction, series of transactions, or merger other than an IPO or de-SPAC transaction, as defined per the terms of the Notes.
The Holder has the option, at the occurrence of qualified equity financing, transaction, series of transactions, or merger other than an IPO, de-SPAC transaction, or reverse public merger transaction, to convert the outstanding Notes into shares of common stock (“Optional Conversion”), or to receive a prepayment from the Company for the outstanding principal and interest remaining on the Notes (“Optional Redemption”).
TUHURA BIOSCIENSES, INC AND SUBSIDIARY
Notes to the condensed consolidated financial statements
For the nine months ended September 30, 2024, and 2023
(Unaudited)
Note 7—Convertible promissory notes (continued)
Under an IPO or de-SPAC transaction, the Notes convert at the sum of (a) the outstanding principal balance and unpaid accrued interest at the time of the transaction, plus (b) a “Make-Whole Amount” premium, defined in the Notes as additional interest to be incurred until the next period end date as defined in the Notes, divided by the common stock price per share at the time of the public offering (for IPO) or at closing (for de-SPAC transaction). Under a reverse public merger transaction, the Notes convert at the sum of (a) the outstanding principal balance and unpaid accrued interest at the time of the transaction, plus (b) a Make-Whole Amount premium, defined in the Notes as additional interest to be incurred until the next period end date as defined in the Notes, divided by a conversion price equal to $0.68. Upon closing of the merger, the Notes were converted into shares of common stock.
The Company evaluated the terms of the Notes for embedded conversion features in accordance with ASC 815-15-25 and determined that the conversion features meet the definition of an embedded derivative liability that is required to be bifurcated from the host instrument and measured at fair value, with subsequent changes in fair value recognized in the condensed consolidated statement of operations.
The 2024 Warrants were identified as freestanding financial instruments and determined to be indexed to the Company’s own stock. Further, the 2024 Warrants were not precluded from being classified within equity. As such, the proceeds received upon issuing the Notes were first allocated to the fair value of the bifurcated embedded derivative with the remainder allocated to the debt host instrument and 2024 Warrants (within additional paid in capital) on a relative fair value basis. Subsequent fair value measurement is not required as long as the instrument continues to be classified in equity. The Company determined that the fair value of the 2024 Warrants in connection with Notes issued as of September 30, 2024 amounted to $6,520,056 and recognized as a debt discount with an offset to additional paid in capital.
Management used a scenario-based analysis to estimate the fair value of the bifurcated embedded derivative liability at issuance of the Notes. The Company recognized debt discount of $2,539,227 upon issuance of the Notes. There was a gain of $21,229 and a loss of $313,772 for the three and nine months ended September 30, 2024, due to the estimated change in fair value of the bifurcated embedded derivative liability. The related discount is amortized to interest expense over the term of the debt using the effective yield method. Amortization expense related to the debt discount totaled $668,838 and $1,107,010 for the three and nine months ended September 30, 2024. Interest expense, inclusive of the debt discount amortization, on the Notes totaled $2,002,886 and $3,615,466 for the three and nine months ended September 30, 2024.
Note 8—Stockholders’ equity
As of September 30, 2024, the Company had two classes of stock defined in its Amended and Restated Articles of Incorporation (the “Articles).
Common Stock – The Company is authorized to issue up to 300,000,000 shares of Common Stock based on the Articles. Holders of common stock are entitled to one vote for each share of common stock.
Preferred Stock – The Company is authorized to issue up to 150,000,000 shares of Preferred Stock based on the Articles. The Company has three classes of Preferred Stock: Series A, Series A-1, and Series B. See below for a summary of the rights and preferences for the Company’s Preferred Stock:
TUHURA BIOSCIENSES, INC AND SUBSIDIARY
Notes to the condensed consolidated financial statements
For the nine months ended September 30, 2024, and 2023
(Unaudited)
Note 8—Stockholders’ equity (continued)
i.Accrues dividends whether or not declared, are cumulative, and are payable only if declared by the Board of Directors. The Series A and Series A-1 preferred stock accrue dividends at a rate of $0.0208 and $0.0264, per annum respectively. Accrued, but unpaid Series A and A-1 dividends totaled approximately $6,530,000 as of September 30, 2024. The Series B preferred stock accrues dividends at a rate of $0.066 for the first two years. After the second anniversary, Series B stock accrues dividends at a rate of $0.0264 per annum. Accrued but unpaid Series B dividends totaled approximately $6,160,000 as of September 30, 2024.
ii.Has liquidation preferences over common stock;
iii.Is convertible into common stock, at the option of the holder, subject to adjustments, as defined; and
iv.For purposes of voting, each holder of outstanding shares of Preferred Stock is entitled to cast the number of votes equal to the number of whole shares of common stock into which the shares are convertible to.
v.The holders of Series A and Series A-1 Preferred Stock exclusively and voting together as a single class have the right to elect one Director of the Company.
vi.A majority of all three classes of Preferred stock are required to amend the Articles of Incorporation or Bylaws, issue shares (unless they are junior to the Preferred stock), issue debt greater than $250,000, liquidate or dissolve the Company, or hold stock in an unaffiliated Company.
In August 2024, the Company extended the exercise period of its common stock purchase warrants issued in connection with its Series A Preferred Stock (the “Series A Warrants”) for an additional six months, with a new expiry date of February 12, 2025. There were no other changes in the terms of the Series A Warrants. As a result, a deemed dividend to the holders of the Series A Warrants in the amount of $965,177 was recorded as an increase in the net loss attributable to the common stockholders for the nine months ended September 30, 2024. The incremental value associated with the warrant modification was determined using a Black-Sholes pricing model using the original terms of the warrants and the modified terms of the warrants and the following assumptions: expected term of approximately 0.1 - 0.6 years, dividend yield of 0.0%, volatility of 75% -112%, and a risk free rate of 5.4% to 5.5%.
As of September 30, 2024, the Company has 59,648,400 warrants outstanding, of which 7,835,300 warrants were for services performed with respect to historical offerings and 18,797,800 warrants for the most recent convertible promissory notes offering (see note 7). The remaining 33,015,300 warrants were issued to Series A, A-1, and B preferred investors. There were 3,587,800 warrants that were exercised in August and September 2024 with proceeds in the amount of $1,931,004. All outstanding warrants entitle the holder thereof to purchase one shares of Company common stock.
Immediately prior to the closing of the Merger, all outstanding shares of Company preferred stock were converted into shares of Company common stock (which were converted into shares of Kintara common stock in the Merger), and upon completion of the merger, all warrants of the Company were converted into warrants to purchase Kintara common stock.
TUHURA BIOSCIENSES, INC AND SUBSIDIARY
Notes to the condensed consolidated financial statements
For the nine months ended September 30, 2024, and 2023
(Unaudited)
Note 9—Stock option plans
The Company uses the Black-Scholes option pricing model to estimate the fair value of stock-based awards on the date of grant. The assumptions employed in the calculation of the fair value of share-based compensation expense were calculated as follows for all periods presented:
|
|
|
|
|
|
|
|
|
2024 |
|
2023 |
|
|
|
|
|
|
Common stock fair value |
|
|
$0.72 |
|
$0.66 |
Risk free interest rate |
|
|
4.1% - 4.27% |
|
4.05% - 4.89% |
Expected dividend yield |
|
|
0% |
|
0% |
Expected term |
|
|
5.9 years |
|
4.9 years |
Expected stock volatility |
|
|
101.0% - 102.0% |
|
91.9% - 99.7% |
Below is a summary of stock option activity for the period ending September 30, 2024:
|
|
|
|
|
|
|
|
|
Weighted |
|
Weighted |
|
Number |
|
Average |
|
Average |
|
of options |
|
Exercise Price |
|
Contractual Life |
|
|
|
|
|
|
Outstanding at December 31, 2023 |
15,545,363 |
|
$0.53 |
|
4.43 years |
Forfeited and cancelled |
(1,217,186) |
|
$0.61 |
|
|
Exercised |
(510,000) |
|
$0.45 |
|
|
Granted |
4,638,471 |
|
$0.72 |
|
|
Outstanding at September 30, 2024 |
18,456,648 |
|
$0.58 |
|
4.98 years |
Exercisable at September 30, 2024 |
12,879,981 |
|
$0.53 |
|
3.21 years |
Options outstanding had an intrinsic value of $2,933,000 and $1,964,000 as of September 30, 2024 and December 31, 2023, respectively. As of September 30, 2024, there was $2,263,000 of unrecognized stock compensation, which will be recognized over the next three years.
Note 10—Commitments and contingencies
Lease Commitments – The Company leases facilities under non-cancelable operating leases for the laboratory and offices in Tampa, Florida. The current lease expires in February 2026.
TUHURA BIOSCIENSES, INC AND SUBSIDIARY
Notes to the condensed consolidated financial statements
For the nine months ended September 30, 2024, and 2023
(Unaudited)
Note 10—Commitments and contingencies (continued)
Future minimum lease payments under these leases are as follows:
|
|
Year ending December 31, 2024 |
$ 42,697 |
Year ending December 31, 2025 |
172,931 |
Year ending December 31, 2026 |
43,411 |
Interest portion of right of use liability |
(19,482) |
Operating lease liabilities |
$ 239,557 |
Total lease expense was approximately $72,000 and $49,000 for the three months ending September 30, 2024 and 2023, respectively, and approximately $205,000 and $143,000 for the nine months ending September 30, 2024 and 2023, respectively.
Employment Agreements – In March 2024, the Company signed a consulting agreement with an entity owned by the former CEO and President. In May 2023, and amended in March 2024, the Company signed employment agreements with the CEO and CFO.
Future minimum payments under these employment and consulting agreements are as follows:
|
|
Year ending December 31, 2024 |
$ 275,709 |
Year ending December 31, 2025 |
877,835 |
|
$ 1,153,544 |
Note 11—Subsequent events
Subsequent events – The Company has evaluated subsequent events through November 14, 2024 in connection with the preparation of these financial statements, which is the date the financial statements were available to be issued.
Exercise of extension rights and program expenses with Kineta
In October 2024, the Company made additional payments of $300,000 to exercise its extension rights with Kineta under the Exclusivity Agreement. Although the exclusivity right under the Exclusivity Agreement terminated in October, the Company is currently still collaborating with Kineta on Kineta’s ongoing Phase 1 clinical program in patients with advanced solid tumor cancer.
Merger with Kintara Therapeutics, Inc.
On October 18, 2024, the Company completed the transactions contemplated by the Merger Agreement with Kintara. Pursuant to the Merger Agreement, Merger Sub merged with and into the Company, with the Company surviving as a direct wholly owned subsidiary of Kintara and the surviving corporation of the Merger. In connection with the completion of the Merger, effective at 12:01 a.m. Eastern Time on October 18, 2024, Kintara effected a 1-for-35 reverse stock split of its common stock (the “Reverse Stock Split”). Effective at 12:03 a.m. Eastern Time on October 18, 2024, the Company completed the Merger, and effective at 12:04 a.m. Eastern Time on October 18, 2024, Kintara changed its name to “TuHURA Biosciences, Inc.”
TUHURA BIOSCIENSES, INC AND SUBSIDIARY
Notes to the condensed consolidated financial statements
For the nine months ended September 30, 2024, and 2023
(Unaudited)
Note 11—Subsequent events (continued)
Under the terms of the Merger, immediately prior to the effective time of the Merger, shares of the Company’s preferred stock were converted into shares of Company common stock and all of the Notes issued by the Company were converted into shares of Company common stock pursuant to the terms therein. At the effective time of the Merger, (i) Kintara issued an aggregate of approximately 40,441,605 shares of its common stock to Company stockholders, based on an exchange ratio of 0.1789 (after giving effect to the Reverse Stock Split) shares of Kintara’s common stock for each share of Company common stock outstanding immediately prior to the Merger, (ii) each then-outstanding Company stock option was assumed and converted into an option to purchase shares of Kintara common stock subject to certain adjustments based on the exchange ratio as set forth in the Merger Agreement, and (iii) each then-outstanding warrant to purchase shares of Company Common Stock was assumed and converted into and exchangeable based on the exchange ratio for a warrant of like tenor entitling the holder to purchase shares of Kintara common stock
The issuance of the shares of Kintara’s common stock to the former stockholders of the Company was registered with the SEC on the Kintara’s Registration Statement on Form S-4 (File No. 333-279368), as amended.
The shares of Kintara’s common stock listed on the Nasdaq Capital Market, previously trading through the close of business on Thursday, October 17, 2024 under the ticker symbol “KTRA,” commenced trading on the Nasdaq Capital Market on a post-Reverse Stock Split adjusted basis and post-Merger basis under the ticker symbol “HURA” on Friday, October 18, 2024. The Company’s common stock is represented by a new CUSIP number, 898920 103.
In connection with the Merger, Kintara entered into a Contingent Value Rights Agreement (the “CVR Agreement”) with the Rights Agent, pursuant to which the Kintara common stockholders and Kintara common stock warrant holders of record as of immediately prior to the consummation of the Merger and Reverse Stock Split received one contingent value right (“CVR”) for each outstanding share of common stock of Kintara held by such stockholder (or, in the case of warrants, each share of common stock of Kintara for which such warrant is exercisable into). Pursuant to the CVR Agreement, upon the achievement of the Milestone (as defined below), the holders of CVRs are entitled, in aggregate, to receive approximately 1,539,918 shares (post-split basis) of common stock of Kintara (which gives effect to the Reverse Stock Split) (collectively, the “CVR Shares”). Each CVR shall entitle the holder thereof to receive its portion of the CVR Shares if Kintara (i) enrolls a minimum of ten cutaneous metastatic breast cancer patients in a study to determine whether a dose of REM-001 lower than 1.2 mg/kg elicits a treatment effect similar to that seen in prior studies of REM-001 at the 1.2 mg/kg dose and (ii) such patients enrolled in the study complete eight weeks of follow-up, in each case, on or before December 31, 2025 (the “Milestone”). The payment date for the CVR Shares will be within 10 business days after the Rights Agent
receives the CVR Shares as the payment for achievement of the Milestone. In the event that the Milestone is not achieved, holders of the CVRs will not receive any CVR Shares pursuant to the CVR Agreement. There can be no assurances that any holders of CVRs will receive any CVR Shares with respect thereto. Some CVR Shares that would otherwise be received may be withheld on account of taxes if the Company or the applicable withholding agent determines that tax withholding is required in connection with the delivery of CVR Shares or that there was a failure to withhold adequately in respect of the distribution of the CVRs.
v3.24.3
Document And Entity Information
|
Oct. 18, 2024 |
| Cover [Abstract] |
|
| Document Type |
8-K/A
|
| Amendment Flag |
true
|
| Amendment Description |
INTRODUCTORY NOTE This Amendment No. 1 on Form 8-K/A (this “Amendment No. 1”) amends the Current Report on Form 8-K filed by TuHURA Biosciences, Inc. (f/k/a Kintara Therapeutics, Inc.) (the “Company”) on October 21, 2024 (the “Original Report”), in which the Company reported, among other events, the completion of the Merger, pursuant to which Kayak Mergeco, Inc. merged with and into TuHURA Biosciences, Inc., a Delaware corporation (“Private TuHURA”). This Amendment No. 1 is filed to (1) update the Item 2.01 information to reflect Management’s Discussion and Analysis of Financial Condition and Results of Operations of Private TuHURA for the three and nine months ended September 30, 2024 and September 30, 2023; and (2) amend the historical financial statements provided under Items 9.01(a) in the Original Report to include the unaudited interim financial statements of Private TuHURA as of September 30, 2024 and for the three and nine months ended September 30, 2024 and September 30, 2023. This Amendment No. 1 does not amend any other item of the Original Report or purport to provide an update or a discussion of any developments at the Company subsequent to the filing date of the Original Report. Capitalized terms used but not defined herein have the meanings given in the Original Report.
|
| Document Period End Date |
Oct. 18, 2024
|
| Entity Registrant Name |
TuHURA Biosciences, Inc./NV
|
| Entity Central Index Key |
0001498382
|
| Entity Emerging Growth Company |
false
|
| Entity File Number |
001-37823
|
| Entity Incorporation, State or Country Code |
NV
|
| Entity Tax Identification Number |
99-0360497
|
| Entity Address, Address Line One |
10500 University Dr.
|
| Entity Address, Address Line Two |
Suite 110
|
| Entity Address, City or Town |
Tampa
|
| Entity Address, State or Province |
FL
|
| Entity Address, Postal Zip Code |
33612
|
| City Area Code |
813
|
| Local Phone Number |
875-6600
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Common Stock, par value $0.001 per share
|
| Trading Symbol |
HURA
|
| Security Exchange Name |
NASDAQ
|
| X |
- DefinitionDescription of changes contained within amended document.
| Name: |
dei_AmendmentDescription |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14a
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Kintara Therapeutics (NASDAQ:KTRA)
Historical Stock Chart
Von Mär 2025 bis Apr 2025

Kintara Therapeutics (NASDAQ:KTRA)
Historical Stock Chart
Von Apr 2024 bis Apr 2025


