Award-winning actor Ted Danson, who lives with plaque psoriasis,
teams up with Bristol Myers Squibb for the inspiring “SO, Have You
Found It?” campaign. This initiative spotlights the resilience of
around two million Americans with moderate to severe plaque
psoriasis, aiming to amplify their voices, underscoring the
strength of their inner vibe, the “it” factor that makes someone
uniquely them.i The campaign signifies a shift in dialogue from the
challenges associated with the condition to empowering individuals
to confidently navigate their plaque psoriasis in partnership with
their dermatologists.
This press release features multimedia. View
the full release here:
https://www.businesswire.com/news/home/20240315988620/en/
Actor Ted Danson and SOTYKTU patient
Emily both living with moderate to severe plaque psoriasis. (Photo:
Bristol Myers Squibb)
Expanding the “Found It” Conversation
“Navigating plaque psoriasis is both deeply personal and often
challenging for many patients,” acknowledged Carlos Dortrait,
senior vice president and general manager of U.S. Immunology and
Neuroscience at Bristol Myers Squibb. “‘SO, Have You Found It?’
builds on the empowering narrative of our ‘Found It’ consumer
campaign. It aims to address not just the visible skin symptoms,
but also the hidden burden of the condition. Our goal is to inspire
patients to talk with their dermatologists to explore treatment
options that work for them and to highlight their strength, their
‘it’ factor, as they navigate their condition."
Psoriasis is a chronic, systemic, immune-mediated disease that
most often manifests as plaque psoriasis.i Psoriasis doesn’t just
affect the skin — it starts within and can touch many aspects of
your life — from dating and work to travel and fashion. Many with
plaque psoriasis find themselves altering their lifestyles,
sometimes avoiding social situations and personal connections.
Recognizing Every Individual’s Journey
“Being part of ‘SO, Have You Found It?’ hits close to home for
me,” shared Ted Danson, the renowned actor known for his humor and
sincerity, and someone who lives with moderate to severe plaque
psoriasis. “This isn't just another role; it's a part of my life.
I've dealt with the ups and downs of plaque psoriasis for decades.
This campaign gives so many of us living with the condition a
platform to openly share our stories and feel empowered to
self-advocate and honor our identity.”
A new video featuring Ted Danson was released as part of the
“SO, Have You Found It?” campaign. The video focuses on the lived
experiences of those with moderate to severe plaque psoriasis,
blending real stories of triumph, resilience and self-advocacy,
complemented by Ted's signature subtle humor. Future episodes aim
to deepen the connection with viewers, offering a mix of genuine
perspectives and expert insights, and capturing the multifaceted
journey of living with moderate to severe plaque psoriasis.
Emily, who is living with the condition and was featured in the
premiere video, is being treated with SOTYKTU™ (deucravacitinib),
an FDA-approved, once-daily pill for adults with moderate to severe
plaque psoriasis. She shared, “The road to managing my plaque
psoriasis has been hard in many aspects of my life, like in certain
social situations and spending time everyday covering up my
plaques. It was because of the continuous conversations with my
dermatologist and advocating for my health, that I have a newfound
sense of self-acceptance and empowerment. Finding a treatment that
worked for me was such an important point in my life.”
Please see Important Safety Information for SOTYKTU below,
including serious and most common side effects.
About the “SO, Have You Found It?” Campaign
“SO, Have You Found It?” honors the resilience and individuality
of approximately two million Americans living with moderate to
severe plaque psoriasis. In partnership with Ted Danson, Bristol
Myers Squibb seeks to support and uplift the community by
amplifying their voices, showcasing their “it” factor, that inner
vibe that makes someone uniquely them. The initiative encourages
those with moderate to severe plaque psoriasis to openly discuss
their condition and treatment options with their dermatologist,
including whether a systemic oral treatment, like SOTYKTU, may be
right for them. Learn more at SoHaveYouFoundIt.com.
About SOTYKTU™ (deucravacitinib)
SOTYKTU™ (deucravacitinib) is a prescription medicine used to
treat adults with moderate to severe plaque psoriasis who may
benefit from taking pills or injections (systemic therapy) or
treatment using ultraviolet or UV light (phototherapy). It is not
known if SOTYKTU is safe or effective in children under 18 years of
age. As the only once-daily pill of its kind, SOTYKTU selectively
targets and inhibits TYK2, a molecule which plays a key role in
passing signals in plaque psoriasis. It is not currently known how
blocking TYK2 signals works to reduce plaque psoriasis symptoms. Of
the 1684 adults in the studies, 841 received SOTYKTU, 421 received
placebo, and 422 received Otezla® (apremilast). In one study, half
of the participants receiving SOTYKTU achieved clear or almost
clear skin with 16 weeks of treatment compared to 9% of those on
placebo. Furthermore, more than half (53%) of SOTYKTU users
experienced 75% clearer skin compared to 9% of those on placebo. In
the same study, 32% of people taking SOTYKTU saw 90% clearer skin
vs 20% taking the leading alternative pill, Otezla.
SOTYKTU may cause serious side effects, including severe
allergic reactions. Patients are advised to seek immediate medical
help if they exhibit symptoms of a serious allergic reaction: feel
faint; swelling of your face, eyelids, lips, mouth, tongue or
throat; trouble breathing or throat tightness; chest tightness;
skin rash, hives. The most common side effects of SOTYKTU include
common cold, sore throat and sinus infection, cold sores, canker
sores on inner lips, gums, tongue, or roof of the mouth, inflamed
hair pores and acne. These are not all of the possible side effects
of SOTYKTU. See additional Important Safety Information below. For
complete safety information and detailed prescribing guidelines,
the U.S. Full Prescribing Information and Medication Guide for
SOTYKTU are available.
About Ted Danson
Ted Danson, acclaimed actor and comedian, known for his roles in
Cheers and The Good Place, lends his voice to the “SO, Have You
Found It?” campaign as an advocate for those living with plaque
psoriasis. Through his involvement, Ted aims to inspire
conversations between dermatologists and patients, and celebrate
the unique journeys of individuals managing plaque psoriasis. Ted
is not taking SOTYKTU.
About Plaque Psoriasis
Psoriasis is a widely prevalent, chronic, systemic
immune-mediated disease that substantially impairs patients’
physical health, quality of life and work productivity.ii Psoriasis
is a serious global problem, with at least 100 million people
worldwide impacted by some form of the disease,iii including
approximately 7.5 million people in the U.S.i Nearly one-quarter of
people with psoriasis have cases that are considered moderate to
severe.i Up to 90 percent of patients with psoriasis have psoriasis
vulgaris, or plaque psoriasis,iv which is characterized by distinct
round or oval plaques typically covered by silvery-white
scales.v
INDICATION AND IMPORTANT SAFETY INFORMATION
INDICATION
SOTYKTU™ (deucravacitinib) is a prescription medicine used to
treat adults with moderate-to-severe plaque psoriasis who may
benefit from taking injections or pills (systemic therapy) or
treatment using ultraviolet or UV light (phototherapy).
It is not known if SOTYKTU is safe and effective in children
under 18 years of age.
IMPORTANT SAFETY INFORMATION about
SOTYKTU (deucravacitinib)
SOTYKTU may cause serious side effects, including:
Serious allergic reactions. Stop taking SOTYKTU and get
emergency medical help right away if you develop any of the
following symptoms of a serious allergic reaction:
- feel faint
- swelling of your face, eyelids, lips, mouth, tongue, or
throat
- trouble breathing or throat tightness
- chest tightness
- skin rash, hives
Infections. SOTYKTU is a medicine that affects your
immune system. SOTYKTU can lower the ability of your immune system
to fight infections and can increase your risk of infections. Some
people have had serious infections while taking SOTYKTU, such as
infections of the lungs, including pneumonia and tuberculosis (TB),
and COVID-19.
- Your healthcare provider should check you for infections and TB
before starting treatment with SOTYKTU and watch you closely for
signs and symptoms of TB during SOTYKTU treatment.
- You may be treated for TB before you begin SOTYKTU treatment if
you have a history of TB or have active TB.
- If you get a serious infection, your healthcare provider may
tell you to stop taking SOTYKTU until your infection is
controlled.
SOTYKTU should not be used in people with an active, serious
infection, including localized infections. You should not start
taking SOTYKTU if you have any kind of infection unless your
healthcare provider tells you it is okay.
You may be at a higher risk of developing shingles (herpes
zoster).
Before starting SOTYKTU, tell your healthcare provider if
you:
- are being treated for an infection, or have had an infection
that does not go away or keeps coming back
- have TB or have been in close contact with someone with TB
- have or have had hepatitis B or C
- think you have an infection or have symptoms of an infection
such as:
- fever, sweats, or chills
- muscle aches
- weight loss
- cough
- shortness of breath
- blood in your phlegm (mucus)
- warm, red, or painful skin or sores on your body different from
your psoriasis
- diarrhea or stomach pain
- burning when you urinate or urinating more often than
normal
- feeling very tired
After you start taking SOTYKTU, call your healthcare provider
right away if you have an infection or have symptoms of an
infection.
SOTYKTU can make you more likely to get infections or make any
infections you have worse.
Cancer. Certain kinds of cancer including lymphoma have
been reported in people taking SOTYKTU. Tell your healthcare
provider if you have ever had any type of cancer.
Muscle problems (rhabdomyolysis). SOTYKTU can cause
muscle problems that can be severe. Treatment with SOTYKTU may
increase the level of an enzyme in your blood called creatine
phosphokinase (CPK) and can be a sign of muscle damage. Increased
CPK is common in people taking SOTYKTU. Your healthcare provider
may tell you to stop taking SOTYKTU if the amount of CPK in your
blood gets too high or if you have signs and symptoms of severe
muscle problems. Tell your healthcare provider right away if you
have any of these signs or symptoms of severe muscle problems:
unexplained muscle pain, tenderness, or weakness, feeling very
tired, fever, or dark-colored urine.
Do not take SOTYKTU if you are allergic to
deucravacitinib or any of the ingredients in SOTYKTU.
Before taking SOTYKTU, tell your healthcare provider about
all of your medical conditions, including if you: have liver
problems or kidney problems, have high levels of fat in your blood
(triglycerides), or have recently received or are scheduled to
receive an immunization (vaccine), as you should avoid receiving
live vaccines during treatment with SOTYKTU.
Tell your healthcare provider if you are pregnant, plan to
become pregnant, or if you are breastfeeding or plan to
breastfeed. It is not known if SOTYKTU can harm your unborn
baby or if SOTYKTU passes into your breast milk.
- Report pregnancies to the Bristol Myers Squibb Company’s
Adverse Event reporting line at 1-800-721-5072.
Tell your healthcare provider about all the medicines you
take, including prescription medicines, over-the-counter
medicines, vitamins, and herbal supplements. Keep a list of them to
show your healthcare provider and pharmacist when you get a new
medicine.
Take SOTYKTU exactly as your healthcare provider tells you to
take it. Take SOTYKTU 1 time every day, with or without food.
Do not crush, cut, or chew the SOTYKTU tablets.
SOTYKTU may cause serious side effects, including:
- Changes in certain laboratory test results. Changes in
laboratory tests have happened in some people taking SOTYKTU. Your
healthcare provider may do blood tests before you start taking
SOTYKTU and during treatment with SOTYKTU to check for the
following:
- Increased triglycerides. Too much fat in your blood can
cause problems with your heart.
- Increased liver enzymes. If your liver enzymes increase
too much, your healthcare provider may need to do additional tests
on your liver and may tell you to stop taking SOTYKTU if they think
that SOTYKTU is harming your liver.
- Potential risks from Janus kinase (JAK) inhibition.
SOTYKTU is a tyrosine kinase 2 (TYK2) inhibitor. TYK2 is in the JAK
family. It is not known whether taking SOTYKTU has the same risks
as taking JAK inhibitors. Increased risk of death (all causes) has
happened in people who were 50 years of age and older with at least
1 heart disease (cardiovascular) risk factor who were taking a JAK
inhibitor used to treat rheumatoid arthritis (RA) compared to
people taking another medicine in a class of medicines called TNF
blockers. SOTYKTU is not for use in people with RA.
The most common side effects of SOTYKTU include: common
cold, sore throat and sinus infection (upper respiratory
infections), cold sores (herpes simplex), sores on inner lips,
gums, tongue, or roof of the mouth (canker sores), inflamed hair
pores (folliculitis) and acne.
These are not all of the possible side effects of SOTYKTU.
Call your doctor for medical advice about side effects. You may
report side effects to FDA at 1-800-FDA-1088.
Please see the U.S. Full Prescribing Information and Medication
Guide for SOTYKTU.
About Bristol Myers Squibb
Bristol Myers Squibb is a global biopharmaceutical company whose
mission is to discover, develop and deliver innovative medicines
that help patients prevail over serious diseases. For more
information about Bristol Myers Squibb, visit us at BMS.com or
follow us on LinkedIn, Twitter, YouTube, Facebook and
Instagram.
corporatefinancial-news
i Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence
in adults in the United States. JAMA Dermatol. Published online
June 30, 2021. doi:10.1001/jamadermatol.2021.2007. ii Armstrong AW,
Schupp C, Wu J, Bebo B. Quality of life and work productivity
impairment among psoriasis patients: findings from the National
Psoriasis Foundation survey data 2003–2011. PloS One.
2012;7(12):e52935. iiI World Health Organization. Global report on
psoriasis. 2016. Accessed May 12, 2022.
https://apps.who.int/iris/bitstream/handle/10665/204417/9789241565189_eng.pdf.psoriasis?sequence=1
iv Menter A, Gottlieb A, Feldman SR, Van Voorhees AS et al.
Guidelines of care for the management of psoriasis and psoriatic
arthritis: Section 1. Overview of psoriasis and guidelines of care
for the treatment of psoriasis with biologics. J Am Acad Dermatol.
2008 May;58(5):826-50. v Nair PA, Badri T. Psoriasis. [Updated 2023
Apr 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls
Publishing; 2024 Jan-. Available from:
https://www.ncbi.nlm.nih.gov/books/NBK448194/
View source
version on businesswire.com: https://www.businesswire.com/news/home/20240315988620/en/
Bristol Myers Squibb Media Inquiries:
media@bms.com Investors: investor.relations@bms.com
Bristol Myers Squibb (NYSE:BMY)
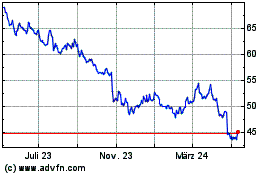
Historical Stock Chart
Von Jan 2025 bis Feb 2025
Bristol Myers Squibb (NYSE:BMY)
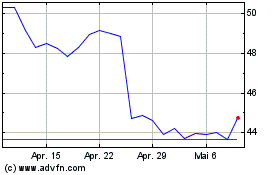
Historical Stock Chart
Von Feb 2024 bis Feb 2025