UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
Report of Foreign Private Issuer Pursuant to Rule
13a-16 or 15d-16
Under the Securities Exchange Act of 1934
For the Month of January 2024
Commission File Number: 001-37353
SCINAI IMMUNOTHERAPEUTICS LTD.
(Translation of registrant’s name into English)
Jerusalem BioPark, 2nd Floor
Hadassah Ein Kerem Campus
Jerusalem, Israel
(Address of principal executive office)
Indicate by check mark whether the registrant files
or will file annual reports under cover Form 20-F or Form 40-F.
Form 20-F ☒
Form 40-F ☐
Explanatory Note
Scinai Immunotherapeutics Ltd. (the
“Company”) has made available an updated presentation about its business, a copy of which is furnished
herewith as Exhibit 99.1 and incorporated by reference. The new updates in the presentation are not an admission as to the
materiality of any information therein. The information contained in the presentation is summary information that should be
considered in the context of the Company’s filings with the Securities and Exchange Commission and other public announcements
the Company may make by press release or otherwise from time to time.
Exhibit Index
SIGNATURES
Pursuant to the requirements
of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto
duly authorized.
| |
Scinai Immunotherapeutics Ltd. |
| |
|
|
| Date: January 18, 2024 |
By: |
/s/ Amir Reichman |
| |
|
Amir Reichman |
| |
|
Chief Executive Officer |
3
Exhibit
99.1

CLICK TO EDIT MASTER TITLE STYLE CORPORATE PRESENTATION | JANUARY 2024 | NASDAQ: SCNI

CLICK TO EDIT MASTER TITLE STYLE 2 SAFE HARBOR STATEMENT This communication contains forward - looking statements within the meaning of the Private Litigation Reform Act of 1995 . Words such as “expect,” “believe,” “intend,” “plan,” “continue,” “may,” “will,” “anticipate,” and similar expressions are intended to identify such forward - looking statements . All statements, other than statements of historical facts, included in this communication regarding strategy, future operations, future financial position, future revenue, projected expenses, prospects, plans and objectives of the management of Scinai Immunotherapeutics Ltd . ("Scinai") are forward - looking statements . Examples of such statements include, but are not limited to, statements regarding the therapeutic and commercial potential of nanosized antibodies (NanoAbs) ; the pipeline market potential ; and the timing of NanoAb proof - of - concept studies and clinical trials . These forward - looking statements reflect management’s current views with respect to certain current and future events and are subject to various risks, uncertainties and assumptions that could cause results to differ materially from those expressed or implied by such forward - looking statements . Such risks and uncertainties include, but are not limited to, those related to : the possibility that the therapeutic and commercial potential of NanoAbs will not be met ; potential changes in the pipeline market potential ; a delay in the preclinical and clinical data for NanoAbs, if any ; Scinai’s ability to secure additional capital on attractive terms, if at all ; Scinai’s ability to acquire rights to additional product opportunities ; Scinai’s ability to enter into collaborations on terms acceptable to Scinai or at all ; timing of receipt of regulatory approval of Scinai’s manufacturing facility in Jerusalem, if at all or when required ; the manufacturing facility will not be able to be used for a wide variety of applications and other pharmaceutical technologies ; and those inherent in drug development, which involves a lengthy and expensive process with uncertain outcomes . More detailed information about such risks and uncertainties can be found in the Company's filings with the Securities and Exchange Commission (the "SEC"), including those set forth in the section entitled “Risk Factors” in the Company's Annual Report on Form 10 - K filed with the SEC on April 17 , 2023 . Scinai undertakes no obligation to revise or update any forward - looking statement .
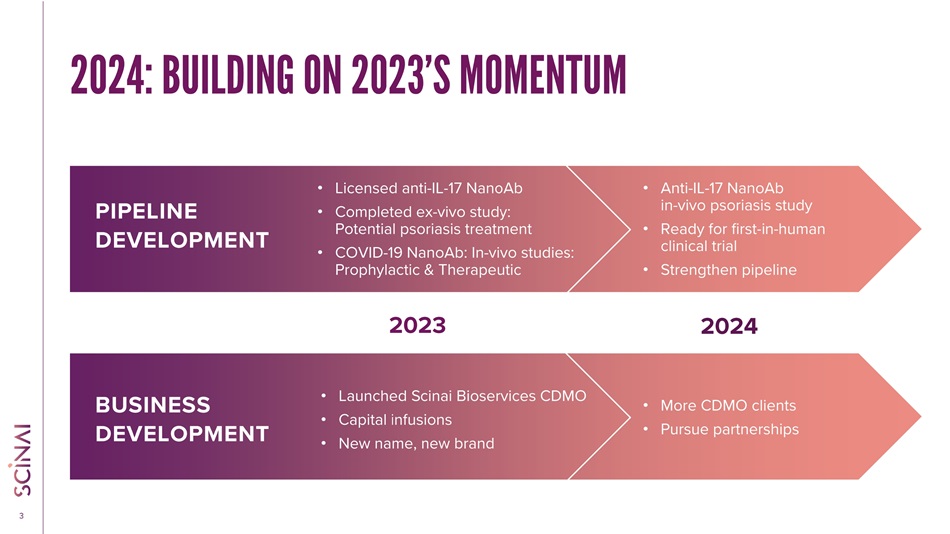
CLICK TO EDIT MASTER TITLE STYLE 3 2024: BUILDING ON 2023’S MOMENTUM 2023 2024 BUSINESS DEVELOPMENT • Launched Scinai Bioservices CDMO • Capital infusions • New name, new brand • More CDMO clients • Pursue partnerships PIPELINE DEVELOPMENT • Licensed anti - IL - 17 NanoAb • Completed ex - vivo study: Potential psoriasis treatment • COVID - 19 NanoAb: In - vivo studies: Prophylactic & Therapeutic • Anti - IL - 17 NanoAb in - vivo psoriasis study • Ready for first - in - human clinical trial • Strengthen pipeline

CLICK TO EDIT MASTER TITLE STYLE 4 TWO COMPLEMENTARY BUSINESS UNITS End - to - end boutique CDMO services to help bring products to market by leveraging Scinai’s GMP and non - GMP drug development and manufacturing capabilities Development of inflammation and immunology (I&I) biological therapeutic products beginning with pipeline of nanosized VHH antibodies (NanoAbs) targeting diseases with large unmet medical needs
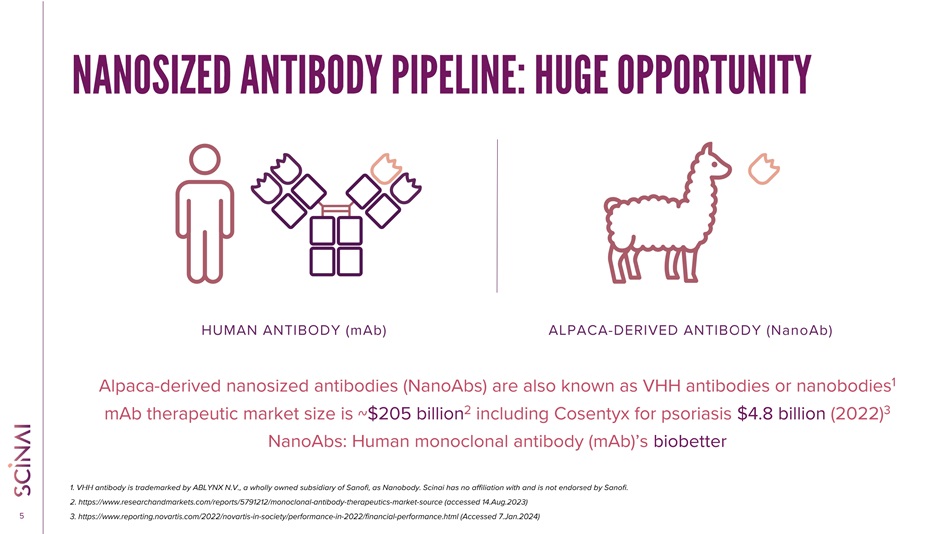
CLICK TO EDIT MASTER TITLE STYLE 5 Alpaca - derived nanosized antibodies (NanoAbs) are also known as VHH antibodies or nanobodies 1 mAb therapeutic market size is ~ $205 billion 2 including Cosentyx for psoriasis $4.8 billion (2022) 3 NanoAbs: Human monoclonal antibody (mAb)’s biobetter 1. VHH antibody is trademarked by ABLYNX N.V., a wholly owned subsidiary of Sanofi, as Nanobody. Scinai has no affiliation wi th and is not endorsed by Sanofi. 2. https://www.researchandmarkets.com/reports/5791212/monoclonal - antibody - therapeutics - market - source (accessed 14.Aug.2023) 3. https://www.reporting.novartis.com/2022/novartis - in - society/performance - in - 2022/financial - performance.html (Accessed 7.Jan.20 24) HUMAN ANTIBODY (mAb) ALPACA - DERIVED ANTIBODY (NanoAb) NANOSIZED ANTIBOD Y PIPELINE: HUGE OPPORTUNITY

CLICK TO EDIT MASTER TITLE STYLE 6 The Max Planck Institute & UMG 1 bring… • Recombinant protein drug development experience from lab to Phase 3 clinical trial • Manufacturing, quality, international regulatory experience • GMP biologics manufacturing facility • Best - in - class equipped labs • Top - tier big pharma & biotech leadership expertise • World - class science & access to leading scientists • NanoAb platform for the development of promising potent therapeutics • Patents covering NanoAbs & their manufacturing Professor Dr Dirk Görlich Director of Max Planck Institute for Multidisciplinary Sciences Winner of inaugural World Laureates Association (WLA) Prize in Life Sciences or Medicine Professor Dr Matthias Dobbelstein Fellow at Max Planck Institute for Multidisciplinary Sciences UMG Head of Department Covering discovery and initial characterization of NanoAbs aimed at predefined list of molecular targets. Designed to create significant clinical and commercial advantages. Scinai brings… MAX PLANCK , UMG, SCINAI COLLABORATION 1. Max Planck Institute for Multidisciplinary Sciences and the University Medical Center Göttingen (UMG)

CLICK TO EDIT MASTER TITLE STYLE 7 NanoAbs’ unique physicochemical attributes can generate multiple crucial advantages vs human monoclonal antibodies (mAbs) Manufacturing • 10 - times more active pharmaceutical ingredients (API) per gram of manufactured protein vs. mAbs • Faster and lower cost production in yeast (pichia) vs mammalian cells • Quicker antibody discovery and optimization due to massive libraries • De - risked pipeline development leveraging approved mAb targets • Hyper - thermostable = longer shelf life, easier storage & distribution • Superior specificity & affinity to target potentially enables lower dose, fewer adverse events, lower cost • Adaptable half life • Multiple, easier routes of administration • Lower immunogenicity • Fewer contraindications • Potentially safer & lower dose R&D Product Patient Safety & Convenience PLATFORM VALUE PROPOSITION
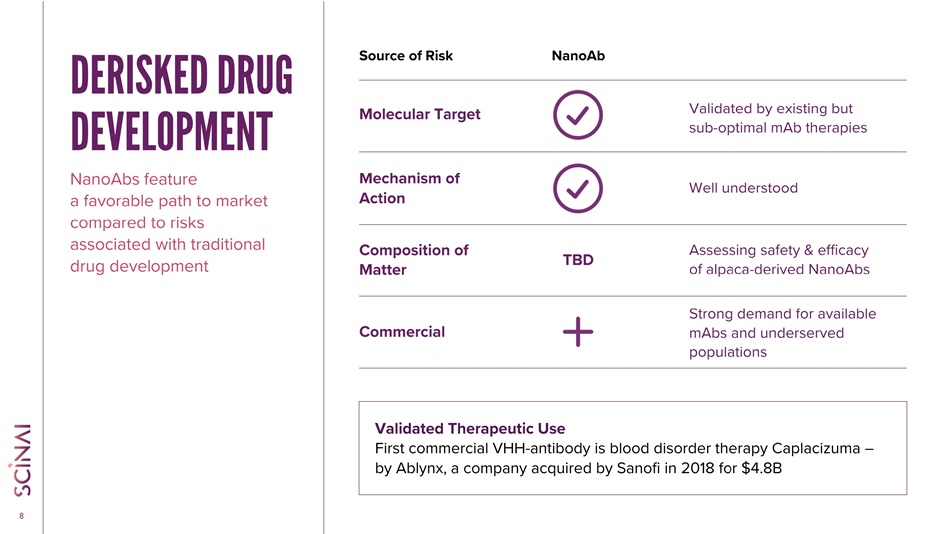
CLICK TO EDIT MASTER TITLE STYLE 8 Source of Risk Molecular Target Mechanism of Action Composition of Matter TBD Commercial Validated by existing but sub - optimal mAb therapies Well understood Assessing safety & efficacy of alpaca - derived NanoAbs Strong demand for available mAbs and underserved populations NanoAb Validated Therapeutic Use First commercial VHH - antibody is blood disorder therapy Caplacizuma – by Ablynx, a company acquired by Sanofi in 2018 for $4.8B DERISKED DRUG DEVELOPMENT NanoAbs feature a favorable path to market compared to risks associated with traditional drug development

CLICK TO EDIT MASTER TITLE STYLE 9 SUPERIOR ROUTES OF ADMINISTRATION P aper covers several aspects of Scinai’s anti - COVID - 19 NanoAbs, including: • S tructure • Mechanism of action • Neutralization of a wide range of SARS - CoV - 2 variants including Omicron • Production in yeast • Formulation into aerosols Describes in vivo studies indicating that “ exposing hamsters to these aerosols, before or even 24 h after infection with SARS - CoV - 2, significantly reduced virus load, weight loss and pathogenicity,” concluding that these results show the significant potential of aerosolized NanoAbs for the prevention and treatment of coronavirus infections. Proof - of - concept: Aerosolized NanoAbs Antiviral Research. January 2024. https://doi.org/10.1016/j.antiviral.2023.105778
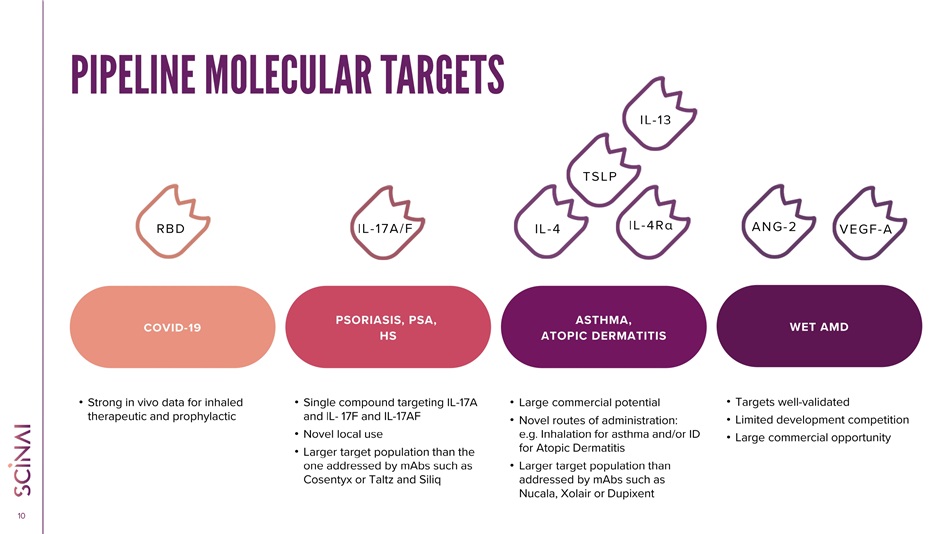
CLICK TO EDIT MASTER TITLE STYLE 10 RBD IL - 4R α ANG - 2 IL - 17A/F • Strong in vivo data for inhaled therapeutic and prophylactic • Single compound targeting IL - 17A and IL - 17F and IL - 17AF • Novel local use • Larger target population than the one addressed by mAbs such as Cosentyx or Taltz and Siliq • Large commercial potential • Novel routes of administration: e.g. Inhalation for asthma and/or ID for Atopic Dermatitis • Larger target population than addressed by mAbs such as Nucala, Xolair or Dupixent • Targets well - validated • Limited development competition • Large commercial opportunity COVID - 19 PSORIASIS, PSA, HS ASTHMA, ATOPIC DERMATITIS WET AMD PIPELINE MOLECULAR TARGETS IL - 4 TSLP VEGF - A IL - 13

CLICK TO EDIT MASTER TITLE STYLE 11 Psoriasis $17.4B Macular Degeneration (AMD) $6.9B Psoriatic arthritis $8.1B $10.4B Asthma Autoimmune Market Sizes • Validated targets of existing mAb treatments • Short time to value generation, lower risk than mAbs • Large markets growing at attractive CAGRs PIPELINE ADDRESSING LARGE MARKETS WITH UNDERSERVED NEEDS • Common diseases (e.g. COVID - 19, Influenza) • Platform potential for response to emerging pandemic pathogens Atopic Dermatitis $9.2B Source: GlobalData, 7 major markets (US, 5EU, Japan) 2023 estimates Respiratory Infectious Diseases
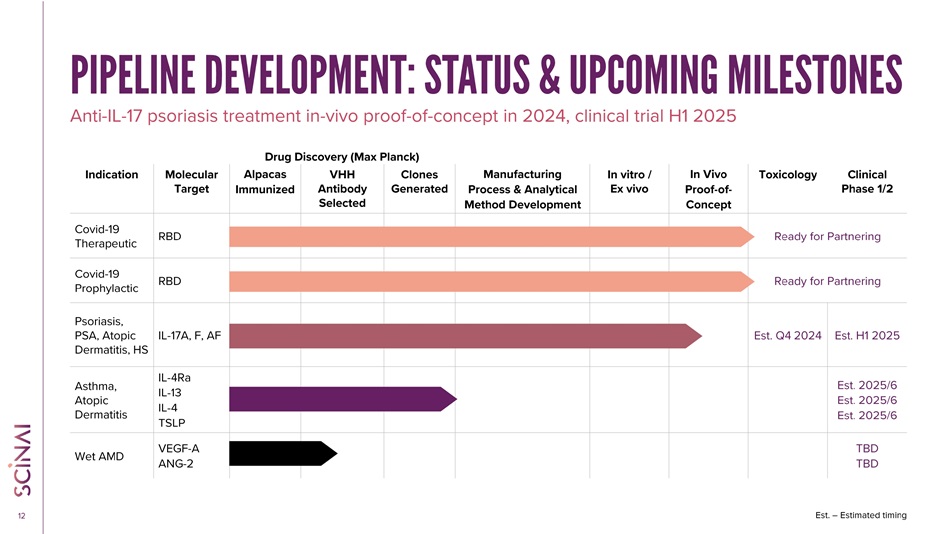
CLICK TO EDIT MASTER TITLE STYLE 12 PIPELINE DEVELOPMENT: STATUS & UPCOMING MILESTONES Anti - IL - 17 psoriasis treatment in - vivo proof - of - concept in 2024, clinical trial H1 2025 Clinical Phase 1/2 Toxicology In Vivo Proof - of - Concept In vitro / Ex vivo Manufacturing Process & Analytical Method Development Clones Generated VHH Antibody Selected Alpacas Immunized Molecular Target Indication Ready for Partnering RBD Covid - 19 Therapeutic Ready for Partnering RBD Covid - 19 Prophylactic Est. H1 2025 Est. Q4 2024 IL - 17A, F, AF Psoriasis, PSA, Atopic Dermatitis, HS Est. 2025/6 Est. 2025/6 Est. 2025/6 IL - 4Ra IL - 13 IL - 4 TSLP Asthma, Atopic Dermatitis TBD TBD VEGF - A ANG - 2 Wet AMD Drug Discovery (Max Planck) Est. – Estimated timing

CLICK TO EDIT MASTER TITLE STYLE 13 R&D STRATEGIC GUIDING PRINCIPLES • Inflammation and Immunology • Platform: NanoAbs • Research Collaboration Agreement (RCA) with MPG/UMG • CMC activities done in - house using Scinai’s CDMO business unit • Leverage learnings, Route of Administration experience • Partner with multinational pharma companies • Commercial manufacturing

CLICK TO EDIT MASTER TITLE STYLE 14 PSORIARIS: 78% UNDERSERVED POPULATION Psoriasis prevalence and severity SEVERE MODERATE MILD 22% of patients. 28% of patients. 50% of patients. >10% of the body Typically has a severe impact on quality of life 3 - 10% of the body Generally affects quality of life <3% of the body Typically occasional effect on quality of life e.g. aesthetic discomfort Sources: Canadian Psoriasis Network; National Psoriasis Foundation; https://link.springer.com/article/10.1007/s13555 - 021 - 00518 - 8 • 125 million patients, including 15.7 million in the 7 major markets (US, EU5 and Japan); 80 - 90% is plaque psoriasis • Current biologica l therapies targeted only to moderate & severe patients, administered systemically • Mild patients may suffer from considerable and visible lesions which may be uncomfortable, painful, and impact social and men tal well - being • Mild patients are ineligible for biological treatments; and moderate psoriatic patients are often reluctant to receive system ic biological treatments due to side effects and costs Mild to moderate patients underserved by current treatments
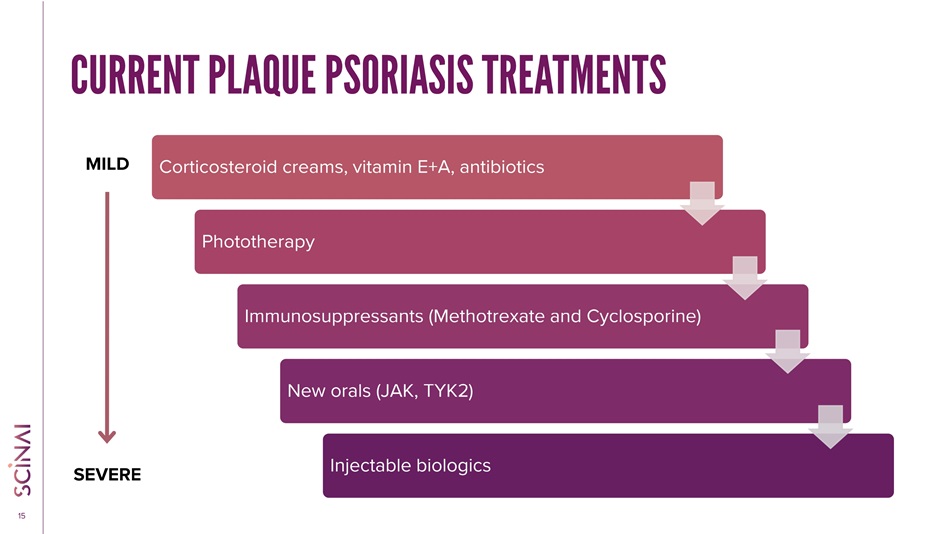
CLICK TO EDIT MASTER TITLE STYLE 15 CURRENT PLAQUE PSORIASIS TREATMENTS Corticosteroid creams, vitamin E+A, antibiotics Phototherapy Immunosuppressants (Methotrexate and Cyclosporine) New orals (JAK, TYK2) Injectable biologics MILD SEVERE
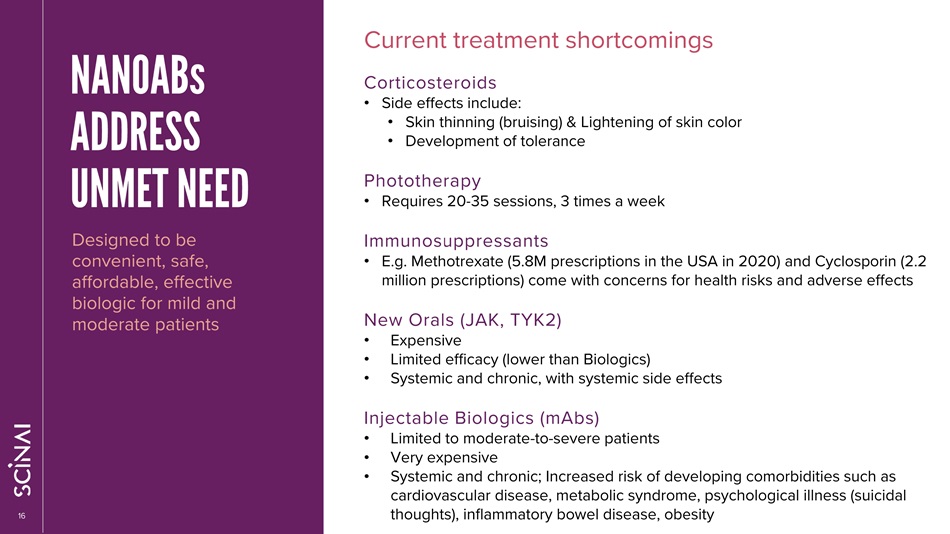
CLICK TO EDIT MASTER TITLE STYLE 16 NANOABs ADDRESS UNMET NEED Designed to be convenient, safe, affordable, effective biologic for mild and moderate patients Current treatment shortcomings Corticosteroids • Side effects include: • Skin thinning (bruising) & Lightening of skin color • Development of tolerance Phototherapy • Requires 20 - 35 sessions, 3 times a week Immunosuppressants • E.g. Methotrexate (5.8M prescriptions in the USA in 2020) and Cyclosporin (2.2 million prescriptions) come with concerns for health risks and adverse effects New Orals (JAK, TYK2) • Expensive • Limited efficacy (lower than Biologics) • Systemic and chronic, with systemic side effects Injectable Biologics (mAbs) • Limited to moderate - to - severe patients • Very expensive • Systemic and chronic; Increased risk of developing comorbidities such as cardiovascular disease, metabolic syndrome, psychological illness (suicidal thoughts), inflammatory bowel disease, obesity
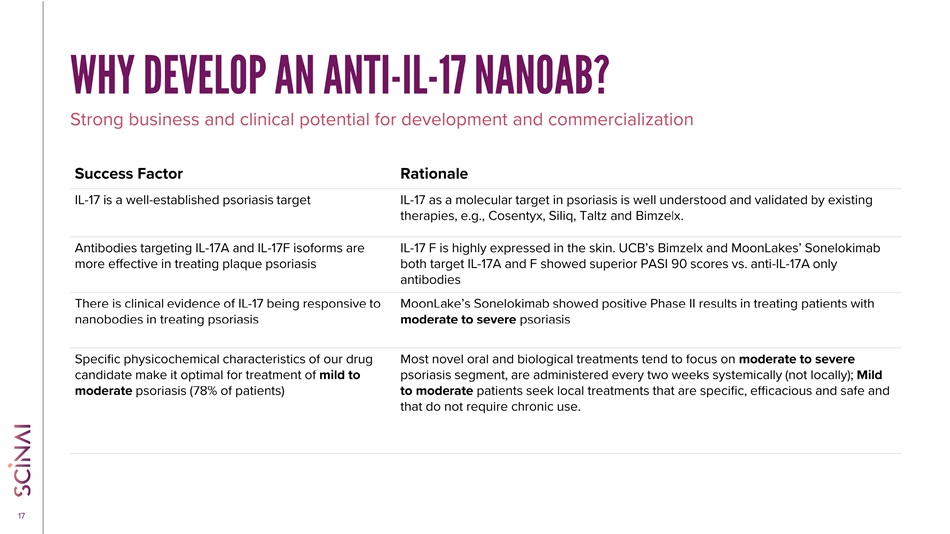
CLICK TO EDIT MASTER TITLE STYLE 17 WHY DEVELOP AN ANTI - IL - 17 NANOAB? Rationale Success Factor IL - 17 as a molecular target in psoriasis is well understood and validated by existing therapies, e.g., Cosentyx, Siliq, Taltz and Bimzelx . IL - 17 is a well - established psoriasis target IL - 17 F is highly expressed in the skin. UCB’s Bimzelx and MoonLakes’ Sonelokimab both target IL - 17A and F showed superior PASI 90 scores vs. anti - IL - 17A only antibodies Antibodies targeting IL - 17A and IL - 17F isoforms are more effective in treating plaque psoriasis MoonLake’s Sonelokimab showed positive Phase II results in treating patients with moderate to severe psoriasis There is clinical evidence of IL - 17 being responsive to nanobodies in treating psoriasis Most novel oral and biological treatments tend to focus on moderate to severe psoriasis segment , are administered every two weeks systemically (not locally); Mild to moderate patients seek local treatments that are specific, efficacious and safe and that do not require chronic use. Specific physicochemical characteristics of our drug candidate make it optimal for treatment of mild to moderate psoriasis (78% of patients) Strong business and clinical potential for development and commercialization
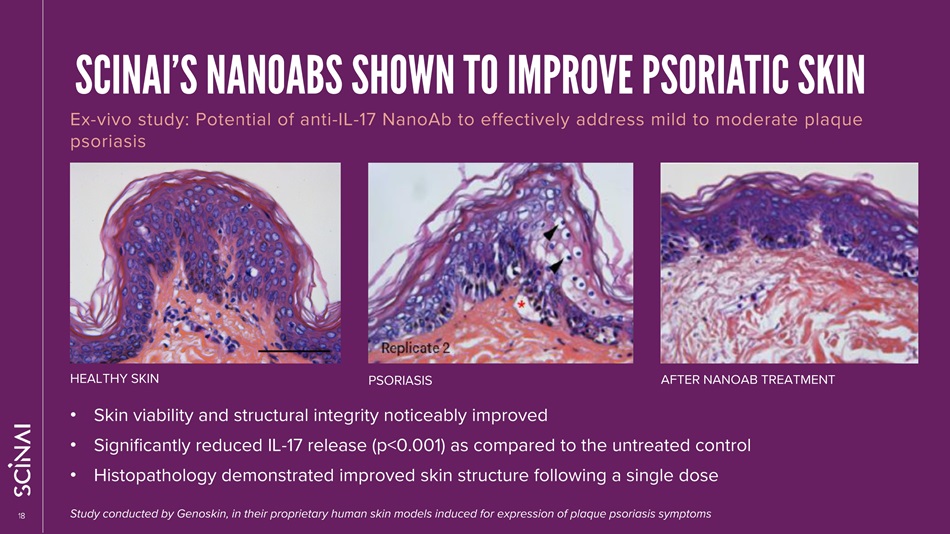
CLICK TO EDIT MASTER TITLE STYLE 18 SCINAI’S NANOABS SHOWN TO IMPROVE PSORIATIC SKIN Ex - vivo study: Potential of anti - IL - 17 NanoAb to effectively address mild to moderate plaque psoriasis • Skin viability and structural integrity noticeably improved • Significantly reduced IL - 17 release (p<0.001) as compared to the untreated control • Histopathology demonstrated improved skin structure following a single dose HEALTHY SKIN PSORIASIS AFTER NANOAB TREATMENT Study conducted by Genoskin, in their proprietary human skin models induced for expression of plaque psoriasis symptoms

CLICK TO EDIT MASTER TITLE STYLE 19 EX - VIVO PROOF OF CONCEPT: NANOABS SHOWN TO BLOCK IL - 17 Similar impact as current leading treatments Betamethasone and Cosentyx Designed to be safer, more convenient *** = p<0.001 **** = p<0.0001 ** = p<0.01 **** *** **** *** Healthy skin Betameth - asone Cosentyx Scinai’s anti - IL - 17 NanoAb (Targeting underserved 78% of mild to moderate patients) IL - 17 Concentration (pg/mL) Untreated psoriatic skin Current standards of care (Approved only for moderate to severe psoriasis) 1 dose 3 doses ** 0 50 100 150

CLICK TO EDIT MASTER TITLE STYLE 20 SCINAI’S ANTI - IL - 17 NANOAB THERAPEUTIC POTENTIAL Scinai’s NanoAbs displayed “potential as anti - inflammatory agents in psoriasis, particularly in improving skin viability and structure.” Genoskin study results indicated that just a single dose “might have been potent enough to block all IL - 17, effectively 'shutting down' the immediate flare - up. This immediate response suggests a direct and effective inhibition of the existing IL - 17 cytokines.” Professor Amos Gilhar, dermatologist Technion Israel Institute of Technology Head of Skin Research Laboratory Professor Gilhar was not involved in this ex - vivo study but has been contracted by Scinai to conduct an in - vivo study

CLICK TO EDIT MASTER TITLE STYLE 21 PSORIASIS IN - VIVO STUDY: RESULTS IN Q2’24 What is being studied? Impact of psoriasis treatments on in - vivo xenograft mouse model of human skin. Why is it important? Genoskin’s ex - vivo study showed Scinai’s anti - IL - 17 NanoAb reduces IL - 17 and improves skin appearance. This study will help measure duration of treatment effectiveness compared to current standards of care and provide additional safety information.
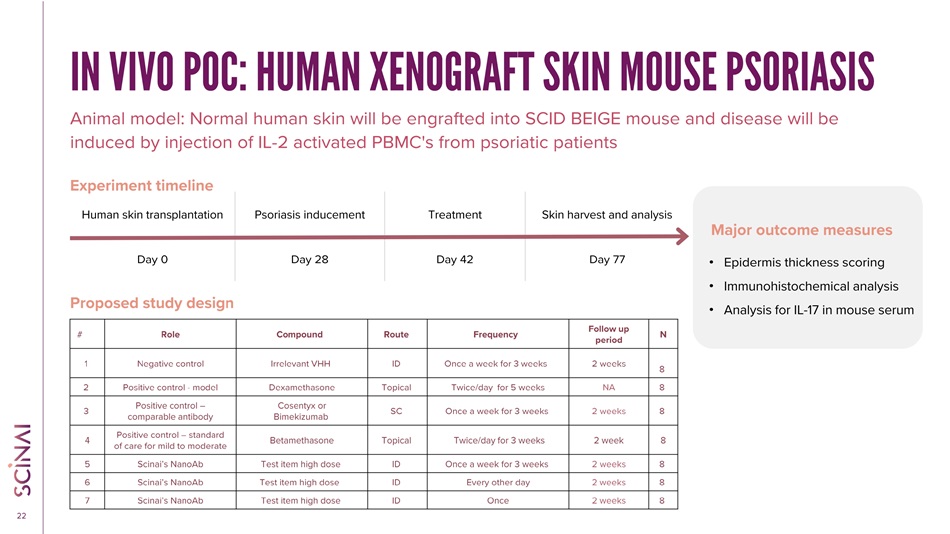
CLICK TO EDIT MASTER TITLE STYLE 22 IN VIVO POC: HUMAN XENOGRAFT SKIN MOUSE PSORIASIS Animal model: Normal human skin will be engrafted into SCID BEIGE mouse and disease will be induced by injection of IL - 2 activated PBMC's from psoriatic patients Proposed study design Major outcome measures Skin harvest and analysis Treatment Psoriasis inducement Human skin transplantation Day 77 Day 42 Day 28 Day 0 Experiment timeline • Epidermis thickness scoring • Immunohistochemical analysis • Analysis for IL - 17 in mouse serum N Follow up period Frequency Route Compound Role # 8 2 weeks Once a week for 3 weeks ID Irrelevant VHH Negative control 1 8 NA Twice/day for 5 weeks Topical Dexamethasone Positive control - model 2 8 2 weeks Once a week for 3 weeks SC Cosentyx or Bimekizumab Positive control – comparable antibody 3 8 2 week Twice/day for 3 weeks Topical Betamethasone Positive control – standard of care for mild to moderate 4 8 2 weeks Once a week for 3 weeks ID Test item high dose Scinai’s NanoAb 5 8 2 weeks Every other day ID Test item high dose Scinai’s NanoAb 6 8 2 weeks Once ID Test item high dose Scinai’s NanoAb 7
CLICK TO EDIT MASTER TITLE STYLE 23 IP STATUS ANTI IL - 17 NANOAB Status • Priority patent application: Filed Dec. 28, 2022 • International patent application (PCT): Filed December 27, 2023 Covers • The patent application encompasses novel VHH antibodies directed against IL - 17 isomers and their use for therapeutic and diagnostic applications. The VHH antibodies, characterized by specific sequences, can block the IL - 17A and - F that are on the critical path for Psoriasis and other diseases. Exclusive license • Scinai has exclusive license from the Max Planck Society for worldwide development and commercialization.

CLICK TO EDIT MASTER TITLE STYLE 24 BOUTIQUE CDMO SERVICES De - risking Scinai’s internal R&D investments by leveraging internal capabilities ASEPTIC GMP MANUFACTURING SUITES STATE - OF - THE - ART R & D AND QC LABORATORIES PHARMA CMC EXPERIENCE

CLICK TO EDIT MASTER TITLE STYLE 25 GMP MANUFACTURING AND R&D LABS Industry standard aseptic facility: Labs, cleanroom, warehouses, offices • Analytical methods development combined with best - in - class QC capabilities and equipment • Labs for manufacturing process development and scale - up allow for the implementation of quality by design and design of experiment principles • cGMP suites for upstream fermentation, downstream purification, media and buffer preparations, formulation and aseptic automated filling of PFS & vials • Designed to meet FDA and EMA regulatory standards • Single - use equipment enables: • Adaptable manufacturing processes for a pipeline of different products • Quicker lead times • Faster time - to - market for new products Scinai’s 1850m 2 (20,000 sq.ft) cGMP Biologics Manufacturing Facility | Jerusalem

CLICK TO EDIT MASTER TITLE STYLE 26 FIRST TWO CDMO CLIENTS SIGNED Q4’23 OHAD LAVI, CEO VOYAGER MEDICAL RESEARCH, LTD. We are excited about the collaboration between Voyager Medical Research and Scinai Bioservices . In our experience so far, Scinai has demonstrated a very high level of professionalism combined with attention to detail and a positive team spirit . We are looking forward to starting this part of our development with Scinai at our side .

CLICK TO EDIT MASTER TITLE STYLE 27 CDMO STRATEGIC GUIDING PRINCIPLES Scinai’s CDMO value proposition: Experienced and professional team available to execute drug development projects at high - speed while adhering to high (EU) quality standards using new and modern equipment located in a well - maintained site, offered at competitive pricing attractive to young biotech start - ups • Focus on serving Israel, Europe and USA • Target services: Early - stage biopharma drug development projects from preclinical studies to clinical phase 2 • Target customers: Early - stage biotech companies at pre - clinical stage

CLICK TO EDIT MASTER TITLE STYLE 28 CDMO EXPECTED CHALLENGES AND NEXT STEPS Challenges: • Cash flow • Capital for investments • Building a track record • Developing a service provider culture for the CDMO BU Focus areas: • Expand the business beyond Israel into the EU and the USA • Business development team, processes and systems • Marketing and sales • Operational excellence and service provider culture • CAPEX projects

CLICK TO EDIT MASTER TITLE STYLE 29 DEEP PHARMA EXPERIENCE & CAPABILITIES AMIR REICHMAN – CEO Senior global pharma leadership positions: Pharmaceutical engineering & supply chain at GSK Vaccines, Belgium; Large projects building vaccine manufacturing sites in Belgium, Italy, Germany, Hungary & US; NeuroDerm (R&D); Novartis Vaccines (Global Supply Chain). DR. TAMAR BEN - YEDIDIA – CSO Co - invented and guided vaccine candidate through 8 clinical trials including pivotal Phase 3. PhD from Department of Immunology, Weizmann Institute of Science . ELAD MARK – COO Led scale - up, tech transfer, manufacturing of recombinant proteins in China, mAbs for Novartis Singapore. Principal bioprocess engineer; Novartis (Technical Project Manager – Process). DR. DALIT WEINSTEIN - FISCHER – VP TECHNICAL R&D Leadership roles at Merck kGaA Israel. Directed Biological Processes at NanoSpun Technologies Ltd. and CTO at VAYU Sense AG, specializing in improving bio - based fermentation processes with an AI - based controller. Led the Natural Biotechnology Systems Department at Sigma Aldrich. PhD Molecular Genetics and Microbiology. 30 STAFF MEMBERS • 5 PhDs • Manufacturing, engineering, technical R&D, upstream & downstream process development, QC, QA, clinical and non - clinical, procurement • Outsourced finance, legal, regulatory
CLICK TO EDIT MASTER TITLE STYLE 30 Mark Germain, Chairman Aentib Group (Managing Director). Founder, director, chairman, and/or investor in over 20 biotech companies including Alexion, Incyte, Neurocrine, Ariad, ChromaDex. Amir Reichman, CEO NeuroDerm Ltd (Senior Scientist), Novartis Vaccines USA (R&D and Global Supply chain), GSK Vaccines Belgium (Global Supply Chain and Global Engineering) Adi Raviv, External Director Experienced in Wall Street investment banking; Capacity Funding LLC (Principal) Samuel Moed, Director Bristol Myers Squibb (NYSE: BMY) (Senior Vice President, Corporate Strategy) Morris C. Laster, Director BioLineRx (CEO, Director), OurCrowd (Partner), Clil Medical (CEO), Vital Spark (CEO), Kitov Pharmaceuticals (Co - founder, Director) Jay Green, External Director Glaxo SmithKline (NYSE: GSK) Global Vaccines (Senior Vice President Finance and CFO), Gavi (Advisor for COVAX) Yael Margolin, PhD, External Director Gamida Cell Ltd. (Nasdaq: GMDA) (President, CEO, Director), Denali Ventures LLC (VP) Avner Rotman, PhD, Director Biodar (CEO), Rodar (Founder) NORTH AMERICA ISRAEL BOARD BRINGS SIGNIFICANT EXPERTISE
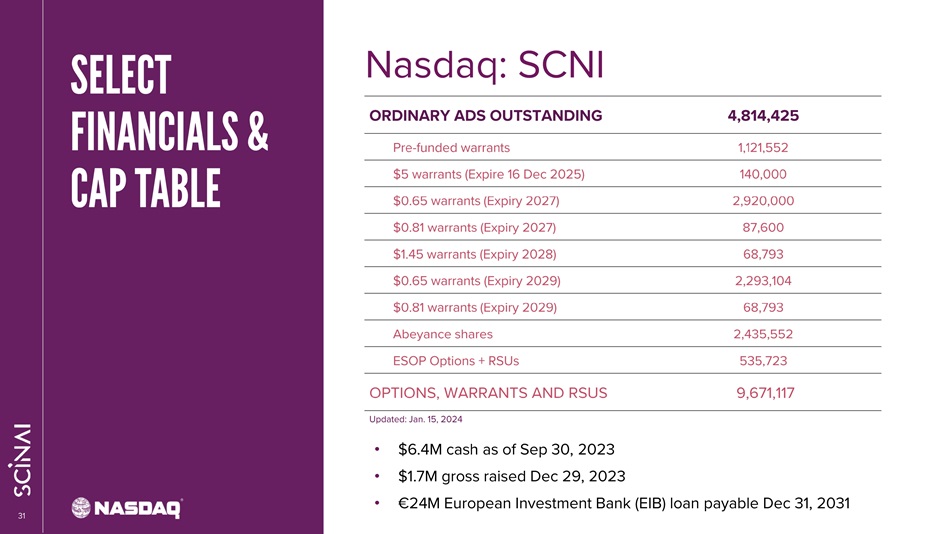
CLICK TO EDIT MASTER TITLE STYLE 31 4,814,425 ORDINARY ADS OUTSTANDING 1,121,552 Pre - funded warrants 140,000 $5 warrants (Expire 16 Dec 2025) 2,920,000 $0.65 warrants (Expiry 2027) 87,600 $0.81 warrants (Expiry 2027) 68,793 $1.45 warrants (Expiry 2028) 2,293,104 $0.65 warrants (Expiry 2029) 68,793 $0.81 warrants (Expiry 2029) 2,435,552 Abeyance shares 535,723 ESOP Options + RSUs 9,671,117 OPTIONS, WARRANTS AND RSU S Updated: Jan. 15, 2024 SELECT FINANCIALS & CAP TABLE • $ 6 . 4 M cash as of Sep 30 , 2023 • $ 1 . 7 M gross raised Dec 29 , 2023 • € 24 M European Investment Bank (EIB) loan payable Dec 31 , 2031 Nasdaq: SCNI

CLICK TO EDIT MASTER TITLE STYLE 32 SIGNIFICANT POTENTIAL FOR VALUE CREATION Pipeline of NanoAb - based drugs Promising preclinical results Preparing for first - in - human clinical trial of anti - IL - 17 NanoAb Collaboration with Max Planck and UMG Targeting diseases with large underserved needs and attractive commercial opportunities CDMO business unit buffers R&D risk

CLICK TO EDIT MASTER TITLE STYLE NASDAQ: SCNI www.scinai.com JANUARY 2024 Contact: Joshua Phillipson Investor Relations ir@scinai.com +972 - 8 - 930 - 2529
Scinai Immunotherapeutics (NASDAQ:SCNI)
Historical Stock Chart
Von Apr 2024 bis Mai 2024

Scinai Immunotherapeutics (NASDAQ:SCNI)
Historical Stock Chart
Von Mai 2023 bis Mai 2024
