UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
Report of Foreign Private Issuer Pursuant to Rule 13a-16
or 15d-16
Under the Securities Exchange Act of 1934
For the Month of September 2023
Commission File Number: 001-41084
NeuroSense Therapeutics Ltd.
(Translation of registrant’s name into English)
11 HaMenofim Street,
Building B
Herzliya 562 Israel
+972-9-9531142
(Address of principal executive offices)
Indicate by check mark whether the registrant files
or will file annual reports under cover Form 20-F or Form 40-F.
Form 20-F ☒ Form 40-F ☐
NeuroSense
Therapeutics Ltd. (the “Company”) has made available an updated presentation about its business (the “Presentation”),
a copy of which is furnished herewith as Exhibit 99.1 to this Report on Form 6-K. The Presentation now contains the design for the AD
trial on slide 18.
The new
update in the Presentation is not an admission as to the materiality of any information therein. The information contained in the Presentation
is summary information that should be considered in the context of the Company’s filings with the Securities and Exchange Commission
and other public announcements the Company may make by press release or otherwise from time to time.
Exhibit Index
SIGNATURES
Pursuant to the requirements
of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto
duly authorized.
| |
NeuroSense Therapeutics Ltd. |
| |
|
|
| Date: September 12, 2023 |
By: |
/s/ Alon Ben-Noon |
| |
|
Alon Ben-Noon |
| |
|
Chief Executive Officer |
2
Exhibit 99.1

Investor Webinar September 2023 Nasdaq: NRSN

Forward - Looking Statements This presentation and oral statements made regarding the subject of this presentation contain "forward - looking statements" within the meaning of the U.S. Private Securities Litigation Reform Act of 1995 that involve substantial risks and uncertainties. All statements contained in this presentation other than statements of historical facts, including our business strategy and plans and objectives for future operations, including our financial performance, are forward looking statements. The words " anticipate"," believe," "continue," "estimate," "expect," "intend," "may," "will" and similar expressions are intended to identify forward looking statements. We have based these forward - looking statements largely on our current expectations and projections about future events and trends that we believe may affect our financial condition, results of operations, business strategy, short term and long - term business operations and objectives and financial needs. Forward looking statements made in this presentation include statements about the timing of reporting top - line results from our ALS Phase 2b clinical trial and of other clinical and regulatory milestones, target market and opportunities for our product candidates; our expectations regarding our competitive advantages; the planned development timeline of our product candidates; and characterizations of the pre - clinical and clinical trial results of our product candidates. Forward looking statements are subject to a number of risks and uncertainties and represent our views only as of the date of the presentation. The future events and trends discussed in this presentation may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward - looking statements due to, among other things, a delay in the reporting of top - line results from our ALS Phase 2b clinical trial and a delay in other clinical and regulatory milestones and changes in market conditions. More information about the risks and uncertainties affecting the Company is contained under the heading "Risk Factors" in the Annual Report on Form 20 - F filed with the Securities and Exchange Commission on March 22, 2023. We undertake no obligation or duty to update information contained in these forward - looking statements, whether as a result of new information, future events or otherwise. Trademarks in this presentation are the property of their respective owners and used for informational and educational purposes only.
NeuroSense Highlights Developing novel therapies for neurodegenerative diseases of high unmet need Multiple near - term catalysts including Phase 2 b topline results for ALS (1) expected in Q4 2023 Existing partnership with big pharma and fully funded through Q2 2024 zed 1. ALS - Amyotrophic Lateral Sclerosis and also referred to as Lou Gehrig’s Disease Phase 2a Results Intro PrimeC Pre - clinical Phase 2b Summary
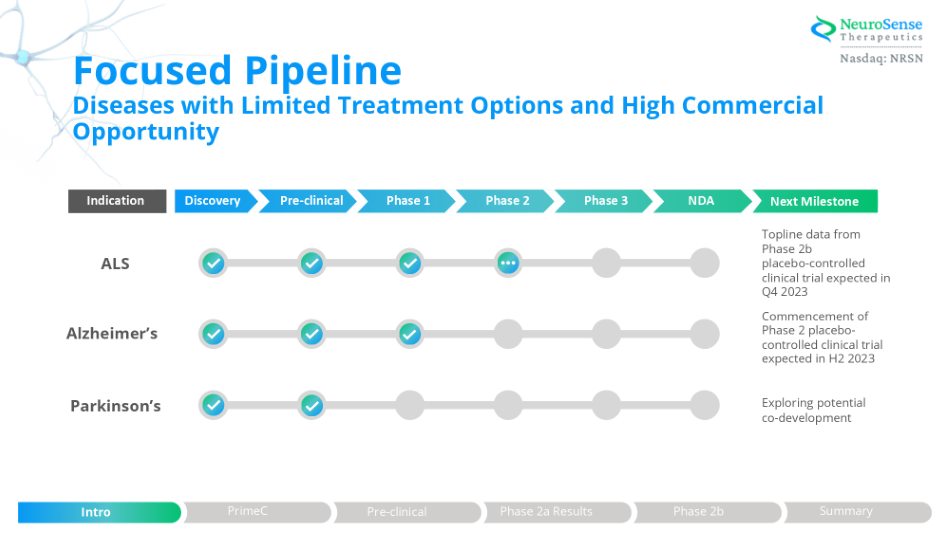
Focused Pipeline Diseases with Limited Treatment Options and High Commercial Opportunity ALS Alzheimer’s Parkinson’s Topline data from Phase 2b placebo - controlled clinical trial expected in Q4 2023 Commencement of Phase 2 placebo - controlled clinical trial expected in H2 2023 Exploring potential co - development Discovery Pre - clinical Phase 1 Phase 2 Phase 3 NDA Indication Next Milestone Phase 2a Results Intro PrimeC Pre - clinical Phase 2b Summary
PrimeC: At a Glance Novel combination therapy of approved products optimized for PK and synergistic effects to address ALS and potentially other disease targets Differentiated efficacy and excellent safety profile observed from ALS Phase 2a clinical trial ALS Phase 2b top - line results from placebo - controlled clinical trial expected in Q4 2023 Expedited and de - risked regulatory pathway (orphan drug designation / 505(b)2 pathway) Patent coverage for novel formulation, method & combination (until 2038) Phase 2a Results Intro PrimeC Pre - clinical Phase 2b Summary
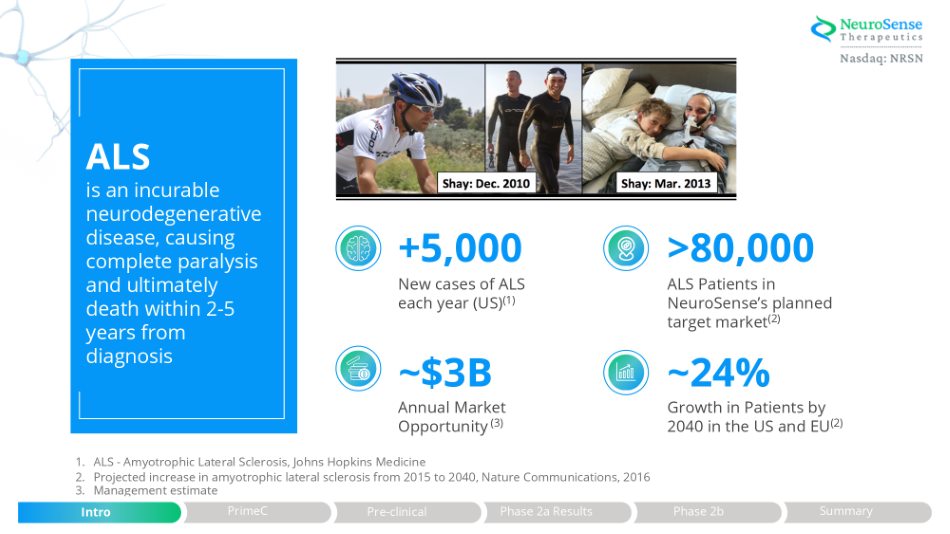
>80,000 ALS Patients in NeuroSense’s planned target market (2) ~24% Growth in Patients by 2040 in the US and EU (2) +5,000 New cases of ALS each year (US) (1) ~$3B Annual Market Opportunity (3) 1. ALS - Amyotrophic Lateral Sclerosis, Johns Hopkins Medicine 2. Projected increase in amyotrophic lateral sclerosis from 2015 to 2040, Nature Communications, 2016 3. Management estimate ALS is an incurable neurodegenerative disease, causing complete paralysis and ultimately death within 2 - 5 years from diagnosis Phase 2a Results Intro PrimeC Pre - clinical Phase 2b Summary
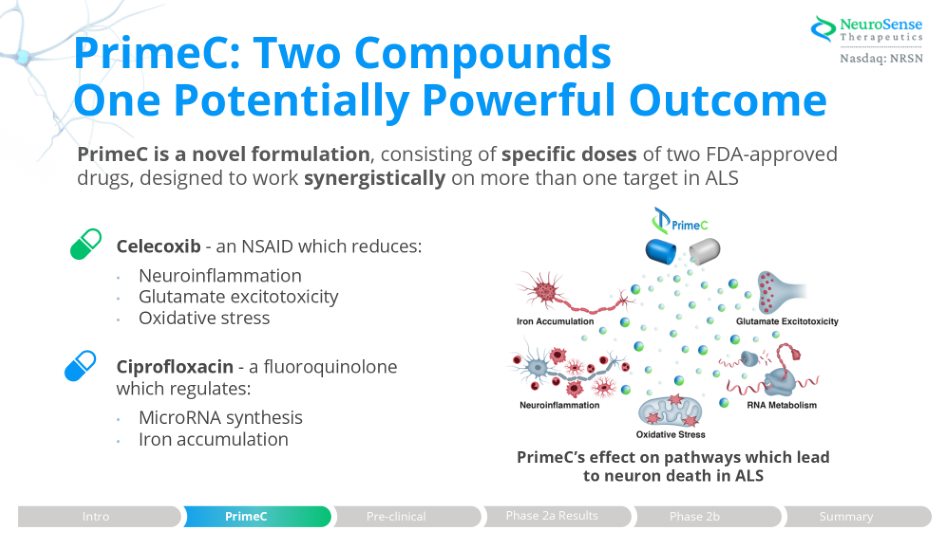
PrimeC: Two Compounds One Potentially Powerful Outcome PrimeC is a novel formulation , consisting of specific doses of two FDA - approved drugs, designed to work synergistically on more than one target in ALS Celecoxib - an NSAID which reduces: • Neuroinflammation • Glutamate excitotoxicity • Oxidative stress Ciprofloxacin - a fluoroquinolone which regulates: • MicroRNA synthesis • Iron accumulation PrimeC’s effect on pathways which lead to neuron death in ALS Phase 2a Results Intro PrimeC Pre - clinical Phase 2b Summary
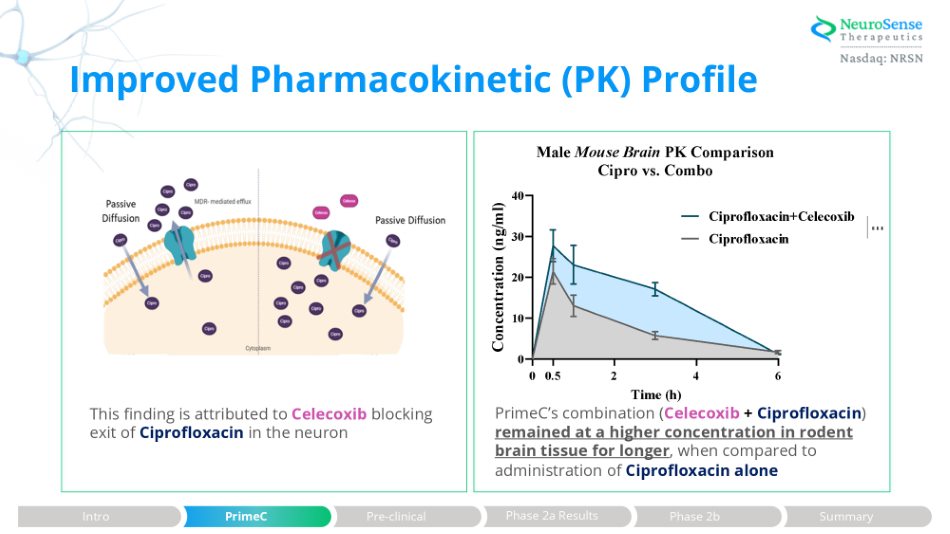
Improved Pharmacokinetic (PK) Profile This finding is attributed to Celecoxib blocking exit of Ciprofloxacin in the neuron PrimeC’s combination ( Celecoxib + Ciprofloxacin ) remained at a higher concentration in rodent brain tissue for longer , when compared to administration of Ciprofloxacin alone Phase 2a Results Intro PrimeC Pre - clinical Phase 2b Summary
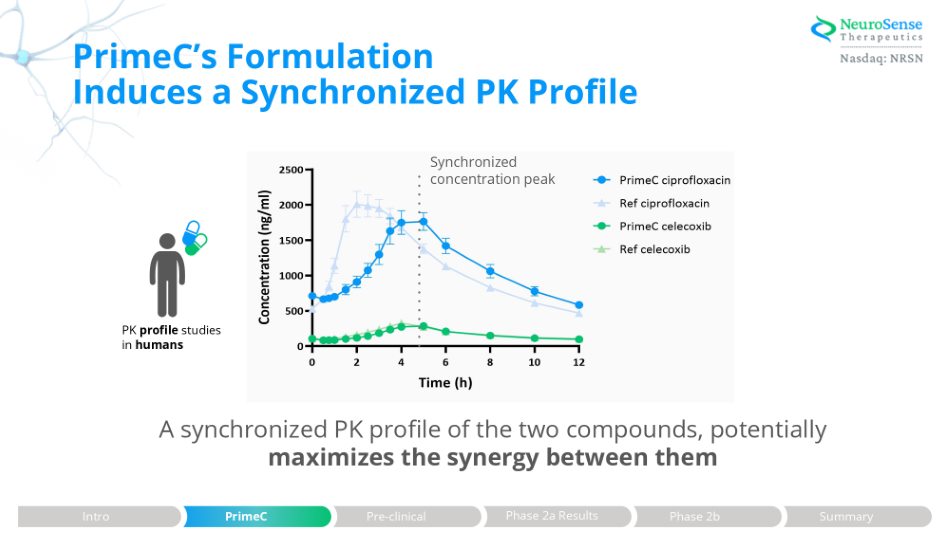
PrimeC’s Formulation Induces a Synchronized PK Profile A synchronized PK profile of the two compounds, potentially maximizes the synergy between them PK profile studies in humans Synchronized concentration peak Phase 2a Results Intro PrimeC Pre - clinical Phase 2b Summary
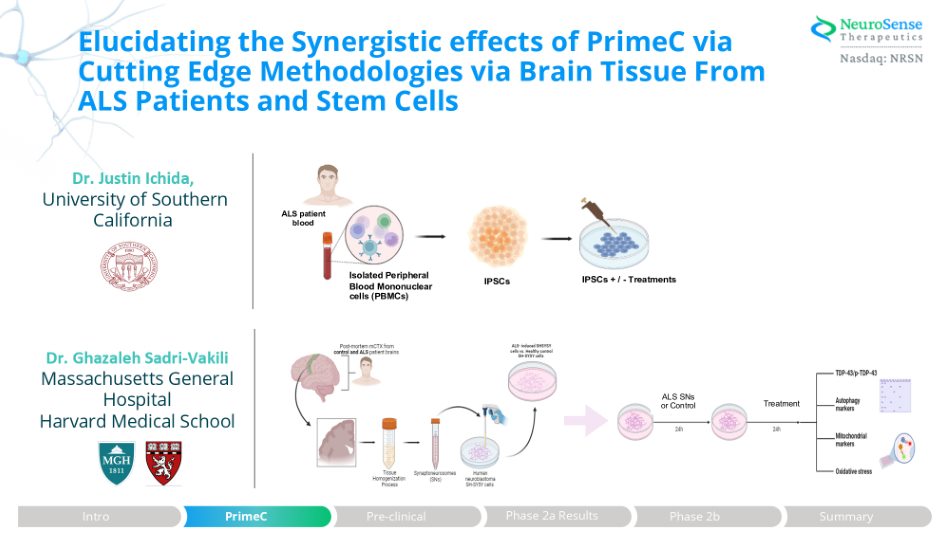
Elucidating the Synergistic effects of PrimeC via Cutting Edge Methodologies via Brain Tissue From ALS Patients and Stem Cells ALS patient blood Isolated Peripheral Blood Mononuclear cells (PBMCs) IPSCs IPSCs + / - Treatments Dr. Justin Ichida, University of Southern California Dr. Ghazaleh Sadri - Vakili Massachusetts General Hospital Harvard Medical School Treatment ALS SNs or Control Phase 2a Results Intro PrimeC Pre - clinical Phase 2b Summary
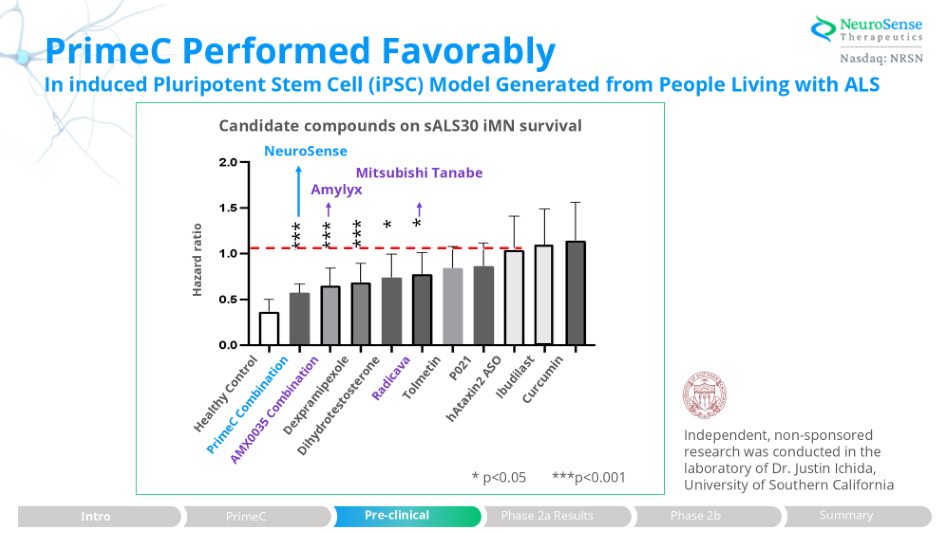
Independent, non - sponsored research was conducted in the laboratory of Dr. Justin Ichida, University of Southern California PrimeC Performed Favorably * = p<0.05, *** = p<0.001 *** *** *** * * In induced Pluripotent Stem Cell (iPSC) Model Generated from People Living with ALS Candidate compounds on sALS30 iMN survival NeuroSense Mitsubishi Tanabe Amylyx Hazard ratio * p<0.05 ***p<0.001 Phase 2a Results Intro PrimeC Pre - clinical Phase 2b Summary
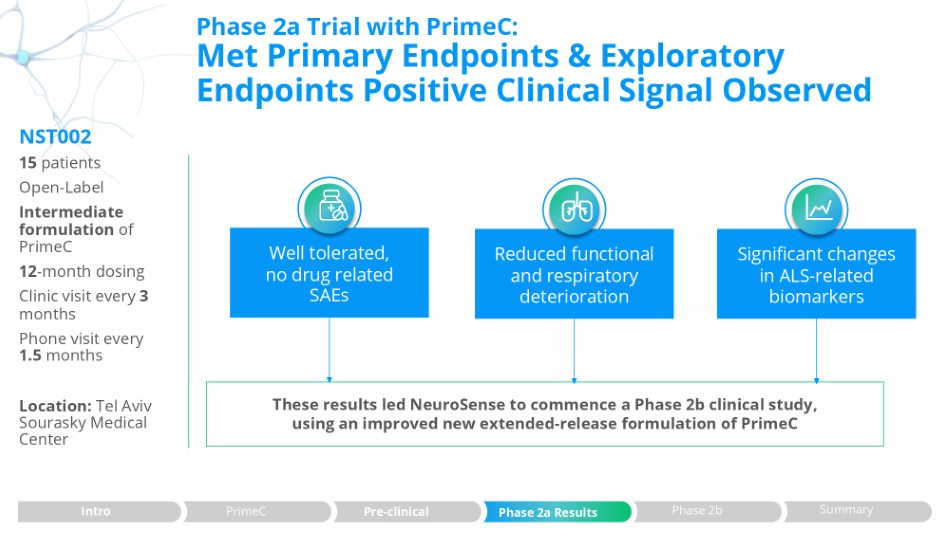
Phase 2a Trial with PrimeC: Met Primary Endpoints & Exploratory Endpoints Positive Clinical Signal Observed These results led NeuroSense to commence a Phase 2b clinical study, using an improved new extended - release formulation of PrimeC Significant changes in ALS - related biomarkers Well tolerated, no drug related SAEs Reduced functional and respiratory deterioration NST002 15 patients Open - Label Intermediate formulation of PrimeC 12 - month dosing Clinic visit every 3 months Phone visit every Phase 2a Results Intro PrimeC Pre - clinical Phase 2b Summary 1.5 months Location : Tel Aviv Sourasky Medical Center
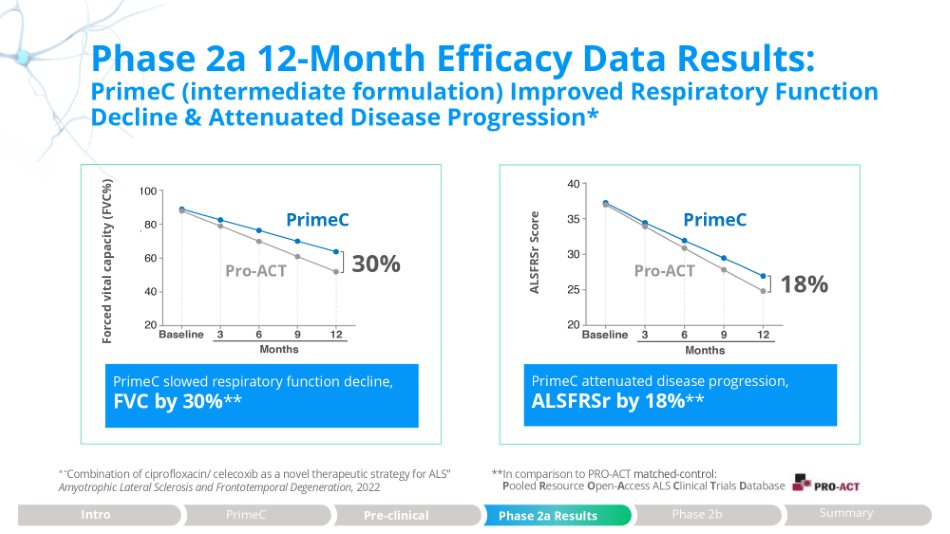
Phase 2a 12 - Month Efficacy Data Results: PrimeC (intermediate formulation) Improved Respiratory Function Decline & Attenuated Disease Progression* ALSFRSr Score Forced vital capacity (FVC%) **In comparison to PRO - ACT matched - control : P ooled R esource O pen - A ccess ALS C linical T rials D atabase * “ Combination of ciprofloxacin/ celecoxib as a novel therapeutic strategy for ALS” Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration , 2022 PrimeC attenuated disease progression, ALSFRSr by 18% ** PrimeC slowed respiratory function decline, FVC by 30% ** 18% 30% PrimeC PrimeC Pro - ACT Pro - ACT Phase 2a Results Intro PrimeC Pre - clinical Phase 2b Summary
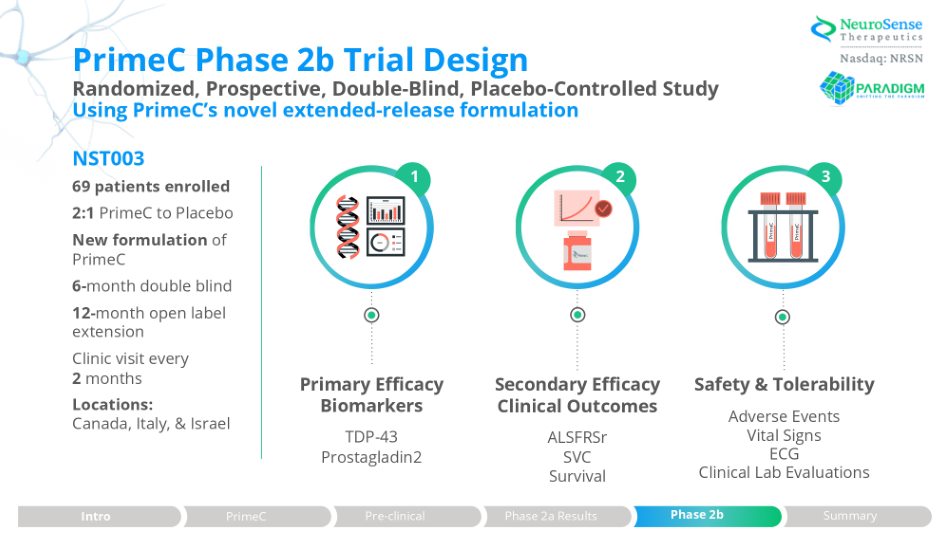
PrimeC Phase 2b Trial Design Randomized, Prospective, Double - Blind, Placebo - Controlled Study Using PrimeC’s novel extended - release formulation NST003 69 patients enrolled 2:1 PrimeC to Placebo New formulation of PrimeC 6 - month double blind 12 - month open label extension Clinic visit every 2 months Locations: Canada, Italy, & Israel Primary Efficacy Biomarkers TDP - 43 Prostagladin2 Secondary Efficacy Clinical Outcomes ALSFRSr SVC Survival Safety & Tolerability Adverse Events Vital Signs ECG Clinical Lab Evaluations 1 3 PrimeC PrimeC 2 Phase 2a Results Intro PrimeC Pre - clinical Phase 2b Summary
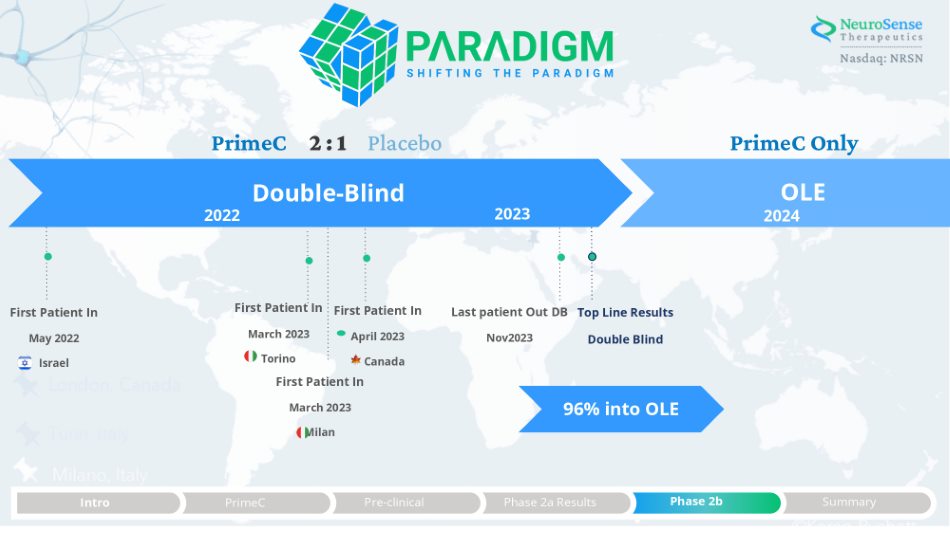
Double - Blind PrimeC 2 : 1 Placebo First Patient In May 2022 Israel First Patient In March 2023 Torino 2022 2023 First Patient In March 2023 Milan First Patient In April 2023 Canada Nov2023 PrimeC Only OLE 2024 Last patient Out DB Top Line Results Double Blind Summary Phase 2b Phase 2a Results Pre - clinical PrimeC Intro 96% into OLE
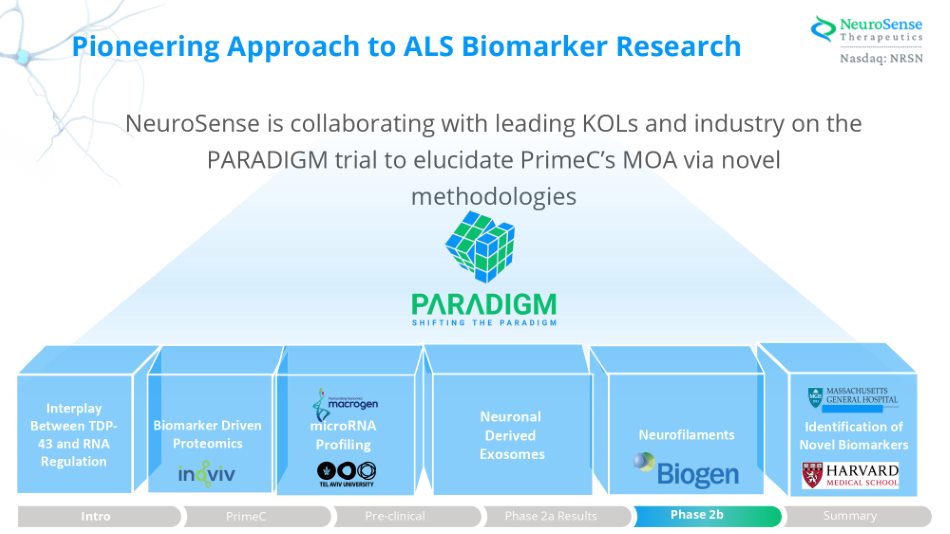
Pioneering Approach to ALS Biomarker Research NeuroSense is collaborating with leading KOLs and industry on the PARADIGM trial to elucidate PrimeC’s MOA via novel methodologies Summary Phase 2a Results Pre - clinical Identification of Novel Biomarkers Neurofilaments Neuronal Derived Exosomes Interplay Between TDP - 43 and RNA Regulation Biomarker Driven Proteomics microRNA Profiling Summary P P h h a a s s e e 2 2 b b Phase 2a Results Pre - clinical PrimeC Intro
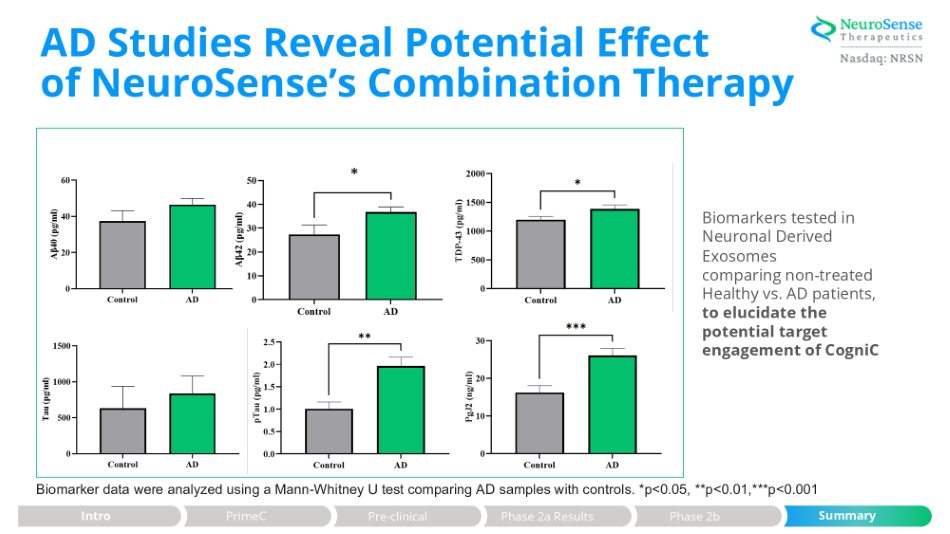
AD Studies Reveal Potential Effect of NeuroSense’s Combination Therapy Biomarkers tested in Neuronal Derived Exosomes comparing non - treated Healthy vs. AD patients, to elucidate the potential target engagement of CogniC Biomarker data were analyzed using a Mann - Whitney U test comparing AD samples with controls. *p<0.05, **p<0.01,***p<0.001 Intro PrimeC Summary Pre - clinical Phase 2a Results Phase 2b *
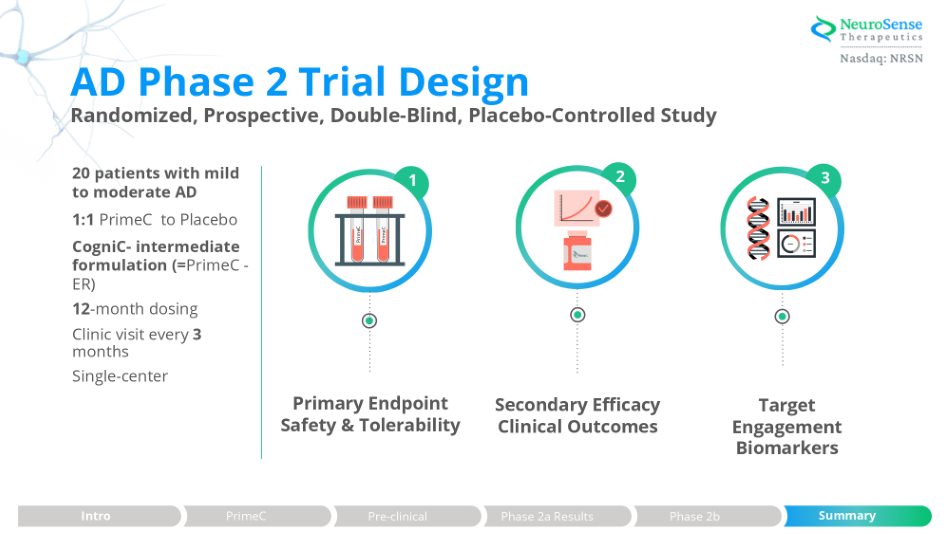
AD Phase 2 Trial Design Randomized, Prospective, Double - Blind, Placebo - Controlled Study 20 patients with mild to moderate AD 1:1 PrimeC to Placebo CogniC - intermediate formulation (= PrimeC - ER) 12 - month dosing Clinic visit every 3 months Single - center Target Engagement Biomarkers Secondary Efficacy Clinical Outcomes Primary Endpoint Safety & Tolerability 3 1 PrimeC PrimeC 2 Summary Phase 2b Phase 2a Results Pre - clinical Intro PrimeC Summary Pre - clinical Phase 2a Results Phase 2b
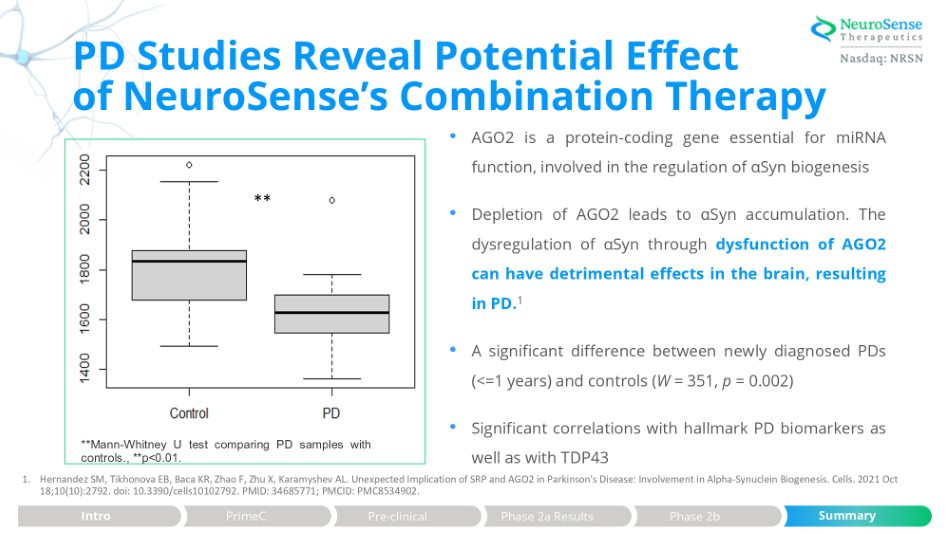
• AGO2 is a protein - coding gene essential for miRNA function, involved in the regulation of α Syn biogenesis • Depletion of AGO 2 leads to α Syn accumulation . The dysregulation of α Syn through dysfunction of AGO 2 can have detrimental effects in the brain, resulting in PD . 1 • A significant difference between newly diagnosed PDs (<= 1 years) and controls ( W = 351 , p = 0 . 002 ) • Significant correlations with hallmark PD biomarkers as well as with TDP 43 1. Hernandez SM, Tikhonova EB, Baca KR, Zhao F, Zhu X, Karamyshev AL. Unexpected Implication of SRP and AGO2 in Parkinson's Disease: Involvement in Alpha - Synuclein Biogenesis. Cells. 2021 Oct 18;10(10):2792. doi: 10.3390/cells10102792. PMID: 34685771; PMCID: PMC8534902. PD Studies Reveal Potential Effect of NeuroSense’s Combination Therapy ** **Mann - Whitney U test comparing PD samples with controls., **p<0.01. Pre - clinical Phase 2a Results Phase 2b Summary Intro PrimeC Summary Pre - clinical Phase 2a Results Phase 2b
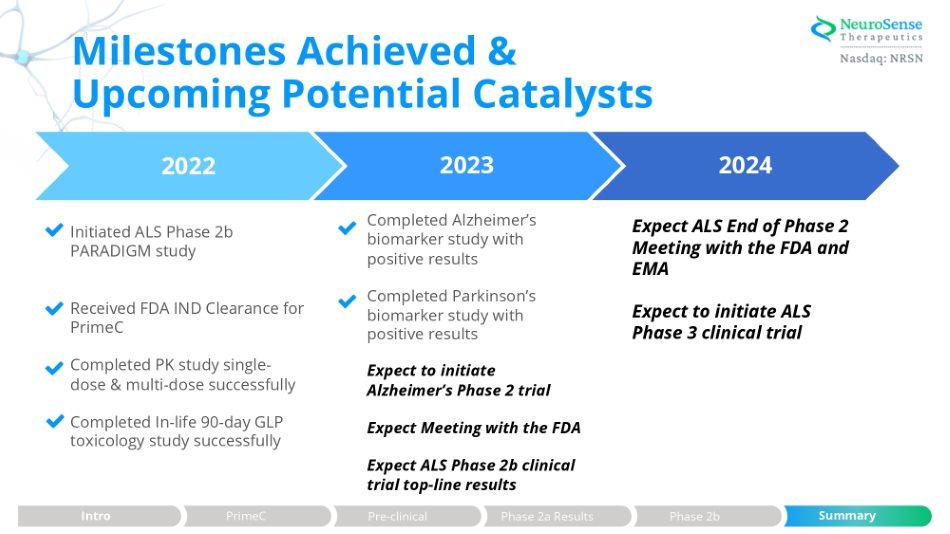
Milestones Achieved & Upcoming Potential Catalysts Expect ALS End of Phase 2 Meeting with the FDA and EMA Expect to initiate ALS Phase 3 clinical trial Initiated ALS Phase 2b PARADIGM study Received FDA IND Clearance for PrimeC Completed PK study single - dose & multi - dose successfully Completed In - life 90 - day GLP toxicology study successfully 2022 2023 2024 Completed Alzheimer’s biomarker study with positive results Completed Parkinson’s biomarker study with positive results Summary Phase 2b Expect to initiate Alzheimer’s Phase 2 trial Expect Meeting with the FDA Expect ALS Phase 2b clinical trial top - line results Pre - clinical Phase 2a Results Intro PrimeC Summary Pre - clinical Phase 2a Results Phase 2b

of Neurology Prof. Jeremy Shefner (Chair) Senior VP at the Barrow Neurological Institute Chair of the Department Dr. Jinsy Andrews Associate Professor of Neurology, Division of Neuromuscular Medicine, Columbia University Director of Neuromuscular Clinical Trials Prof. Merit Cudkowicz Chief of Neurology at Mass General and Director, Sean M. Healey & AMG Center for ALS Professor of Neurology at Harvard Medical School Prof. Jeffrey Rosenfeld Professor of Neurology and Associate Chairman of Neurology at Loma Linda University School of Medicine Medical Director of Center for Restorative Neurology at Loma Linda University Prof. Orla Hardiman Head of Academic Unit of Neurology at Trinity College Dublin and Consultant Neurologist at Beaumont Co - Chair of the European Consortium to Cure ALS and Chair of the Scientific Committee of ENCALS Scientific Advisory Board Summary Phase 2b Phase 2a Results Pre - clinical Intro PrimeC Summary Pre - clinical Phase 2a Results Phase 2b

Key Collaborations Intro PrimeC Summary Pre - clinical Phase 2a Results Phase 2b
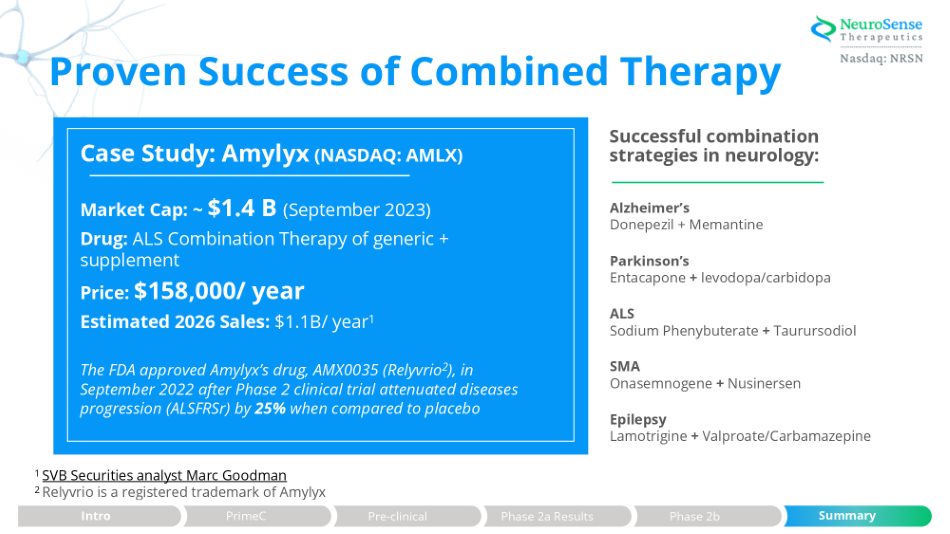
Proven Success of Combined Therapy Successful combination strategies in neurology: Alzheimer’s Donepezil + Memantine Parkinson’s Entacapone + levodopa/carbidopa ALS Sodium Phenybuterate + Taurursodiol SMA Onasemnogene + Nusinersen Epilepsy Lamotrigine + Valproate/Carbamazepine Case Study: Amylyx (NASDAQ: AMLX) Market Cap: ~ $1.4 B (September 2023) Drug: ALS Combination Therapy of generic + supplement Price: $158,000/ year Estimated 2026 Sales: $1.1B/ year 1 The FDA approved Amylyx’s drug, AMX0035 (Relyvrio 2 ), in September 2022 after Phase 2 clinical trial attenuated diseases progression (ALSFRSr) by 25% when compared to placebo 1 SVB Securities analyst Marc Goodman 2 Relyvrio is a registered trademark of Amylyx Intro PrimeC Summary Pre - clinical Phase 2a Results Phase 2b

NeuroSense Summary Novel combination therapy designed to address multiple disease targets in a synergistic manner Promising efficacy and biomarker results with an excellent safety profile observed from 12 - month ALS Phase 2a clinical trial Top - line results from ALS Phase 2b placebo - controlled clinical trial expected in Q4 2023 Initiation of phase 2 Alzheimer’s study expected in 2H 2023 Existing relationship with pharma and fully funded through several near - term catalysts 24 Well - Positioned for Transformative Growth Summary Phase 2b Phase 2a Results Pre - clinical PrimeC Intro
Nasdaq: NRSN Q&A For more information: info@neurosense - tx.com
NeuroSense Therapeutics (NASDAQ:NRSN)
Historical Stock Chart
Von Apr 2024 bis Mai 2024

NeuroSense Therapeutics (NASDAQ:NRSN)
Historical Stock Chart
Von Mai 2023 bis Mai 2024
