Filed Pursuant to Rule 424(b)(5)
Registration Statement No. 333-256100
PROSPECTUS SUPPLEMENT
(To Prospectus dated May 21, 2021)
Phio Pharmaceuticals
Corp.

862,500 Shares of Common Stock
This prospectus supplement relates to the issuance and sale of up to
862,500 shares of our common stock, par value $0.0001 per share (“Common Stock”) to Triton Funds LP (“Triton”)
pursuant to a purchase agreement entered into on May 16, 2024 (the “Purchase Agreement”), at an offering price of $0.72
per share of Common Stock. This prospectus supplement also covers the resale of these shares by Triton to the public. The 862,500 shares
of Common Stock registered hereunder and issuable to Triton represent approximately 18.8% of the shares of our Common Stock outstanding
on the date of the Purchase Agreement. Pursuant to the terms of the Purchase Agreement, the closing date of any issuance of shares of
Common Stock to Triton shall be no later than three business days after the exercise of our put option. Pursuant to the terms of the Purchase
Agreement, Triton shall have the right, upon delivery of notice to the Company, to reduce the number of shares of Common Stock to be purchased
under the Purchase Agreement if the trading price of the Common Stock, as reported by Nasdaq, falls below $0.72 per share after the date
of the Purchase Agreement and prior to the closing of any sale of Common Stock to Triton. We expect that the offering to Triton will be
completed on the earlier of (i) November 17, 2024, which is the final date of the “commitment period”, as defined in the Purchase
Agreement, and (ii) such time as we have sold to Triton all of the 862,500 shares of our Common Stock under this prospectus supplement
pursuant to the terms of the Purchase Agreement.
The number of shares of Common Stock to be issued and sold to Triton,
and the offering price per share of Common Stock to be paid by Triton to the Company, are each subject to adjustment for any reorganization,
recapitalization, non-cash dividend or stock split, including forward and reverse, or other similar transaction.
The primary offering and sale to Triton under this prospectus supplement
may be deemed to be an “at-the-market-offering” as defined in Rule 415(a)(4) promulgated under the Securities Act of 1933,
as amended (the “Securities Act”). Triton is deemed to be an “underwriter” within the meaning of the Securities
Act, and the compensation and discounts received by Triton will be deemed to be underwriting commissions or discounts. We will pay for
expenses of this offering.
Our Common Stock is listed on The Nasdaq Capital
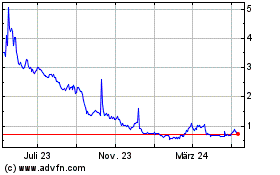
Market under the symbol “PHIO.” The closing price of our Common Stock on May 15, 2024, as reported by Nasdaq, was $0.80 per
share.
As of May 16, 2024, the aggregate market value
of our outstanding common equity held by non-affiliates was approximately $4,884,163, which was calculated based on 4,564,638 shares of
Common Stock held by non-affiliates on May 16, 2024 and a closing price of our Common Stock of $1.07 on March 20, 2024 the highest closing
price of the Company's Common Stock on the Nasdaq Capital Market during the preceding sixty (60) day period. Pursuant to General Instruction
I.B.6 of Form S-3, in no event will we sell the securities described in this prospectus supplement in a public primary offering with a
value exceeding more than one-third (1/3) of the aggregate market value of our Common Stock held by non-affiliates in any twelve (12)-calendar
month period, so long as the aggregate market value of our outstanding Common Stock held by non-affiliates remains below $75,000,000.
During the twelve calendar months prior to and including the date of this prospectus supplement (but excluding this offering), we have
sold $1,000,005 of securities pursuant to General Instruction I.B.6 of Form S-3.
Investing in our securities involves a high
degree of risk. Before making any investment in these securities, you should consider carefully the risks and uncertainties in the section
entitled “Risk Factors” beginning on page S-7 of this prospectus supplement and page 5 of the accompanying prospectus, and
in the other documents that are incorporated by reference and any related free writing prospectus.
Neither the Securities and Exchange Commission
nor any other regulatory body has approved or disapproved of these securities or passed upon the accuracy or adequacy of this prospectus
supplement or the accompanying prospectus. Any representation to the contrary is a criminal offense.
The date of this prospectus supplement is May
16, 2024
TABLE OF CONTENTS
ABOUT THIS PROSPECTUS SUPPLEMENT
This prospectus supplement and the accompanying
prospectus form part of a registration statement on Form S-3 that we filed with the Securities and Exchange Commission (the “SEC”),
under the Securities Act, using a “shelf” registration or continuous offering process. This document is in two parts. The
first part is this prospectus supplement, which describes the specific terms of this offering and certain other matters and may add, update
or change information in the accompanying prospectus, including the documents incorporated by reference into this prospectus supplement.
The second part is the accompanying prospectus, dated May 21, 2021, including the documents incorporated by reference therein, which provides
you with general information about securities we may offer from time to time, some of which may not apply to this offering. Generally,
when we refer to this prospectus, we are referring to both parts of this document combined. To the extent there is a conflict between
the information contained in this prospectus supplement, on the one hand, and the information contained in the accompanying prospectus,
on the other hand, you should rely on the information in this prospectus supplement. These documents contain important information you
should consider when making your investment decision.
You should rely only on the information provided
in this prospectus supplement and the accompanying prospectus, including any information incorporated by reference, and in any free writing
prospectus that we have authorized for use in connection with this offering. We have not authorized anyone to provide you with any other
information and we take no responsibility for any other information that others may give you. The information contained in this prospectus
supplement and the accompanying prospectus speaks only as of the date set forth on the cover page and may not reflect subsequent changes
in our business, financial condition, results of operations and prospects. We may authorize one or more “free writing prospectuses”
(i.e. written communications concerning the offering that are not part of this prospectus supplement) that may contain certain material
information relating to this offering.
We are offering to sell, and are seeking offers
to buy, securities only in jurisdictions where such offers and sales are permitted. We are not making offers to sell these securities
in any jurisdiction in which an offer or solicitation is not authorized or permitted or in which the person making such offer or solicitation
is not qualified to do so or to any person to whom it is unlawful to make such an offer or solicitation. You should read this prospectus
supplement, the accompanying prospectus, including any information incorporated by reference, and any free writing prospectus that we
have authorized for use in connection with this offering, in their entirety before making an investment decision. You should also read
and consider the information in the documents to which we have referred you in the sections entitled “Where You Can Find More Information”
and “Incorporation of Certain Information by Reference.”
In this prospectus supplement and accompanying
prospectus, unless otherwise noted, (1) the term “Phio” refers to Phio Pharmaceuticals Corp. and our subsidiary, MirImmune,
LLC and (2) the terms “Company,” “we,” “us” and “our” refer to the ongoing business operations
of Phio and MirImmune, LLC, whether conducted through Phio or MirImmune, LLC.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus supplement contains forward-looking
statements within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements can be identified by
words such as “intends,” “believes,” “anticipates,” “indicates,” “plans,”
“expects,” “suggests,” “may,” “would,” “should,” “potential,”
“designed to,” “will,” “ongoing,” “estimate,” “forecast,” “target,”
“predict,” “could,” and similar references, although not all forward-looking statements contain these words. Forward-looking
statements are neither historical facts nor assurances of future performance. These statements are based only on our current beliefs,
expectations and assumptions regarding the future of our business, future plans and strategies, projections, anticipated events and trends,
the economy and other future conditions. Because forward-looking statements relate to the future, they are subject to inherent uncertainties,
risks and changes in circumstances that are difficult to predict and many of which are outside of our control. Risks that could cause
actual results to vary from expected results expressed in our forward-looking statements include, but are not limited to:
| |
· |
we are dependent on the success of our INTASYL™ technology platform, and our product candidates based on this platform, which is unproven and may never lead to approved and marketable products; |
| |
· |
our product candidates are in an early stage of development and we may fail, experience significant delays, never advance in clinical development or not be successful in our efforts to identify or discover additional product candidates, which may materially and adversely impact our business; |
| |
· |
if we experience delays or difficulties in identifying and enrolling subjects in clinical trials, it may lead to delays in generating clinical data and the receipt of necessary regulatory approvals; |
| |
· |
topline data may not accurately reflect or may materially differ from the complete results of a clinical trial; |
| |
· |
we rely upon third parties for the manufacture of the clinical supply for our product candidates; |
| |
· |
our business and operations would suffer in the event of computer system failures, cyberattacks or a deficiency in our cybersecurity; |
| |
· |
we are dependent on the patents we own and the technologies we license, and if we fail to maintain our patents or lose the right to license such technologies, our ability to develop new products would be harmed; |
| |
· |
we will require substantial additional funds to complete our research and development activities; |
| |
· |
future financing may be obtained through, and future development efforts may be paid for by, the issuance of debt or equity, which may have an adverse effect on our stockholders or may otherwise adversely affect our business; |
| |
· |
we may not be able to regain compliance with the continued listing requirements of The Nasdaq Capital Market; and |
| |
· |
the price of our Common Stock has been and may continue to be volatile. |
The risks set forth above are not exhaustive and
additional factors, including those identified in this prospectus under the heading “Risk Factors,” for reasons described
elsewhere in this prospectus and in other filings Phio Pharmaceuticals Corp. periodically makes with the Securities and Exchange Commission,
including the other risks identified in Item 1A. Risk Factors in each of our Annual Report on Form 10-K for the year ended December
31, 2023 and Quarterly Report on Form 10-Q for the quarter ended March 31, 2024, could adversely affect our business and financial performance.
Therefore, you should not rely unduly on any of these forward-looking statements. Forward-looking statements contained in this prospectus
speak as of the date hereof and Phio Pharmaceuticals Corp. does not undertake to update any of these forward-looking statements to reflect
a change in its views or events or circumstances that occur after the date of this report, except as required by law.
PROSPECTUS SUPPLEMENT SUMMARY
The following summary highlights certain information
contained elsewhere or incorporated by reference in this prospectus supplement and the accompanying prospectus. This summary provides
an overview of selected information and does not contain all of the information you should consider in making your investment decision.
Therefore, you should read the entire prospectus supplement and the accompanying prospectus, and the documents incorporated by reference
herein carefully before investing in our securities. Investors should carefully consider the information set forth under “Risk Factors”
beginning on page S-7 of this prospectus supplement and the financial statements and other information incorporated by reference in this
prospectus.
Overview
Phio is a clinical stage biotechnology
company whose proprietary INTASYL™ small interfering RNA gene silencing technology is designed to make immune cells more effective
in killing tumor cells. We are developing therapeutics that are designed to leverage INTASYL to precisely target specific proteins that
reduce the body’s ability to fight cancer, without the need for specialized formulations or drug delivery systems.
In 2023, we implemented a
cost rationalization program driven by our transition from a discovery research company to a product development company. This transition
resulted in a decision not to renew the lease for our corporate headquarters and primary research facility in Marlborough, Massachusetts,
which expired on March 31, 2024. As of April 1, 2024, we have continued operations primarily as a remote business with a laboratory facility
in Worcester, Massachusetts. Additionally, we rationalized discovery research personnel resulting in headcount reduction by approximately
36%. These expense reductions have been redirected to funding the Phase 1b clinical trial with PH-762 directed toward skin cancer.
INTASYL Platform
Overall, RNA is involved in
the synthesis, regulation and expression of proteins. RNA takes the instructions from DNA and turns those instructions into proteins within
the body’s cells. RNA interference, or RNAi, is a biological process that inhibits the expression of genes or the production of
proteins. Diseases are often related to the incorrect protein being made, excessive amounts of a specific protein being made, or the correct
protein being made, but at the wrong location or time. RNAi offers a novel approach to drug development because RNAi compounds can be
designed to silence any one of the thousands of human genes, many of which are considered “undruggable” by traditional therapeutics.
Our development efforts are
based on our proprietary INTASYL self-delivering RNAi technology platform. It is a patented platform from which specific patented compounds
are developed. INTASYL compounds are comprised of a unique sequence of chemically modified nucleotides (modified small interfering RNA,
or siRNAs) that target a broad range of cell types and tissues. The compounds are designed to effectively silence genes that tumors use
to evade the immune system.
Since
the initial discovery of RNAi, drug delivery has been the primary challenge in developing RNAi-based therapeutics. Other siRNA technologies
require cell targeting chemical conjugates which limit delivery to specific cell types. INTASYL is based on proprietary chemistry that
is designed to maximize the activity and adaptability of the compound and is unique in that it can be delivered to any cell type or tissue
without the need to modify the chemistry. This is designed to eliminate the need for formulations or delivery systems (for example, nanoparticles
or electroporation). This provides efficient, spontaneous, cellular uptake with potent, long-lasting intracellular activity.
We
believe that our INTASYL platform provides the following benefits including, but not limited to:
| |
· |
Ability to target a broad range of cell types and tissues; |
| |
· |
Ability to target both intracellular and extracellular protein targets; |
| |
· |
Efficient uptake by target cells, avoiding the need for assisted delivery; |
| |
· |
Sustained, or long-term, effect in vivo; |
| |
· |
Ability to target multiple genes in one drug product; |
| |
· |
Favorable clinical safety profile with local administration; and |
| |
· |
Readily manufactured under current good manufacturing practices. |
PH-762
PH-762 is an INTASYL compound
designed to reduce the expression of cell death protein 1 (“PD-1”). PD-1 is a protein that inhibits T cells’
ability to kill cancer cells and is a clinically validated target in immunotherapy. Decreasing the expression of PD-1 can thereby increase
the capacity of T cells, which protect the body from cancer cells and infections, to kill cancer cells.
Our preclinical studies have
demonstrated that direct-to-tumor application of PH-762 resulted in potent anti-tumoral effects and have shown that direct-to-tumor treatment
with PH-762 inhibits tumor growth in a dose dependent fashion in PD-1 responsive and refractory models. Importantly, direct-to-tumor administration
of PH-762 resulted in activity against distant untreated tumors, indicative of a systemic anti-tumor response. We believe these data further
support the potential for PH-762 to provide a strong local immune response without the dose immune-related adverse effects seen with systemic
antibody therapy.
PH-762 is currently being
evaluated in a U.S. multi-center Phase 1b dose-escalating clinical trial through the intratumoral injection of PH-762 for the treatment
of patients with cutaneous squamous cell carcinoma, melanoma and Merkel cell carcinoma. The trial is designed to evaluate the safety and
tolerability of neoadjuvant use of intratumorally injected PH-762, assess the tumor response, and determine the dose or dose range for
continued study of PH-762 and is expected to enroll up to 30 patients. In November 2023, under a previously cleared Investigational New
Drug (“IND”) application by the U.S. Food and Drug Administration. The first two patients enrolled in our first cohort
have completed treatment with PH-762 with no reported adverse events. The trial is currently open for the continued enrollment of patients
and expects to complete enrollment of patients in the second quarter of 2025.
Due to INTASYL’s ease
of administration, we have shown that our compounds can easily be incorporated into current adoptive cell therapy (“ACT”)
manufacturing processes. In ACT, T cells are usually taken from a patient's own blood or tumor tissue, grown in large numbers in a laboratory,
and then given back to the patient to help the immune system fight cancer. By treating T cells with our INTASYL compounds while they are
being grown in the laboratory, we believe our INTASYL compounds can improve these immune cells to make them more effective in killing
cancer. Preclinical data generated in collaboration with AgonOx, Inc. (“AgonOx”), a private company developing a pipeline
of novel immunotherapy drugs targeting key regulators of the immune response to cancer, demonstrated that treating AgonOx’s “double
positive” tumor infiltrating lymphocytes (“DP TIL”) with PH-762 increased their tumor killing activity by two-fold.
In
February 2021, we entered into a clinical co-development collaboration agreement (the “Clinical Co-Development Agreement”)
with AgonOx to develop a T cell-based therapy using PH-762 and AgonOx’s DP TIL. Under the Clinical Co-Development Agreement, we
agreed to reimburse AgonOx up to $4 million in expenses incurred to conduct a Phase 1 clinical trial of PH-762 treated DP TIL in patients
with advanced melanoma and other advanced solid tumors. We were also eligible to receive certain future development milestones and low
single-digit sales-based royalty payments from AgonOx’s licensing of its DP TIL technology.
On
May 8, 2024, we terminated the Clinical Co-Development Agreement with AgonOx, effective immediately. Effective as of the date of the termination,
the Clinical Co-Development Agreement and our continuing obligations and those of AgonOx thereunder were terminated in their entirety.
We will no longer be required to provide financial support for the development costs incurred under the Clinical Co-Development Agreement
or entitled to certain future development milestones and low single-digit sales-based royalty payments from AgonOx’s licensing of
its DP TIL technology.
Prior
to the termination of the Clinical Co-Development Agreement with AgonOx, PH-762 treated DP TIL were being evaluated in a Phase 1 clinical
trial in the United States with up to 18 patients with advanced melanoma and other advanced solid tumors by AgonOx. The primary trial
objectives were to evaluate the safety and to study the potential for enhanced therapeutic benefit from the administration of PH-762 treated
DP TIL. AgonOx had enrolled three patients. The first two patients were treated with DP TIL only and the third patient was treated with
a combination of DP TIL and PH-762.
Corporate Information
We
were incorporated in the state of Delaware in 2011 as RXi Pharmaceuticals Corporation. On November 19, 2018, the Company changed its name
to Phio Pharmaceuticals Corp., to reflect its transition from a platform company to one that is fully committed to developing groundbreaking
immuno-oncology therapeutics. Our principal mailing address is 11 Apex Drive, Suite 300A, PMB 2006, Marlborough, Massachusetts 01752,
and our telephone number is (508) 767-3861. The Company’s website address is http://www.phiopharma.com. Our website and the
information contained on that site, or connected to that site, is not part of or incorporated by reference into this prospectus.
THE OFFERING
| |
|
|
| Common Stock offered: |
|
862,500 shares of Common Stock. |
| |
|
| Offering price: |
|
$0.72 per share of Common Stock. |
| |
|
| Common Stock outstanding after this offering: |
|
5,454,2001, which assumes the sale of 862,500 shares of Common Stock to Triton at a price of $0.72 per share. The actual number of shares of our Common Stock issued will vary depending on how many shares of Common Stock we elect to sell to Triton under the Purchase Agreement, but will not be greater than 862,500 shares, representing approximately 18.8% of the shares of Common Stock outstanding as of the date of the Purchase Agreement. |
| |
|
|
| Termination of this offering: |
|
We expect that the offering to Triton will be completed on the earlier of (i) November 17, 2024, which is the final date of the “commitment period”, as defined in the Purchase Agreement, and (ii) such time as we have sold to Triton all of the 862,500 shares of our Common Stock under this prospectus supplement pursuant to the terms of the Purchase Agreement. |
| |
|
|
| Purchaser: |
|
Triton Funds LP |
| |
|
| Use of proceeds: |
|
We estimate that the net proceeds from this offering will be approximately $0.5 million after deducting the estimated offering expenses payable by us. We intend to use the proceeds from this offering to fund the development of the Company’s product candidates, other research and development activities and for general working capital needs. See “Use of Proceeds” on page S-9 of this prospectus supplement. |
| |
|
| Risk factors: |
|
You should read the “Risk Factors” section of this prospectus supplement and the accompanying prospectus for a discussion of factors to consider carefully before deciding to invest in shares of our Common Stock. |
| |
|
| Nasdaq Capital Market symbol: |
|
“PHIO.” |
1 The number of shares of Common Stock
to be outstanding after this offering is based on 4,591,700 shares of Common Stock outstanding as of March 31, 2024, and excludes:
| |
· |
5,504,918 shares of Common Stock issuable upon the exercise of warrants to purchase shares of our Common Stock outstanding as of March 31, 2024, having a weighted average exercise price of $4.03 per share; |
| |
· |
10,061 shares of Common Stock issuable upon the exercise of stock options outstanding as of March 31, 2024, having a weighted average exercise price of $104.21 per share; |
| |
· |
18,582 shares of Common Stock issuable upon the vesting of restricted stock units outstanding as of March 31, 2024; |
| |
· |
140,500 shares of Common Stock reserved for future issuance under our 2020 Long-Term Incentive Plan as of March 31, 2024; and |
| |
· |
661 shares of Common Stock reserved for future issuance under our Employee Stock Purchase Plan as of March 31, 2024. |
RISK FACTORS
Investing in our Common Stock involves a high
degree of risk. Before investing in our Common Stock, you should carefully consider the risks, uncertainties and assumptions described
below and in the accompanying prospectus, in the section under the heading “Risk Factors” included in our Annual Report on
Form 10-K for our most recent fiscal year, any amendment or updates thereto reflected in subsequent
filings with the SEC, and in other reports we file with the SEC that are incorporated by reference herein, before making an investment
decision. Our business, financial condition, results of operations and future growth prospects could be materially and adversely
affected by any of these risks. In these circumstances, the market price of our Common Stock could decline, and you may lose all or part
of your investment.
Risks Related to this Offering
We have a significant number of outstanding warrants and the
future issuance of the underlying shares of Common Stock could adversely affect the market price of our Common Stock.
As of May 16, 2024, we had outstanding warrants
exercisable for 5,504,918 shares of our Common Stock at a weighted average exercise price of $4.03 per share, all of which warrants are
currently exercisable. Upon exercise of these warrants, we would issue additional shares of our Common Stock. As a result, our current
stockholders as a group would own a substantially smaller interest in us and may have less influence on our management and policies than
they now have. Furthermore, the holders may sell these shares of Common Stock in the public markets from time to time, without limitations
on the timing, amount or method of sale. As our stock price rises, the holders may exercise more of their warrants and sell a large number
of shares of Common Stock. This could cause the market price of our Common Stock to decline.
We have broad discretion in how we use the
net proceeds of this offering, and we may not use these proceeds effectively or in ways with which you agree.
Our management will have broad
discretion as to the application of the net proceeds of this offering and could use them for purposes other than those contemplated at
the time of the offering. We intend to use the net proceeds, if any, from this offering for general corporate purposes, which may include,
among other things, the development of the Company’s product candidates, other research and development activities and for general
working capital needs. Our stockholders may not agree with the manner in which our management chooses to allocate and spend the net proceeds.
Moreover, our management may use the net proceeds for corporate purposes that may not increase the market price of our Common Stock.
You may experience further dilution if we issue additional equity
securities in future fundraising transactions.
To raise additional capital,
we may in the future offer additional shares of our Common Stock or other securities convertible into or exchangeable for our Common Stock
at prices that may not be the same as the price per share in this offering. We may sell shares or other securities in any other offering
at a price per share that is less than the price per share paid by investors in this offering, and investors purchasing shares or other
securities in the future could have rights superior to existing stockholders. The price per share at which we sell additional shares of
our Common Stock, or securities convertible or exchangeable into Common Stock, in future transactions may be higher or lower than the
price per share paid by investors in this offering. Further, the exercise of outstanding warrants and equity awards may result in further
dilution of your investment.
Triton will pay less than the then-prevailing market price of
our Common Stock, which could cause the price of our Common Stock to decline.
The shares of our Common Stock
to be issued to Triton pursuant to the terms of the Purchase Agreement will be purchased and sold at $0.72 per share, which is lower than
the market price of our Common Stock as of the date of this prospectus supplement. To the extent that our market price is higher than
$0.72 per share, Triton has a financial incentive to sell our shares immediately upon receiving them to realize the profit between the
discounted price and the market price. If Triton sells our shares, the price of our Common Stock may decline.
We have no history of paying dividends on
our Common Stock, and we do not anticipate paying dividends in the foreseeable future.
We have not previously paid
dividends on our Common Stock. We currently anticipate that we will retain all of our available cash, if any, for purposes, which may
include, among other things, the development of the Company’s product candidates, other research and development activities and
for general working capital needs. Any payment of future dividends will be at the discretion of our board of directors and will depend
upon, among other things, our earnings, financial condition, capital requirements, level of indebtedness, statutory and contractual restrictions
applicable to the payment of dividends and other considerations that our board of directors deems relevant. Investors must rely on sales
of their Common Stock after price appreciation, which may never occur, as the only way to realize a return on their investment.
USE OF PROCEEDS
We estimate that we will receive
net proceeds of approximately $0.5 million from this offering, after deducting administrative fees and estimated offering expenses payable
by us.
We intend to use the net proceeds
from this offering to fund the development of our product candidates, other research and development activities and for general working
capital needs. We may also use a portion of the net proceeds to acquire or invest in complementary businesses, products and technologies
or to fund the development of any such complementary businesses, products or technologies. We currently have no plans for any such acquisitions.
DIVIDEND POLICY
We have never declared or
paid cash dividends on our capital stock. We currently intend to retain our future earnings, if any, for use in our business and therefore
do not anticipate paying cash dividends in the foreseeable future. Payment of future dividends, if any, will be at the discretion of our
board of directors after taking into account various factors, including our financial condition, operating results, current and anticipated
cash needs and plans for expansion.
PLAN OF DISTRIBUTION
Pursuant to this prospectus supplement, we are
offering up to 862,500 shares of our Common Stock to Triton pursuant to the Purchase Agreement. This prospectus supplement also covers
the resale of these shares by Triton to the public.
We may, from time to time and at our sole
discretion, direct Triton to purchase shares of our Common Stock, pursuant to the terms of the Purchase Agreement. The aggregate sale
of shares of Common Stock to Triton will be limited to no more than the number of shares of Common Stock that would result in the direct
or indirect beneficial ownership by Triton of more than 19.99% of the then-outstanding shares of Common Stock. Triton will purchase the
shares of Common Stock under the Purchase Agreement at the price of $0.72 per share. The actual number of shares of our Common Stock issued
will not be greater than 862,500 shares of our Common Stock, representing 18.8% of the shares of our Common Stock outstanding on the date
of the Purchase Agreement. Neither we nor Triton may assign or transfer its rights and obligations under the Purchase Agreement without
the other party’s written consent.
The shares of Common Stock
that we may from time to time issue to Triton under this prospectus supplement may be subsequently sold or distributed from time to time
by Triton, as a selling stockholder, directly to one or more purchasers or through brokers, dealers, or underwriters who may act solely
as agents at market prices prevailing at the time of sale, at prices related to the prevailing market prices, at negotiated prices, or
at fixed prices, which may be changed. Any resale of the shares of our Common Stock offered by this prospectus supplement could be effected
in one or more of the following methods:
| · | ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers; |
| · | block trades in which the broker-dealer will attempt to sell the shares as agent but may position and
resell a portion of the block as principal to facilitate the transaction; |
| · | purchases by a broker-dealer as principal and resale by the broker-dealer for its account; |
| · | an exchange distribution in accordance with the rules of the applicable exchange; |
| · | privately negotiated transactions; |
| · | in transactions through broker-dealers that agree with the selling stockholder to sell a specified number
of such securities at a stipulated price per security; |
| · | through the writing or settlement of options, whether through an options exchange or otherwise; |
| · | a combination of any such methods of sale; or |
| · | any other method permitted pursuant to applicable law. |
Triton may also sell the share
of Common Stock under Rule 144 under the Securities Act, if available, rather than under this prospectus supplement.
In order to comply with the securities laws of
certain states, if applicable, the shares may be sold through registered or licensed brokers or dealers. In addition, in certain states,
the shares may not be sold unless they have been registered or qualified for sale in the state or an exemption from the state’s
registration or qualification requirement is available and complied with.
Triton is deemed an “underwriter”
within the meaning of Section 2(a)(11) of the Securities Act.
Triton has informed us that
it intends to use an unaffiliated broker-dealer to effectuate sales, if any, of the Common Stock that it may acquire from us pursuant
to the Purchase Agreement. Such sales will be made at prices and at terms then prevailing or at prices related to the then current market
price. Each such unaffiliated broker-dealer will be an underwriter within the meaning of Section 2(a)(11) of the Securities Act. Triton
has informed us that each such broker-dealer will receive commissions from Triton that will not exceed customary brokerage commissions.
Brokers, dealers, underwriters,
or agents participating in any distribution of the shares of our Common Stock offered by this prospectus supplement may receive compensation
in the form of commissions, discounts, or concessions from the seller and/or purchasers of the Common Stock for whom the broker-dealers
may act as agent. The compensation paid to a particular broker-dealer may be less than or in excess of customary commissions. Neither
we nor Triton can presently estimate the amount of compensation that any agent will receive.
We know of no existing arrangements
between Triton or any other stockholder, broker, dealer, underwriter or agent relating to the sale or distribution of the shares of Common
Stock offered by this prospectus supplement. At the time a particular offer of shares is made, a prospectus supplement, if required under
the Securities Act, will be distributed that will set forth the names of any agents, underwriters or dealers and any compensation from
the selling stockholder, and any other required information.
We will pay our expenses incident
to this offering, which we estimate to be approximately $125,000. We have agreed to indemnify Triton and certain other persons against
certain liabilities in connection with the offering of shares of our Common Stock offered hereby, and if such indemnity is unavailable,
to contribute amounts required to be paid in respect of such liabilities.
Triton has covenanted that
neither Triton nor any affiliate of Triton acting on its behalf or pursuant to any understanding with it, will execute any short sales
of our Common Stock during the term of the Purchase Agreement.
We have advised Triton that
it is required to comply with Regulation M promulgated under the Securities Exchange Act of 1934, as amended (the “Exchange Act”).
With certain exceptions, Regulation M precludes a selling stockholder, any affiliated purchasers, and any broker-dealer or other person
who participates in a distribution from bidding for or purchasing, or attempting to induce any person to bid for or purchase any security
which is the subject of the distribution until the entire distribution is complete. Regulation M also prohibits any bids or purchases
made in order to stabilize the price of a security in connection with the distribution of that security. All of the foregoing may affect
the marketability of the securities offered by this prospectus supplement.
This offering will terminate
upon the earlier of the sale of 862,500 shares of our Common Stock under the Purchase Agreement and November 17, 2024.
This
summary of the material provisions of the Purchase Agreement does not purport to be a complete statement of its terms and conditions.
A copy of the Purchase Agreement is filed as an exhibit to a current report on Form 8-K filed with the SEC under the Exchange Act, and
incorporated by reference in this prospectus supplement. See “Incorporation of Certain Documents by Reference” and “Where You Can Find More Information.”
No
action has been or will be taken in any jurisdiction (except in the United States) that would permit a public offering of the securities
offered by this prospectus supplement and accompanying prospectus, or the possession, circulation, or distribution of this prospectus
supplement and accompanying prospectus or any other material relating to us or the securities offered hereby in any jurisdiction where
action for that purpose is required. Accordingly, the securities offered hereby may not be offered or sold, directly or indirectly, and
neither of this prospectus supplement and accompanying prospectus nor any other offering material or advertisements in connection with
the securities offered hereby may be distributed or published, in or from any country or jurisdiction except in compliance with any applicable
rules and regulations of any such country or jurisdiction. Triton may arrange to sell securities offered by this prospectus supplement
and accompanying prospectus in certain jurisdictions outside the United States, either directly or through affiliates, where they are
permitted to do so.
Our Common Stock is listed on The Nasdaq Capital
Market under the symbol “PHIO.”
LEGAL MATTERS
Certain legal matters relating
to the issuance of the securities offered hereby will be passed upon for us by Hogan Lovells US LLP.
EXPERTS
The
consolidated financial statements of Phio Pharmaceuticals Corp. (the Company) as of December 31,
2023 and 2022 and for each of the two years in the period ended December 31, 2023 incorporated by reference in this Prospectus and in
the Registration Statement have been so incorporated in reliance on the report of BDO USA, P.C., an independent registered public accounting
firm, given on the authority of said firm as experts in auditing and accounting. The report on the consolidated financial statements contains
an explanatory paragraph regarding the Company’s ability to continue as a going concern.
Where
You Can Find More Information
We
are required to file annual, quarterly and current reports, proxy statements and other information with the SEC. Our filings with the
SEC are available to the public at the SEC’s Internet web site at http://www.sec.gov. Copies of certain information
filed by us with the SEC are also available on our website at www.phiopharma.com. Our website is not a part of this prospectus
and is not incorporated by reference in this prospectus, and you should not consider the contents of our website in making an investment
decision with respect to our Common Stock.
We
have filed a registration statement, of which this prospectus is a part, covering the securities offered hereby. As allowed by SEC rules,
this prospectus does not include all of the information contained in the registration statement and the included exhibits, financial statements
and schedules. You are referred to the registration statement, the included exhibits, financial statements and schedules for further information.
You should review the information and exhibits in the registration statement for further information about us and our subsidiaries
and the securities we are offering. Statements in this prospectus concerning any document we filed as an exhibit to the registration statement
or that we otherwise filed with the SEC are not intended to be comprehensive and are qualified by reference to these filings. You should
review the complete document to evaluate these statements.
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The SEC allows us to “incorporate
by reference” the information we have filed with them, which means that we can disclose important information to you by referring
you to those documents. The information we incorporate by reference is an important part of this prospectus supplement, and information
that we file later with the SEC will automatically update and supersede this information. The documents we are incorporating by reference
are:
| |
· |
our Annual Report on Form 10-K for the year ended December 31, 2023, filed with the SEC on April 1, 2024; |
| |
|
|
| |
· |
Our Quarterly Report on Form 10-Q for the quarter ended March 31, 2024, filed with the SEC on May 9, 2024; |
| |
|
|
| |
· |
our Current Reports on Form 8-K, filed with the SEC on January 26, 2024 and May 17, 2024; and |
| |
|
|
| |
· |
the description of our Common Stock contained in our registration statement on Form 8-A12B filed with the SEC on February 7, 2014, as updated by the description of our Common Stock filed as Exhibit 4.16 to our Annual Report on Form 10-K for the year ended December 31, 2023, including any amendment or report filed for the purpose of updating such description. |
All
documents we file with the SEC pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act, except as to any portion of any report
or document that is not deemed filed under such provisions, on or after the date of this prospectus supplement until the earlier of the
date on which all of the securities registered hereunder have been sold or the registration statement of which this prospectus supplement
is a part has been withdrawn, shall be deemed incorporated by reference in this prospectus supplement and to be a part of this prospectus
supplement from the date of filing of those documents and will be automatically updated and, to the extent described above, supersede
information contained or incorporated by reference in this prospectus and previously filed documents that are incorporated by reference
in this prospectus supplement.
Nothing in this prospectus
shall be deemed to incorporate information furnished but not filed with the SEC pursuant to Item 2.02, 7.01 or 9.01 of Form 8-K.
Upon written or oral request,
we will provide without charge to each person, including any beneficial owner, to whom a copy of the prospectus is delivered a copy of
any or all of the reports or documents incorporated by reference herein (other than exhibits to such documents, unless such exhibits are
specifically incorporated by reference herein). You may request a copy of these filings, at no cost, by writing or telephoning us at the
following address: Phio Pharmaceuticals Corp., 11 Apex Drive, Suite 300A, PMB 2006, Marlborough, Massachusetts 01752, Attention: Investor
Relations, telephone: (508) 767-3861. We maintain a website at www.phiopharma.com. You may access our definitive
proxy statements on Schedule 14A, annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on
Form 8-K and periodic amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange
Act with the SEC free of charge at our website as soon as reasonably practicable after such material is electronically filed with, or
furnished to, the SEC. The information contained in, or that can be accessed through, our website is not incorporated by reference in,
and is not part of, this prospectus. We have not authorized anyone to provide you with any information that differs from that contained
in this prospectus. Accordingly, you should not rely on any information that is not contained in this prospectus. You should not assume
that the information in this prospectus is accurate as of any date other than the date of the front cover of this prospectus.
PROSPECTUS
$100,000,000
Phio Pharmaceuticals
Corp.

Common Stock
Preferred Stock
Debt Securities
Warrants
Units
We may offer and sell an indeterminate number of shares of our common
stock and preferred stock, debt securities, warrants and/or units from time to time under this prospectus. We will describe in a prospectus
supplement or sales agreement prospectus the securities we are offering and selling, as well as the specific terms of the securities.
We may offer these securities in amounts, at prices and on terms determined
at the time of offering. We may sell the securities directly to you, through agents we select, or through underwriters and dealers we
select. If we use agents, underwriters or dealers to sell the securities, we will name them and describe their compensation in a prospectus
supplement or sales agreement prospectus.
Our common stock trades on the Nasdaq Capital Market under the symbol
“PHIO”. On May 12, 2021, the closing price for our common stock, as reported on the Nasdaq Capital Market, was $2.05 per share.
Investing in our securities involves certain risks. See “Risk
Factors” beginning on Page 5 of this prospectus and in the applicable prospectus supplement or sales agreement prospectus for
certain risks you should consider. You should read the entire prospectus carefully before you make your investment decision.
Neither the Securities and Exchange Commission nor any state securities
commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation
to the contrary is a criminal offense.
The date of this prospectus is May 21, 2021.
TABLE OF CONTENTS
ABOUT THIS PROSPECTUS
This prospectus is part of a registration statement that we filed with
the Securities and Exchange Commission, or SEC, utilizing a shelf registration process. Under the shelf registration process, we may offer
shares of our common stock and preferred stock, various series of debt securities, warrants and units to purchase any of such securities
with a total value of up to $100,000,000 from time to time under this prospectus at prices and on terms to be determined by market conditions
at the time of offering. This prospectus provides you with a general description of the securities we may offer. Each time we offer a
type or series of securities, we will provide a prospectus supplement (which term includes, as applicable, the sales agreement prospectus
filed with the registration statement of which this prospectus forms a part) that will describe the specific amounts, prices and other
important terms of the securities, including, to the extent applicable:
| |
· |
|
designation or classification; |
| |
· |
|
aggregate principal amount or aggregate offering price; |
| |
· |
|
original issue discount, if any; |
| |
· |
|
rates and times of payment of interest, dividends or other payments, if any; |
| |
· |
|
redemption, conversion, exchange, settlement or sinking fund terms, if any; |
| |
· |
|
conversion, exchange or settlement prices or rates, if any, and, if applicable, any provisions for changes to or adjustments in the conversion, exchange or settlement prices or rates and in the securities or other property receivable upon conversion, exchange or settlement; |
| |
· |
|
restrictive covenants, if any; |
| |
· |
|
voting or other rights, if any; and |
| |
· |
|
important federal income tax considerations. |
A prospectus supplement may include a discussion of risks or other
special considerations applicable to us or the offered securities. A prospectus supplement may also add, update or change information
in this prospectus. If there is any inconsistency between the information in this prospectus and the applicable prospectus supplement,
you must rely on the information in the prospectus supplement. Please carefully read both this prospectus and the applicable prospectus
supplement in their entirety together with additional information described under the heading “Where You Can Find More Information”
in this prospectus. This prospectus may not be used to offer or sell any securities unless accompanied by a prospectus supplement.
The registration statement containing this prospectus, including exhibits
to the registration statement, provides additional information about us and the securities offered under this prospectus. The registration
statement can be read on the SEC’s website or at the SEC’s public reading room mentioned under the heading “Where You Can Find More Information” in this prospectus.
We have not authorized any broker-dealer, salesperson or other person
to give any information or to make any representation other than those contained or incorporated by reference in this prospectus and the
accompanying prospectus supplement. You must not rely upon any information or representation not contained or incorporated by reference
in this prospectus or the accompanying prospectus supplement. This prospectus and the accompanying prospectus supplement do not constitute
an offer to sell or the solicitation of an offer to buy securities, nor do this prospectus and the accompanying prospectus supplement
constitute an offer to sell or the solicitation of an offer to buy securities in any jurisdiction to any person to whom it is unlawful
to make such offer or solicitation. The information contained in this prospectus and the accompanying prospectus supplement speaks only
as of the date set forth on the cover page and may not reflect subsequent changes in our business, financial condition, results of operations
and prospects even though this prospectus and any accompanying prospectus supplement is delivered or securities are sold on a later date.
PROSPECTUS SUMMARY
In this prospectus, unless otherwise noted, (1) the term “Phio”
refers to Phio Pharmaceuticals Corp. and our subsidiary, MirImmune, LLC and (2) the terms “Company,” “we,” “us,”
and “our” refer to the ongoing business operations of Phio and MirImmune, LLC, whether conducted through Phio or MirImmune,
LLC.
Overview
Phio Pharmaceuticals Corp. is a biotechnology company developing
the next generation of immuno-oncology therapeutics based on our self-delivering RNAi (“INTASYL™”) therapeutic platform.
Our efforts are focused on silencing tumor-induced suppression of the immune system through our proprietary INTASYL platform with utility
in immune cells and the tumor micro-environment. Our goal is to develop powerful INTASYL therapeutic compounds that can weaponize immune
effector cells to overcome tumor immune escape, thereby providing patients a potentially powerful new treatment option that goes beyond
current treatment modalities.
Our development efforts are based on our broadly patented INTASYL
technology platform. Our INTASYL compounds do not require a delivery vehicle to penetrate into tissues and cells and are designed to “silence”
or down-regulate, the expression of a specific gene which is over-expressed in cancer. We believe that our INTASYL platform has the potential
to uniquely position the Company in the field of immuno-oncology for the following reasons:
| · | Efficient uptake of INTASYL by target cells obviating the need for facilitated delivery (mechanical or formulation); |
| · | Does not require permanent genetic modification; |
| · | Can target multiple genes (i.e. multiple immunosuppression pathways) in a single therapeutic entity; |
| · | Gene silencing by
INTASYL has been shown to have a sustained, or long-term, effect in vivo; |
| · | Favorable clinical safety profile of INTASYL with local administration; and |
| · | Can be readily manufactured under current good manufacturing practices. |
In contrast to other RNA technologies and platforms, the self-delivering
nature of our INTASYL platform makes it ideally suited for use with adoptive cell therapy (“ACT”) treatments as well
as for direct therapeutic use. ACT consists of the infusion of immune cells with antitumor properties, after growing them in a lab to
large numbers. These cells can be derived from unmodified (i.e. naturally occurring) immune cells, immune cells isolated from resected
tumors, or genetically engineered immune cells that recognize tumor cells. Regardless of the source of immune cells (or naturally
occurring immune cells), in patients with solid tumors, these cells have several shortcomings that inhibit their full therapeutic potential.
By using INTASYL technology during the manufacturing of such ACT cell products we can improve the phenotype and function of these cells,
potentially leading to better therapeutic outcomes. Multiple inhibitory mechanisms restrain immune cells from effectively eradicating
tumors, including immune checkpoints, reduced cell fitness and cell persistence. Furthermore, the immunosuppressive tumor microenvironment
(the “TME”) can pose a formidable barrier to immune cell infiltration and function. By using INTASYL based drugs administered
directly, we can also reprogram cells in the TME to help overcome these immunosuppressive mechanisms.
We have developed a product platform based on our INTASYL technology
that allows easy, precise, rapid, and selective non-genetically modified programming of ACT cells (ex vivo, during manufacturing)
and of the TME (in vivo, by local application), resulting in reduced immune inhibition and in improved immunotherapy.
INTASYL Use To Improve Adoptive Cell Therapy Products
ACT is a form of immune therapy based on the use of immune cells,
isolated from patients, donors or retrieved from allogeneic immune cell banks. They are grown in a lab to large numbers, followed by administering
them to the patient to fight cancer. Sometimes, immune cells that naturally recognize a tumor are used, while other times immune cells
are modified or “genetically engineered” to make them recognize and kill the cancer cells. There are several types of ACT,
including: a.) non-engineered cell therapy in which immune cells are grown from the patient’s tumor or blood, such as tumor infiltrating
lymphocytes (“TILs”), or from donor blood or tissue such as natural killer (“NK”) cells, dendritic cells (“DC”)
and macrophages, and b.) genetically engineered immune cells that are genetically modified to recognize specific tumor proteins and to
remain in an activated state (such as T cell receptor technology (“TCRs”), chimeric antigen receptor (“CAR”) T
cells, or CAR-NK cells).
Multiple inhibitory mechanisms restrain immune cells used in ACT
from effectively eradicating tumors, including immune checkpoints, reduced cell fitness and cell persistence, and other barriers to immune
cell infiltration and function mainly in solid tumors. We believe our INTASYL compounds are ideally suited to be used in ACT products.
With INTASYL compounds, we can unlock the full potential of ACT, by improving the immune cell function, differentiation and metabolism,
in order to make these immune cells more effective without the need for additional complicated manufacturing steps and/or genetic engineering.
Our approach builds on well-established methodologies of ACT
and involves the treatment of immune cells with our INTASYL compounds ex vivo while they are grown in the lab and before
administering them to the patient. Because our INTASYL compounds do not require a delivery vehicle, in contrast to other RNA
technologies, to penetrate into the cells, we are able to enhance the function of these cells by merely adding our INTASYL compounds
during the expansion process and without the need for genetic engineering, without the need for complex delivery vehicles or
formulations, and without additional needed complex manufacturing steps. By adding INTASYL to the cell culture media used during the
cell expansion, we can reduce or eliminate the expression of genes that make the immune cells less effective. For example, with our
INTASYL compounds, we can reduce the expression of immunosuppressive proteins by the therapeutic immune cells, potentially enabling
them to overcome tumor resistance mechanisms and thus improving their ability to destroy the tumor cells. In various types of immune
cells tested to date, INTASYL treatment results in potent silencing with close to 100% transfection efficiency and while maintaining
cell viability and cell growth rate. After expanding these cells and enhancing them with INTASYL ex vivo, they are returned to
the patient for treatment.
Our lead product candidate and most advanced program being developed
in ACT is PH-762, an INTASYL compound that targets the checkpoint protein PD-1. Checkpoint proteins, such as PD-1, normally act as a type
of “off switch” that prevent T cells from attacking certain cells, such as cancer cells, in the body. T cells are immune
cells that protect the body from cancer cells and infections.
Data developed by Phio and with collaborators has shown that
PH-762 silences PD-1 checkpoint expression, thereby removing the “off switch” and resulting in enhanced T cell
activation and tumor cytotoxicity. Experimental data shows that PH-762 can silence the expression of PD-1 in target human T cells in
a potent and durable manner, and can increase the function of patient derived TILs for use in ACT and of CAR-T cells used in ACT,
showing that PH-762 is applicable for use in both ACT and as a standalone direct therapeutic.
In March 2021, the Company announced that it entered into a clinical
development collaboration with AgonOx, Inc. (“AgonOx”), a private company developing a pipeline of novel immunotherapy drugs
targeting key regulators of the immune response to cancer. Under the agreement, the companies will collaborate on the development of novel
T cell-based therapies using PH-762 and AgonOx’s “double positive” (DP) TIL technology. AgonOx has demonstrated that
their DP CD8+ T cells isolated from human solid tumors (DP TILs) have increased tumor killing activity when compared to TILs that were
not enriched prior to expansion. Preclinical data from AgonOx in collaboration with Phio has shown that treating DP TILs with PH-762 increases
the tumor killing activity of the DP TILs even further (a two-fold increase). As a result, the use of PH-762 treated DP TILs is expected
to enhance therapeutic responses in cancer data. Based on these data showing that the combination of our technologies can result in TIL
therapeutics, our collaboration will focus on conducting a clinical study for PH-762 treated DP TILs. Under the terms of the collaboration
agreement, AgonOx will receive financial support for the clinical trial from Phio and Phio will be entitled to certain future development
milestones and sales-related royalty payments from AgonOx’s DP TIL technology. In collaboration with AgonOx, we are planning a clinical
trial in ACT with PH-762 and AgonOx’s DP TIL technology. We are in the process of finalizing the investigational new drug-enabling
studies and preparing for regulatory submission in the third quarter of 2021 and anticipate commencing the start of the clinical trial
soon thereafter.
Our second product candidate in ACT is PH-894, an INTASYL compound
that targets BRD4 which is a regulator of gene expression impacting cell differentiation. In previous studies, PH-894 has been shown to
improve T cell function and persistence by differentiating T cells into a more active state (stem-cell like memory phenotype). Data, completed
in partnership with the Karolinska Institutet in Sweden, demonstrated that the application of PH-894, was shown to silence BRD4 in human
T cells during expansion for ACT, which has the potential to confer superior anti-tumor activity, for example by improving T cell persistence.
With this data, we expanded our collaboration with the Karolinska Institutet to build upon these findings and develop INTASYL compounds
for additional targets and cell types toward clinical application in areas of the Karolinska Institutet’s ongoing clinical research.
We are also developing our INTASYL compound PH-804 for use in ACT.
PH-804 targets the suppressive immune receptor TIGIT, which is a checkpoint protein present on T cells and NK cells. We have shown that
PH-804 can silence the expression of TIGIT in NK cells and T cells, overcoming their “off switch” and the cells becoming “weaponized”
to kill cancer cells.
Direct Therapeutic Use of INTASYL Towards the Tumor Micro-Environment
The TME is the environment that surrounds and feeds a tumor, including
normal cells, blood vessels, immune cells, and the extracellular matrix. The TME is an immunosuppressive environment that inhibits the
immune system’s natural ability to recognize and destroy tumor cells by negatively impacting how immunosuppressive cells are being
attracted and activated. Reprogramming different components of the TME may overcome resistance to immunotherapy. Such reprogramming of
the TME by INTASYL compounds through direct local administration into the tumor could potentially become an important form of therapy.
The Company has previously shown in a clinical setting that our INTASYL compounds are safe and well-tolerated following local administration,
therefore we believe that our INTASYL technology can not only be used with ACT, but can also be used as an independent therapeutic platform.
We are developing our PH-762, PH-894 and PH-804 INTASYL
compounds also for use as direct therapeutics to reprogram the TME, for example by in situ transfection and activation of
immune cells in the TME.
Animal studies conducted by the Company showed that local administration
of PH-894 or the mouse version of PH-762 through intra-tumoral injection resulted in potent anti-tumoral effects. The treated animals
showed a complete and statistically significant inhibition of tumor growth, whereas placebo treated animals displayed exponential tumor
growth. In vivo studies performed by the Company with PH-804 showed that intra-tumoral injection of a mouse version of PH-804
reduced the tumor growth in colorectal carcinoma tumor bearing mice, which was shown to inhibit tumor growth and was correlated with the
silencing of TIGIT mRNA expression and in increase in cytotoxic effector T cells in the TME.
The combined PH-804, PH-762, and PH-894 data further shows that INTASYL
compounds can trigger associated changes in the TME such as an increase of TILs, including CD8+ T cells responsible for tumor cell killing,
and an increase of activation markers on these cells. These preclinical findings demonstrate that direct injection of INTASYL compounds
can successfully infiltrate solid tumors and impact the TME by activating the immune response in animal models of solid tumors resulting
in reduced tumor growth. A key challenge for many other immunotherapy platforms is to be able to achieve an adequate therapeutic effect
in solid tumors with an acceptable safety profile. Many of the available systemic immuno-therapeutics indeed come with dose limiting immune-related
adverse events, which we believe can be mitigated with local INTASYL treatment.
Based on our positive preclinical data, the Company is
preparing for a clinical study with PH-762 using intra-tumoral administration for patients with advanced melanoma. The required
preclinical studies and regulatory submission needed to initiate the clinical trial with PH-762 as a direct therapeutic are being
finalized. The clinical trial will be conducted at the Gustave Roussy Institute, which is one of the largest cancer centers in Europe,
with Dr. Caroline Robert as our lead principal investigator. The Company expects to start the clinical trial evaluating the use of
PH-762 as a direct therapeutic in the fourth quarter of 2021.
Corporate Information
We were incorporated in the state of Delaware in 2011 as RXi Pharmaceuticals
Corporation. On November 19, 2018, the Company changed its name to Phio Pharmaceuticals Corp., to reflect its transition from a platform
company to one that is fully committed to developing groundbreaking immuno-oncology therapeutics. Our executive offices are located at
257 Simarano Drive, Suite 101, Marlborough, MA 01752, and our telephone number is (508) 767-3861. The Company’s website address
is http://www.phiopharma.com. Our website and the information contained on that site, or connected to that site, is not part of or incorporated
by reference into this prospectus.
RISK FACTORS
Before making an investment decision, you should carefully consider
the risks described under “Risk Factors” in the applicable prospectus supplement, together with all of the other information
appearing in this prospectus or incorporated by reference into this prospectus and any applicable prospectus supplement, in light of your
particular investment objectives and financial circumstances. Our business, financial condition or results of operations could be materially
adversely affected by any of these risks. The trading price of our securities could decline due to any of these risks, and you may lose
all or part of your investment. This prospectus and the incorporated documents also contain forward-looking statements that involve risks
and uncertainties. Our actual results could differ materially from those anticipated in these forward-looking statements as a result of
certain factors, including the risks mentioned elsewhere in this prospectus. You should also consider the risks, uncertainties and assumptions
discussed under the heading “Risk Factors” included in our most recent Annual Report on Form 10-K, which is on file with the
SEC and is incorporated herein by reference, and which may be amended, supplemented or superseded from time to time by other reports we
file with the SEC in the future.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements within the meaning
of the Private Securities Litigation Reform Act of 1995. Forward-looking statements can be identified by words such as “intends,”
“believes,” “anticipates,” “indicates,” “plans,” “expects,” “suggests,”
“may,” “would,” “should,” “potential,” “designed to,” “will,”
“ongoing,” “estimate,” “forecast,” “predict,” “could,” and similar references,
although not all forward-looking statements contain these words. Forward-looking statements are neither historical facts nor assurances
of future performance. These statements are based only on our current beliefs, expectations and assumptions regarding the future of our
business, future plans and strategies, projections, anticipated events and trends, the economy and other future conditions. Because forward-looking
statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to
predict and many of which are outside of our control. Risks that could cause actual results to vary from expected results expressed in
our forward-looking statements include, but are not limited to:
| · | our business and operations may be materially and adversely affected by the coronavirus pandemic; |
| · | our product candidates are in an early stage of development and may fail or experience significant delays or may never advance to
the clinic, which may materially and adversely impact our business; |
| · | we are dependent on collaboration partners for the successful development of our adoptive cell therapy product candidates; |
| | · | we rely upon third-party relationships to conduct preclinical studies,
and any future clinical trials, for our product candidates and may not be able to establish or maintain the third-party relationships
that are necessary to support their development; |
| | · | we rely upon third parties for the manufacture of our product candidates; |
| · | the approach we are taking to discover and develop novel therapeutics using RNAi may never lead to marketable products; |
| · | a number of different factors could prevent us from advancing into clinical development, obtaining regulatory approval, and ultimately
commercializing our product candidates on a timely basis, or at all; |
| · | we may be unable to protect our intellectual property rights licensed from other parties; our intellectual property rights may be
inadequate to prevent third parties from using our technologies or developing competing products; and we may need to license additional
intellectual property from others; |
| · | we are subject to significant competition and may not be able to compete successfully; |
| · | if we fail to attract, hire and retain qualified personnel, we may not be able to design, develop, market or sell our products or
successfully manage our business; |
| · | future financing may be obtained through, and future development efforts may be paid for by, the issuance of debt or equity, which
may have an adverse effect on our stockholders or may otherwise adversely affect our business; and |
| · | the price of our common stock has been and may continue to be volatile. |
Our actual results and financial condition may differ materially from
those indicated in the forward-looking statements as a result of the foregoing factors, as well as those identified in this prospectus
or applicable prospectus supplement under the heading “Risk Factors” and in other filings the Company periodically makes with
the SEC. Therefore, you should not rely unduly on any of these forward-looking statements. Forward-looking statements contained in this
prospectus speak as of the date hereof and the Company does not undertake to update any of these forward-looking statements to reflect
a change in its views or events or circumstances that occur after the date of this report.
DESCRIPTION OF SECURITIES
We may offer shares of our common stock and preferred stock, various
series of debt securities, warrants, and units to purchase any such securities with a total value of up to $100,000,000 from time to time
under this prospectus at prices and on terms to be determined by market conditions at the time of offering. Each time we offer a type
or series of securities, we will provide a prospectus supplement that will describe the specific amounts, prices and other important terms
of the securities.
Common Stock.
We may issue shares of our common stock from time to time. Holders
of our common stock are entitled to one vote per share for the election of directors and on all other matters that require stockholder
approval. Subject to any preferential rights of any outstanding preferred stock, in the event of our liquidation, dissolution or winding
up, holders of our common stock are entitled to share ratably in the assets remaining after payment of liabilities and the liquidation
preferences of any outstanding preferred stock. Our common stock does not carry any redemption rights or any preemptive rights enabling
a holder to subscribe for, or receive shares of, any class of our common stock or any other securities convertible into shares of any
class of our common stock.
We are authorized to issue 100,000,000 shares of common stock, par
value $0.0001 per share.
Our common stock is listed on the NASDAQ Capital Market under the symbol
“PHIO”. The transfer agent and registrar for our common stock is Computershare Trust Company, N.A.
Preferred Stock.
We may issue shares of our preferred stock from time to time, in one
or more series. Under our certificate of incorporation, our board of directors has the authority, without further action by stockholders,
to designate up to 10,000,000 shares of preferred stock in one or more series and to fix the rights, preferences, privileges, qualifications
and restrictions granted to or imposed upon the preferred stock, including dividend rights, conversion rights, voting rights, rights and
terms of redemption, liquidation preference and sinking fund terms, any or all of which may be greater than the rights of the common stock.
If we issue preferred stock, we will fix the rights, preferences, privileges,
qualifications and restrictions of the preferred stock of each series that we sell under this prospectus and applicable prospectus supplements
in the certificate of designations relating to that series. If we issue preferred stock, we will incorporate by reference into the registration
statement of which this prospectus is a part the form of any certificate of designations that describes the terms of the series of preferred
stock we are offering before the issuance of the related series of preferred stock. We urge you to read the prospectus supplement related
to any series of preferred stock we may offer, as well as the complete certificate of designations that contains the terms of the applicable
series of preferred stock.
Debt Securities.
The paragraphs below describe the general terms and provisions of the
debt securities we may issue. When we offer to sell a particular series of debt securities, we will describe the specific terms of the
securities in a supplement to this prospectus, including any additional covenants or changes to existing covenants relating to such series.
The prospectus supplement also will indicate whether the general terms and provisions described in this prospectus apply to a particular
series of debt securities. You should read the actual indenture if you do not fully understand a term or the way we use it in this prospectus
or applicable prospectus supplement.
We may offer senior or subordinated debt securities. Each series of
debt securities may have different terms. The senior debt securities will be issued under one or more senior indentures, dated as of a
date prior to such issuance, between us and a trustee, as amended or supplemented from time to time. We will refer to any such indenture
throughout this prospectus as the “senior indenture.” Any subordinated debt securities will be issued under one or more separate
indentures, dated as of a date prior to such issuance, between us and a trustee, as amended or supplemented from time to time. We will
refer to any such indenture throughout this prospectus as the “subordinated indenture” and to the trustee under the senior
or subordinated indenture as the “trustee.” The senior indenture and the subordinated indenture are sometimes collectively
referred to in this prospectus as the “indentures.” The indentures will be subject to and governed by the Trust Indenture
Act of 1939, as amended. We included copies of the forms of the indentures as exhibits to our registration statement and they are incorporated
into this prospectus by reference.
If we issue debt securities at a discount from their principal amount,
then, for purposes of calculating the aggregate initial offering price of the offered securities issued under this prospectus, we will
include only the initial offering price of the debt securities and not the principal amount of the debt securities.
We have summarized below the material provisions of the indentures
and the debt securities, or indicated which material provisions will be described in the related prospectus supplement. The prospectus
supplement relating to any particular securities offered will describe the specific terms of the securities, which may be in addition
to or different from the general terms summarized in this prospectus. Because the summary in this prospectus and in any prospectus supplement
does not contain all of the information that you may find useful, you should read the documents relating to the securities that are described
in this prospectus or in any applicable prospectus supplement. Please read “Where You Can Find More Information” in this prospectus
to find out how you can obtain a copy of those documents. Except as otherwise indicated, the terms of the indentures are identical. As
used under this caption, the term “debt securities” includes the debt securities being offered by this prospectus and all
other debt securities issued by us under the indentures.
General
The indentures:
| |
· |
|
do not limit the amount of debt securities that we may issue; |
| |
· |
|
allow us to issue debt securities in one or more series; |
| |
· |
|
do not require us to issue all of the debt securities of a series at the same time; |
| |
· |
|
allow us to reopen a series to issue additional debt securities without the consent of the holders of the debt securities of such series; and |
| |
· |
|
provide that the debt securities will be unsecured, except as may be set forth in the applicable prospectus supplement. |
Unless we give you different information in the applicable prospectus
supplement, the senior debt securities will be unsubordinated obligations and will rank equally with all of our other unsecured and unsubordinated
indebtedness. Payments on the subordinated debt securities will be subordinated to the prior payment in full of all of our senior indebtedness,
as described under “Description of the Debt Securities—Subordination” and in the applicable prospectus supplement.
Each indenture provides that we may, but need not, designate more than
one trustee under an indenture. Any trustee under an indenture may resign or be removed and a successor trustee may be appointed to act
with respect to the series of debt securities administered by the resigning or removed trustee. If two or more persons are acting as trustee
with respect to different series of debt securities, each trustee shall be a trustee of a trust under the applicable indenture separate
and apart from the trust administered by any other trustee. Except as otherwise indicated in this prospectus, any action described in
this prospectus to be taken by each trustee may be taken by each trustee with respect to, and only with respect to, the one or more series
of debt securities for which it is trustee under the applicable indenture.
The prospectus supplement for each offering will provide the following
terms, where applicable:
| |
· |
|
the title of the debt securities and whether they are senior or subordinated; |
| |
· |
|
the aggregate principal amount of the debt securities being offered, the aggregate principal amount of the debt securities outstanding as of the most recent practicable date and any limit on their aggregate principal amount, including the aggregate principal amount of debt securities authorized; |
| |
· |
|
the price at which the debt securities will be
issued, expressed as a percentage of the principal and, if other than the principal amount thereof, the portion of the principal
amount thereof payable upon declaration of acceleration of the maturity thereof or, if applicable, the portion of the principal amount of such debt securities that is convertible into common stock or preferred stock or the method by which any such portion shall be determined; |
| |
· |
|
if convertible, the terms on which such debt securities are convertible, including the initial conversion price or rate and the conversion period and any applicable limitations on the ownership or transferability of common stock or preferred stock received on conversion; |
| |
· |
|
the date or dates, or the method for determining the date or dates, on which the principal of the debt securities will be payable; |
| |
· |
|
the fixed or variable interest rate or rates of the debt securities, or the method by which the interest rate or rates is determined; |
| |
· |
|
the date or dates, or the method for determining the date or dates, from which interest will accrue; |
| |
· |
|
the dates on which interest will be payable; |
| |
· |
|
the record dates for interest payment dates, or the method by which we will determine those dates; |
| |
· |
|
the persons to whom interest will be payable; |
| |
· |
|
the basis upon which interest will be calculated if other than that of a 360-day year of twelve 30-day months; |
| |
· |
|
any make-whole amount, which is the amount in addition to principal and interest that is required to be paid to the holder of a debt security as a result of any optional redemption or accelerated payment of such debt security, or the method for determining the make-whole amount; |
| |
· |
|
the place or places where the principal of, and any premium, or make-whole amount, and interest on, the debt securities will be payable; |
| |
· |
|
where the debt securities may be surrendered for registration of transfer or conversion or exchange; |
| |
· |
|
where notices or demands to or upon us in respect of the debt securities and the applicable indenture may be served; |
| |
· |
|
the times, prices and other terms and conditions upon which we may redeem the debt securities; |
| |
· |
|
any obligation we have to redeem, repay or purchase the debt securities pursuant to any sinking fund or analogous provision or at the option of holders of the debt securities, and the times and prices at which we must redeem, repay or purchase the debt securities as a result of such an obligation; |
| |
· |
|
the currency or currencies in which the debt securities are denominated and payable if other than United States dollars, which may be a foreign currency or units of two or more foreign currencies or a composite currency or currencies and the terms and conditions relating thereto, and the manner of determining the equivalent of such foreign currency in United States dollars; |
| |
· |
|
whether the principal of, and any premium, or make-whole amount, or interest on, the debt securities of the series are to be payable, at our election or at the election of a holder, in a currency or currencies other than that in which the debt securities are denominated or stated to be payable, and other related terms and conditions; |
| |
· |
|
whether the amount of payments of principal of, and any premium, or make-whole amount, or interest on, the debt securities may be determined according to an index, formula or other method and how such amounts will be determined; |
| |
· |
|
whether the debt securities will be in registered form, bearer form or both and (1) if in registered form, the person to whom any interest shall be payable, if other than the person in whose name the security is registered at the close of business on the regular record date for such interest, or (2) if in bearer form, the manner in which, or the person to whom, any interest on the security shall be payable if otherwise than upon presentation and surrender upon maturity; |
| |
· |
|
any restrictions applicable to the offer, sale or delivery of securities in bearer form and the terms upon which securities in bearer form of the series may be exchanged for securities in registered form of the series and vice versa if permitted by applicable laws and regulations; |
| |
· |
|
whether any debt securities of the series are to be issuable initially in temporary global form and whether any debt securities of the series are to be issuable in permanent global form with or without coupons and, if so, whether beneficial owners of interests in any such permanent global security may or shall be required to exchange their interests for other debt securities of the series, and the manner in which interest shall be paid; |
| |
· |
|
the identity of the depositary for securities in registered form, if such series are to be issuable as a global security; |
| |
· |
|
the date as of which any debt securities in bearer form or in temporary global form shall be dated if other than the original issuance date of the first security of the series to be issued; |
| |
· |
|
the applicability, if any, of the defeasance and covenant defeasance provisions described in this prospectus or in the applicable indenture; |
| |
· |
|
whether and under what circumstances we will pay any additional amounts on the debt securities in respect of any tax, assessment or governmental charge and, if so, whether we will have the option to redeem the debt securities in lieu of making such a payment; |
| |
· |
|
whether and under what circumstances the debt securities being offered are convertible into common stock or preferred stock, as the case may be, including the conversion price or rate or manner or calculation thereof; |
| |
· |
|
the circumstances, if any, specified in the applicable prospectus supplement, under which beneficial owners of interests in the global security may obtain definitive debt securities and the manner in which payments on a permanent global debt security will be made if any debt securities are issuable in temporary or permanent global form; |
| |
· |
|
any provisions granting special rights to holders of securities upon the occurrence of such events as specified in the applicable prospectus supplement; |
| |
· |
|
if the debt securities of such series are to be issuable in definitive form only upon receipt of certain certificates or other documents or satisfaction of other conditions, then the form and/or terms of such certificates, documents or conditions; |
| |
· |
|
the name of the applicable trustee and the nature of any material relationship with us or any of our affiliates, and the percentage of debt securities of the class necessary to require the trustee to take action; |
| |
· |
|
any deletions from, modifications of, or additions to our events of default or covenants and any change in the right of any trustee or any of the holders to declare the principal amount of any of such debt securities due and payable; |
| |
· |
|
applicable CUSIP numbers; and |
| |
· |
|
any other terms of such debt securities not inconsistent with the provisions of the applicable indenture. |
We may issue debt securities at a discount below their principal amount
and provide for less than the entire principal amount thereof to be payable upon declaration of acceleration of the maturity of the debt
securities. We refer to any such debt securities throughout this prospectus as “original issue discount securities.” The applicable
prospectus supplement will describe the United States federal income tax consequences and other relevant considerations applicable to
original issue discount securities.
We also may issue indexed debt securities. Payments of principal of,
and premium and interest on, indexed debt securities are determined with reference to the rate of exchange between the currency or currency
unit in which the debt security is denominated and any other currency or currency unit specified by us, to the relationship between two
or more currencies or currency units or by other similar methods or formulas specified in the prospectus supplement.
Except as described under “—Merger, Consolidation or Sale
of Assets” or as may be set forth in any prospectus supplement, the debt securities will not contain any provisions that (1) would
limit our ability to incur indebtedness or (2) would afford holders of debt securities protection in the event of (a) a highly leveraged
or similar transaction involving us, or (b) a change of control or reorganization, restructuring, merger or similar transaction involving
us that may adversely affect the holders of the debt securities. In the future, we may enter into transactions, such as the sale of all
or substantially all of our assets or a merger or consolidation, that may have an adverse effect on our ability to service our indebtedness,
including the debt securities, by, among other things, substantially reducing or eliminating our assets.
Neither the Delaware General Corporation Law nor our governing instruments
define the term “substantially all” as it relates to the sale of assets. Additionally, Delaware cases interpreting the term
“substantially all” rely upon the facts and circumstances of each particular case. Consequently, to determine whether a sale
of “substantially all” of our assets has occurred, a holder of debt securities must review the financial and other information
that we have disclosed to the public.
We will provide you with more information in the applicable prospectus
supplement regarding any deletions, modifications, or additions to the events of default or covenants that are described below, including
any addition of a covenant or other provision providing event risk or similar protection.
Payment
Unless we give you different information in the applicable prospectus
supplement, the principal of, and any premium, or make-whole amount, and interest on, any series of the debt securities will be payable
at the corporate trust office of the trustee. We will provide you with the address of the trustee in the applicable prospectus supplement.
We may also pay interest by mailing a check to the address of the person entitled to it as it appears in the applicable register for the
debt securities or by wire transfer of funds to that person at an account maintained within the United States.
All monies that we pay to a paying agent or a trustee for the payment
of the principal of, and any premium, or make-whole amount, or interest on, any debt security will be repaid to us if unclaimed at the
end of two years after the obligation underlying payment becomes due and payable. After funds have been returned to us, the holder of
the debt security may look only to us for payment, without payment of interest for the period which we hold the funds.
Denomination, Interest, Registration and Transfer
Unless otherwise described in the applicable prospectus supplement,
the debt securities of any series will be issuable in denominations of $1,000 and integral multiples of $1,000.
Subject to the limitations imposed upon debt securities that are evidenced
by a computerized entry in the records of a depository company rather than by physical delivery of a note, a holder of debt securities
of any series may:
| |
· |
|
exchange them for any authorized denomination of other debt securities of the same series and of a like aggregate principal amount and kind upon surrender of such debt securities at the corporate trust office of the applicable trustee or at the office of any transfer agent that we designate for such purpose; and |
| |
· |
|
surrender them for registration of transfer or exchange at the corporate trust office of the applicable trustee or at the office of any transfer agent that we designate for such purpose. |
Every debt security surrendered for registration of transfer or exchange
must be duly endorsed or accompanied by a written instrument of transfer satisfactory to the applicable trustee or transfer agent. Payment
of a service charge will not be required for any registration of transfer or exchange of any debt securities, but we or the trustee may
require payment of a sum sufficient to cover any tax or other governmental charge payable in connection therewith. If in addition to the
applicable trustee, the applicable prospectus supplement refers to any transfer agent initially designated by us for any series of debt
securities, we may at any time rescind the designation of any such transfer agent or approve a change in the location through which any
such transfer agent acts, except that we will be required to maintain a transfer agent in each place of payment for such series. We may
at any time designate additional transfer agents for any series of debt securities.
Neither we, nor any trustee, will be required to:
| |
· |
|
issue, register the transfer of or exchange debt securities of any series during a period beginning at the opening of business 15 days before the day that the notice of redemption of any debt securities selected for redemption is mailed and ending at the close of business on the day of such mailing; |
| |
· |
|
register the transfer of or exchange any debt security, or portion thereof, so selected for redemption, in whole or in part, except the unredeemed portion of any debt security being redeemed in part; and |
| |
· |
|
issue, register the transfer of or exchange any debt security that has been surrendered for repayment at the option of the holder, except the portion, if any, of such debt security not to be so repaid. |
Merger, Consolidation or Sale of Assets
The indentures provide that we may, without the consent of the holders
of any outstanding debt securities, (1) consolidate with, (2) sell, lease or convey all or substantially all of our assets to, or (3)
merge with or into, any other entity provided that:
| |
· |
|
either we are the continuing entity, or the successor entity, if other than us, assumes the obligations (A) to pay the principal of, and any premium (or make-whole amount) and interest on, all of the debt securities and (B) to duly perform and observe all of the covenants and conditions contained in each indenture; |
| |
· |
|
after giving effect to the transaction, there is no event of default under the indentures and no event which, after notice or the lapse of time, or both, would become such an event of default, occurs and continues; and |
| |
· |
|
an officers’ certificate and legal opinion covering such conditions are delivered to each applicable trustee. |
Covenants
Existence. Except as permitted under “Debt Securities—Merger,
Consolidation or Sale of Assets,” the indentures require us to do or cause to be done all things necessary to preserve and keep
in full force and effect our existence, rights and franchises. However, the indentures do not require us to preserve any right or franchise
if we determine that any right or franchise is no longer desirable in the conduct of our business.
Provision of financial information. The indentures require us
to (1) within 15 days of each of the respective dates by which we are required to file our annual reports, quarterly reports and other
documents with the SEC, file with the trustee copies of the annual report, quarterly report and other documents that we file with the
SEC under Section 13 or 15(d) of the Securities Exchange Act of 1934, as amended, or the Exchange Act, (2) file with the trustee and the
SEC any additional information, documents and reports regarding compliance by us with the conditions and covenants of the indentures,
as required, (3) within 30 days after the filing with the trustee, mail to all holders of debt securities, as their names and addresses
appear in the applicable register for such debt securities, without cost to such holders, summaries of any documents and reports required
to be filed by us pursuant to (1) and (2) above, and (4) supply, promptly upon written request and payment of the reasonable cost of duplication
and delivery, copies of such documents to any prospective holder.
Additional covenants. The applicable prospectus supplement will
set forth any additional covenants relating to any series of debt securities.
Events of Default, Notice and Waiver
Unless the applicable prospectus supplement states otherwise, when
we refer to “events of default” as defined in the indentures with respect to any series of debt securities, we mean:
| |
· |
|
default in the payment of any installment of interest on any debt security of such series continuing for 30 days; |
| |
· |
|
default in the payment of principal of, or any premium, or make-whole amount, on any debt security of such series for five business days at its stated maturity; |
| |
· |
|
default in making any sinking fund payment as required for any debt security of such series for five business days; |
| |
· |
|
default in the performance or breach of any covenant or warranty in the debt securities or in the indenture continuing for 60 days after written notice as provided in the applicable indenture, but not of a covenant added to the indenture solely for the benefit of a series of debt securities issued thereunder other than such series; |
| |
· |
|
bankruptcy, insolvency or reorganization, or court appointment of a receiver, liquidator or trustee of the Company or any significant subsidiary of the Company; and |
| |
· |
|
any other event of default provided with respect to a particular series of debt securities. |
When we use the term “significant subsidiary,” we refer
to the meaning ascribed to such term in Rule 1-02 of Regulation S-X promulgated under the Securities Act of 1933, as amended, or Securities
Act.
If an event of default occurs and is continuing with respect to debt
securities of any series outstanding, then the applicable trustee or the holders of 25% or more in principal amount of the debt securities
of that series will have the right to declare the principal amount of all the debt securities of that series to be due and payable. If
the debt securities of that series are original issue discount securities or indexed securities, then the applicable trustee or the holders
of 25% or more in principal amount of the debt securities of that series will have the right to declare the portion of the principal amount
as may be specified in the terms thereof to be due and payable. However, at any time after such a declaration of acceleration has been
made, but before a judgment or decree for payment of the money due has been obtained by the applicable trustee, the holders of at least
a majority in principal amount of outstanding debt securities of such series or of all debt securities then outstanding under the applicable
indenture may rescind and annul such declaration and its consequences if:
| |
· |
|
we have deposited with the applicable trustee all required payments of the principal, any premium, or make-whole amount, interest and, to the extent permitted by law, interest on overdue installment of interest, plus applicable fees, expenses, disbursements and advances of the applicable trustee; and |
| |
· |
|
all events of default, other than the non-payment of accelerated principal, or a specified portion thereof, and any premium, or make-whole amount, have been cured or waived. |
The indentures also provide that the holders of at least a majority
in principal amount of the outstanding debt securities of any series or of all debt securities then outstanding under the applicable indenture
may, on behalf of all holders, waive any past default with respect to such series and its consequences, except a default:
| |
· |
|
in the payment of the principal, any premium, or make-whole amount, or interest; |
| |
· |
|
in respect of a covenant or provision contained in the applicable indenture that cannot be modified or amended without the consent of the holders of the outstanding debt security that is affected by the default; or |
| |
· |
|
in respect of a covenant or provision for the benefit or protection of the trustee, without its express written consent. |
The indentures require each trustee to give notice to the holders of
debt securities within 90 days of a default unless such default has been cured or waived. However, the trustee may withhold notice if
specified persons of such trustee consider such withholding to be in the interest of the holders of debt securities. The trustee may not
withhold notice of a default in the payment of principal, any premium or interest on any debt security of such series or in the payment
of any sinking fund installment in respect of any debt security of such series.
The indentures provide that holders of debt securities of any series
may not institute any proceedings, judicial or otherwise, with respect to such indenture or for any remedy under the indenture, unless
the trustee fails to act for a period of 60 days after the trustee has received a written request to institute proceedings in respect
of an event of default from the holders of 25% or more in principal amount of the outstanding debt securities of such series, as well
as an offer of indemnity reasonably satisfactory to the trustee. However, this provision will not prevent any holder of debt securities
from instituting suit for the enforcement of payment of the principal of, and any premium, or make-whole amount, and interest on, such
debt securities at the respective due dates thereof.
The indentures provide that, subject to provisions in each indenture
relating to its duties in the case of a default, a trustee has no obligation to exercise any of its rights or powers at the request or
direction of any holders of any series of debt securities then outstanding under the indenture, unless the holders have offered to the
trustee reasonable security or indemnity. The holders of at least a majority in principal amount of the outstanding debt securities of
any series or of all debt securities then outstanding under an indenture shall have the right to direct the time, method and place of
conducting any proceeding for any remedy available to the applicable trustee, or of exercising any trust or power conferred upon such
trustee. However, a trustee may refuse to follow any direction which:
| |
· |
|
is in conflict with any law or the applicable indenture; |
| |
· |
|
upon a good faith determination of a responsible officer of the trustee, may involve the trustee in personal liability; or |
| |
· |
|
upon a good faith determination of a responsible officer of the trustee, may be unduly prejudicial to the holders of debt securities of the series not joining the proceeding. |
Within 120 days after the close of each fiscal year, we will be required
to deliver to each trustee a certificate, signed by one of our several specified officers, stating whether or not that officer has knowledge
of any default under the applicable indenture. If the officer has knowledge of any default, the notice must specify the nature and status
of the default.
Modification of the Indentures
The indentures provide that modifications and amendments may be made
only with the consent of the affected holders of at least a majority in principal amount of all outstanding debt securities issued under
that indenture. However, no such modification or amendment may, without the consent of the holders of the debt securities affected by
the modification or amendment:
| |
· |
|
change the stated maturity of the principal of, or any premium, or make-whole amount, on, or any installment of principal of or interest on, any such debt security; |
| |
· |
|
reduce the principal amount of, the rate or amount of interest on or any premium, or make-whole amount, payable on redemption of any such debt security; |
| |
· |
|
reduce the amount of principal of an original issue discount security that would be due and payable upon declaration of acceleration of the maturity thereof or would be provable in bankruptcy, or adversely affect any right of repayment of the holder of any such debt security; |
| |
· |
|
change the place of payment or the coin or currency for payment of principal of, or any premium, or make-whole amount, or interest on, any such debt security; |
| |
· |
|
impair the right to institute suit for the enforcement of any payment on or with respect to any such debt security; |
| |
· |
|
reduce the percentage in principal amount of any outstanding debt securities necessary to modify or amend the applicable indenture with respect to such debt securities, to waive compliance with particular provisions thereof or defaults and consequences thereunder or to reduce the quorum or voting requirements set forth in the applicable indenture; and |
| |
· |
|
modify any of the foregoing provisions or any of the provisions relating to the waiver of particular past defaults or covenants, except to increase the required percentage to effect such action or to provide that some of the other provisions may not be modified or waived without the consent of the holder of such debt security. |
The holders of a majority in aggregate principal amount of the outstanding
debt securities of each series may, on behalf of all holders of debt securities of that series, waive, insofar as that series is concerned,
our compliance with material restrictive covenants of the applicable indenture.
We and our respective trustee may make modifications and amendments
of an indenture without the consent of any holder of debt securities for any of the following purposes:
| |
· |
|
to evidence the succession of another person to us as obligor under such indenture; |
| |
· |
|
to add to our covenants for the benefit of the holders of all or any series of debt securities or to surrender any right or power conferred upon us in such indenture; |
| |
· |
|
to add events of default for the benefit of the holders of all or any series of debt securities; |
| |
· |
|
to add or change any provisions of an indenture (1) to change or eliminate restrictions on the payment of principal of, or premium, or make-whole amount, or interest on, debt securities in bearer form, or (2) to permit or facilitate the issuance of debt securities in uncertificated form, provided that such action shall not adversely affect the interests of the holders of the debt securities of any series in any material respect; |
| |
· |
|
to change or eliminate any provisions of an indenture, provided that any such change or elimination shall become effective only when there are no debt securities outstanding of any series created prior thereto which are entitled to the benefit of such provision; |
| |
· |
|
to secure the debt securities; |
| |
· |
|
to establish the form or terms of debt securities of any series; |
| |
· |
|
to provide for the acceptance of appointment by a successor trustee or facilitate the administration of the trusts under an indenture by more than one trustee; |
| |
· |
|
to cure any ambiguity, defect or inconsistency in an indenture, provided that such action shall not adversely affect the interests of holders of debt securities of any series issued under such indenture; and |
| |
· |
|
to supplement any of the provisions of an indenture to the extent necessary to permit or facilitate defeasance and discharge of any series of such debt securities, provided that such action shall not adversely affect the interests of the holders of the outstanding debt securities of any series. |
Voting
The indentures provide that in determining whether the holders of the
requisite principal amount of outstanding debt securities of a series have given any request, demand, authorization, direction, notice,
consent or waiver under the indentures or whether a quorum is present at a meeting of holders of debt securities:
| |
· |
|
the principal amount of an original issue discount security that shall be deemed to be outstanding shall be the amount of the principal thereof that would be due and payable as of the date of such determination upon declaration of acceleration of the maturity thereof; |
| |
· |
|
the principal amount of any debt security denominated in a foreign currency that shall be deemed outstanding shall be the United States dollar equivalent, determined on the issue date for such debt security, of the principal amount or, in the case of an original issue discount security, the United States dollar equivalent on the issue date of such debt security of the amount determined as provided in the preceding bullet point; |
| |
· |
|
the principal amount of an indexed security that shall be deemed outstanding shall be the principal face amount of such indexed security at original issuance, unless otherwise provided for such indexed security under such indenture; and |
| |
· |
|
debt securities owned by us or any other obligor upon the debt securities or by any affiliate of ours or of such other obligor shall be disregarded. |
The indentures contain provisions for convening meetings of the
holders of debt securities of a series. A meeting will be permitted to be called at any time by the applicable trustee, and also,
upon request, by us or the holders of at least 25% in principal amount of the outstanding debt securities of such series, in any
such case upon notice given as provided in such indenture. Except for any consent that must be given by the holder of each debt
security affected by the modifications and amendments of an indenture described above, any resolution presented at a meeting or
adjourned meeting duly reconvened at which a quorum is present may be adopted by the affirmative vote of the holders of a majority
of the aggregate principal amount of the outstanding debt securities of that series represented at such meeting.
Notwithstanding the preceding paragraph, except as referred to above,
any resolution relating to a request, demand, authorization, direction, notice, consent, waiver or other action that may be made, given
or taken by the holders of a specified percentage, which is less than a majority of the aggregate principal amount of the outstanding
debt securities of a series, may be adopted at a meeting or adjourned meeting duly reconvened at which a quorum is present by the affirmative
vote of such specified percentage.
Any resolution passed or decision taken at any properly held meeting
of holders of debt securities of any series will be binding on all holders of such series. The quorum at any meeting called to adopt a
resolution, and at any reconvened meeting, will be persons holding or representing a majority in principal amount of the outstanding debt
securities of a series. However, if any action is to be taken relating to a consent or waiver which may be given by the holders of at
least a specified percentage in principal amount of the outstanding debt securities of a series, the persons holding such percentage will
constitute a quorum.
Notwithstanding the foregoing provisions, the indentures provide that
if any action is to be taken at a meeting with respect to any request, demand, authorization, direction, notice, consent, waiver or other
action that such indenture expressly provides may be made, given or taken by the holders of a specified percentage in principal amount
of all outstanding debt securities affected by such action, or of the holders of such series and one or more additional series:
| |
· |
|
there shall be no minimum quorum requirement for such meeting; and |
| |
· |
|
the principal amount of the outstanding debt securities of such series that vote in favor of such request, demand, authorization, direction, notice, consent, waiver or other action shall be taken account in determining whether such request, demand, authorization, direction, notice, consent, waiver or other action has been made, given or taken under such indenture. |
Subordination
Unless otherwise provided in the applicable prospectus supplement,
subordinated securities will be subject to the following subordination provisions.
Upon any distribution to our creditors in a liquidation, dissolution
or reorganization, the payment of the principal of and interest on any subordinated securities will be subordinated to the extent provided
in the applicable indenture in right of payment to the prior payment in full of all senior debt. However, our obligation to make payments
of the principal of and interest on such subordinated securities otherwise will not be affected. No payment of principal or interest will
be permitted to be made on subordinated securities at any time if a default on senior debt exists that permits the holders of such senior
debt to accelerate its maturity and the default is the subject of judicial proceedings or we receive notice of the default. After all
senior debt is paid in full and until the subordinated securities are paid in full, holders of subordinated securities will be subrogated
to the rights of holders of senior debt to the extent that distributions otherwise payable to holders of subordinated securities have
been applied to the payment of senior debt. The subordinated indenture will not restrict the amount of senior debt or other indebtedness
of the Company and its subsidiaries. As a result of these subordination provisions, in the event of a distribution of assets upon insolvency,
holders of subordinated securities may recover less, ratably, than our general creditors.
The term “senior debt” will be defined in the applicable
indenture as the principal of and interest on, or substantially similar payments to be made by us in respect of, other outstanding indebtedness,
whether outstanding at the date of execution of the applicable indenture or subsequently incurred, created or assumed. The prospectus
supplement may include a description of additional terms implementing the subordination feature.
No restrictions will be included in any indenture relating to subordinated
securities upon the creation of additional senior debt.
If this prospectus is being delivered in connection with the offering
of a series of subordinated securities, the accompanying prospectus supplement or the information incorporated in this prospectus by reference
will set forth the approximate amount of senior debt outstanding as of the end of our most recent fiscal quarter.
Discharge, Defeasance and Covenant Defeasance
Unless otherwise indicated in the applicable prospectus supplement,
the indentures allow us to discharge our obligations to holders of any series of debt securities issued under any indenture when:
| |
· |
|
either (1) all securities of such series have already been delivered to the applicable trustee for cancellation; or (2) all securities of such series have not already been delivered to the applicable trustee for cancellation but (A) have become due and payable, (B) will become due and payable within one year, or (C) if redeemable at our option, are to be redeemed within one year, and we have irrevocably deposited with the applicable trustee, in trust, funds in such currency or currencies, currency unit or units or composite currency or currencies in which such debt securities are payable, an amount sufficient to pay the entire indebtedness on such debt securities in respect of principal and any premium, or make-whole amount, and interest to the date of such deposit if such debt securities have become due and payable or, if they have not, to the stated maturity or redemption date; |
| |
· |
|
we have paid or caused to be paid all other sums payable; and |
| |
· |
|
an officers’ certificate and an opinion of counsel stating the conditions to discharging the debt securities have been satisfied has been delivered to the trustee. |
Unless otherwise indicated in the applicable prospectus supplement,
the indentures provide that, upon our irrevocable deposit with the applicable trustee, in trust, of an amount, in such currency or currencies,
currency unit or units or composite currency or currencies in which such debt securities are payable at stated maturity, or government
obligations, or both, applicable to such debt securities, which through the scheduled payment of principal and interest in accordance
with their terms will provide money in an amount sufficient to pay the principal of, and any premium, or make-whole amount, and interest
on, such debt securities, and any mandatory sinking fund or analogous payments thereon, on the scheduled due dates therefor, the issuing
company may elect either:
| |
· |
|
to defease and be discharged from any and all obligations with respect to such debt securities; or |
| |
· |
|
to be released from its obligations with respect to such debt securities under the applicable indenture or, if provided in the applicable prospectus supplement, its obligations with respect to any other covenant, and any omission to comply with such obligations shall not constitute an event of default with respect to such debt securities. |
Notwithstanding the above, we may not elect to defease and be discharged
from the obligation to pay any additional amounts upon the occurrence of particular events of tax, assessment or governmental charge with
respect to payments on such debt securities and the obligations to register the transfer or exchange of such debt securities, to replace
temporary or mutilated, destroyed, lost or stolen debt securities, to maintain an office or agency in respect of such debt securities,
or to hold monies for payment in trust.
The indentures only permit us to establish the trust described in the
paragraph above if, among other things, it has delivered to the applicable trustee an opinion of counsel to the effect that the holders
of such debt securities will not recognize income, gain or loss for United States federal income tax purposes as a result of such defeasance
or covenant defeasance and will be subject to United States federal income tax on the same amounts, in the same manner and at the same
times as would have been the case if such defeasance or covenant defeasance had not occurred. Such opinion of counsel, in the case of
defeasance, will be required to refer to and be based upon a ruling received from or published by the Internal Revenue Service or a change
in applicable United States federal income tax law occurring after the date of the indenture. In the event of such defeasance, the holders
of such debt securities would be able to look only to such trust fund for payment of principal, any premium, or make-whole amount, and
interest.
When we use the term “government obligations,” we mean
securities that are:
| |
· |
|
direct obligations of the United States or the government that issued the foreign currency in which the debt securities of a particular series are payable, for the payment of which its full faith and credit is pledged; or |
| |
· |
|
obligations of a person controlled or supervised by and acting as an agency or instrumentality of the United States or other government that issued the foreign currency in which the debt securities of such series are payable, the payment of which is unconditionally guaranteed as a full faith and credit obligation by the United States or such other government, which are not callable or redeemable at the option of the issuer thereof and shall also include a depository receipt issued by a bank or trust company as custodian with respect to any such government obligation or a specific payment of interest on or principal of any such government obligation held by such custodian for the account of the holder of a depository receipt. However, except as required by law, such custodian is not authorized to make any deduction from the amount payable to the holder of such depository receipt from any amount received by the custodian in respect of the government obligation or the specific payment of interest on or principal of the government obligation evidenced by such depository receipt. |
Unless otherwise provided in the applicable prospectus supplement,
if after we have deposited funds and/or government obligations to effect defeasance or covenant defeasance with respect to debt securities
of any series, (1) the holder of a debt security of such series is entitled to, and does, elect under the terms of the applicable indenture
or the terms of such debt security to receive payment in a currency, currency unit or composite currency other than that in which such
deposit has been made in respect of such debt security, or (2) a conversion event occurs in respect of the currency, currency unit or
composite currency in which such deposit has been made, the indebtedness represented by such debt security will be deemed to have been,
and will be, fully discharged and satisfied through the payment of the principal of, and premium, or make whole amount, and interest on,
such debt security as they become due out of the proceeds yielded by converting the amount so deposited in respect of such debt security
into the currency, currency unit or composite currency in which such debt security becomes payable as a result of such election or such
cessation of usage based on the applicable market exchange rate.
When we use the term “conversion event,” we mean the cessation
of use of:
| |
· |
|
a currency, currency unit or composite currency both by the government of the country that issued such currency and for the settlement of transactions by a central bank or other public institutions of or within the international banking community; |
| |
· |
|
the European Currency Unit both within the European Monetary System and for the settlement of transactions by public institutions of or within the European Communities; or |
| |
· |
|
any currency unit or composite currency other than the European Currency Unit for the purposes for which it was established. |
Unless otherwise provided in the applicable prospectus supplement,
all payments of principal of, and any premium, or make-whole amount, and interest on, any debt security that is payable in a foreign currency
that ceases to be used by its government of issuance shall be made in United States dollars.
In the event that (1) we effect covenant defeasance with respect to
any debt securities and (2) those debt securities are declared due and payable because of the occurrence of any event of default, the
amount in the currency, currency unit or composite currency in which such debt securities are payable, and government obligations on deposit
with the applicable trustee, will be sufficient to pay amounts due on such debt securities at the time of their stated maturity but may
not be sufficient to pay amounts due on such debt securities at the time of the acceleration resulting from such event of default. However,
the issuing company would remain liable to make payments of any amounts due at the time of acceleration.
If a trustee or paying agent is unable to apply any money in accordance
with the foregoing paragraphs describing discharge and defeasance by reason of any order or judgment of any court or governmental authority
enjoining, restraining or otherwise prohibiting such application, then the obligations under the indentures and such securities from which
we have been discharged or released pursuant to the foregoing shall be revived and reinstated as though no deposit had occurred with respect
to such securities, until such time as the trustee or paying agent is permitted to apply all money held in trust with respect to such
securities in accordance with the foregoing; provided, that if we make any payment of principal of or any premium or interest on any such
security following such reinstatement of its obligations, we shall be subrogated to the rights (if any) of the holders of such securities
to receive such payment from the money so held in trust.
The applicable prospectus supplement may further describe the provisions,
if any, permitting such defeasance or covenant defeasance, including any modifications to the provisions described above, with respect
to the debt securities of or within a particular series.
Conversion Rights
The terms and conditions, if any, upon which the debt securities are
convertible into common stock or preferred stock will be set forth in the applicable prospectus supplement. The terms will include whether
the debt securities are convertible into shares of common stock or preferred stock, the conversion price, or manner of calculation thereof,
the conversion period, provisions as to whether conversion will be at the issuing company’s option or the option of the holders,
the events requiring an adjustment of the conversion price and provisions affecting conversion in the event of the redemption of the debt
securities and any restrictions on conversion.
Global Securities
The debt securities of a series may be issued in whole or in part in
the form of one or more global securities that will be deposited with, or on behalf of, a depository identified in the applicable prospectus
supplement relating to such series. Global securities, if any, issued in the United States are expected to be deposited with The Depository
Trust Company, or DTC, as depository. We may issue global securities in either registered or bearer form and in either temporary or permanent
form. We will describe the specific terms of the depository arrangement with respect to a series of debt securities in the applicable
prospectus supplement relating to such series. We expect that unless the applicable prospectus supplement provides otherwise, the following
provisions will apply to depository arrangements.
All interests in global securities will be subject to the operations
and procedures of the depository for such global securities or its nominee. We provide the following summaries of those operations and
procedures solely for the convenience of investors. Once a global security is issued, we expect that the depository for such global security
or its nominee will credit on its book-entry registration and transfer system the respective principal amounts of the individual debt
securities represented by such global security to the accounts of participants that have accounts with such depository. Such accounts
shall be designated by the underwriters, dealers or agents with respect to such debt securities or by us if we offer such debt securities
directly. Ownership of beneficial interests in such global security will be limited to participants with the depository or persons that
may hold interests through those participants.
We expect that, under procedures established by DTC, ownership of beneficial
interests in any global security for which DTC is the depository will be shown on, and the transfer of that ownership will be effected
only through, records maintained by DTC or its nominee, with respect to beneficial interests of participants with the depository, and
records of participants, with respect to beneficial interests of persons who hold through participants with the depository. Neither we
nor the trustee will have any responsibility or liability for any aspect of the records of DTC or for maintaining, supervising or reviewing
any records of DTC or any of its participants relating to beneficial ownership interests in the debt securities.
So long as the depository for a global security or its nominee is the
registered owner of such global security, such depository or such nominee, as the case may be, will be considered the sole owner or holder
of the debt securities represented by the global security for all purposes under the applicable indenture. Except as described below or
in the applicable prospectus supplement, owners of beneficial interest in a global security will not be entitled to have any of the individual
debt securities represented by such global security registered in their names, will not receive or be entitled to receive physical delivery
of any such debt securities in definitive form and will not be considered the owners or holders thereof under the applicable indenture.
Beneficial owners of debt securities evidenced by a global security will not be considered the owners or holders thereof under the applicable
indenture for any purpose, including with respect to the giving of any direction, instructions or approvals to the trustee under the indenture.
Accordingly, each person owning a beneficial interest in a global security with respect to which DTC is the depository must rely on the
procedures of DTC and, if such person is not a participant with the depository, on the procedures of the participant through which such
person owns its interests, to exercise any rights of a holder under the applicable indenture.
Payments of principal of, and any premium, or make-whole amount, and
interest on, individual debt securities represented by a global security registered in the name of a depository or its nominee will be
made to or at the direction of the depository or its nominee, as the case may be, as the registered owner of the global security under
the applicable indenture. Under the terms of the applicable indenture, we and the trustee may treat the persons in whose name debt securities,
including a global security, are registered as the owners thereof for the purpose of receiving such payments. Consequently, neither we
nor the trustee have or will have any responsibility or liability for the payment of such amounts to beneficial owners of debt securities
including principal, any premium, or make-whole amount, or interest. We believe, however, that it is currently the policy of DTC to immediately
credit the accounts of relevant participants with such payments, in amounts proportionate to their respective holdings of beneficial interests
in the relevant global security as shown on the records of DTC or its nominee. We also expect that payments by participants to owners
of beneficial interests in such global security held through such participants will be governed by standing instructions and customary
practices, as is the case with securities held for the account of customers in bearer form or registered in street name, and will be the
responsibility of such participants. Redemption notices with respect to any debt securities represented by a global security will be sent
to the depository or its nominee. If less than all of the debt securities of any series are to be redeemed, we expect the depository to
determine the amount of the interest of each participant in such debt securities to be redeemed to be determined by lot. Neither we, the
trustee, any paying agent nor the security registrar for such debt securities will have any responsibility or liability for any aspect
of the records relating to or payments made on account of beneficial ownership interests in the global security for such debt securities
or for maintaining any records with respect thereto.
Neither we nor the trustee will be liable for any delay by the holders
of a global security or the depository in identifying the beneficial owners of debt securities, and we and the trustee may conclusively
rely on, and will be protected in relying on, instructions from the holder of a global security or the depository for all purposes. The
rules applicable to DTC and its participants are on file with the SEC.
If a depository for any debt securities is at any time unwilling, unable
or ineligible to continue as depository and we do not appoint a successor depository within 90 days, we will issue individual debt securities
in exchange for the global security representing such debt securities. In addition, we may at any time and in our sole discretion, subject
to any limitations described in the applicable prospectus supplement relating to such debt securities, determine not to have any of such
debt securities represented by one or more global securities and in such event will issue individual debt securities in exchange for the
global security or securities representing such debt securities. Individual debt securities so issued will be issued in denominations
of $1,000 and integral multiples of $1,000.
The debt securities of a series may also be issued in whole or in part
in the form of one or more bearer global securities that will be deposited with a depository, or with a nominee for such depository, identified
in the applicable prospectus supplement. Any such bearer global securities may be issued in temporary or permanent form. The specific
terms and procedures, including the specific terms of the depositary arrangement, with respect to any portion of a series of debt securities
to be represented by one or more bearer global securities will be described in the applicable prospectus supplement.
No Recourse
There is no recourse under any obligation, covenant or agreement in
the applicable indenture or with respect to any security against any of our or our successor’s past, present or future stockholders,
employees, officers or directors.
Warrants
We may issue warrants for the purchase of common stock, preferred stock
and/or debt securities in one or more series, from time to time. We may issue warrants independently or together with common stock, preferred
stock and/or debt securities, and the warrants may be attached to or separate from those securities.
If we issue warrants, they will be evidenced by warrant
agreements or warrant certificates issued under one or more warrant agreements, which are contracts between us and the holders of
the warrants or an agent for the holders of the warrants. We urge you to read the prospectus supplement related to any series of
warrants we may offer, as well as the complete warrant agreement and warrant certificate that contain the terms of the warrants. If
we issue warrants, forms of warrant agreements and warrant certificates relating to warrants for the purchase of common stock,
preferred stock and debt securities will be incorporated by reference into the registration statement of which this prospectus is a
part from reports we would subsequently file with the SEC.
Units
We may issue units comprised of shares of common stock, shares of preferred
stock, debt securities and warrants in any combination. We may issue units in such amounts and in as many distinct series as we wish.
This section outlines certain provisions of the units that we may issue. If we issue units, they may be issued under one or more unit
agreements to be entered into between us and a bank or other financial institution, as unit agent. The information described in this section
may not be complete in all respects and is qualified entirely by reference to the unit agreement with respect to the units of any particular
series. The specific terms of any series of units offered will be described in the applicable prospectus supplement. If so described in
a particular supplement, the specific terms of any series of units may differ from the general description of terms presented below. We
urge you to read any prospectus supplement related to any series of units we may offer, as well as the complete unit agreement and unit
certificate that contain the terms of the units. If we issue units, forms of unit agreements and unit certificates relating to such units
will be incorporated by reference as exhibits to the registration statement, which includes this prospectus.
Each unit that we may issue will be issued so that the holder of the
unit is also the holder of each security included in the unit. Thus, the holder of a unit will have the rights and obligations of a holder
of each included security. The unit agreement under which a unit is issued may provide that the securities included in the unit may not
be held or transferred separately, at any time or at any time before a specified date. The applicable prospectus supplement may describe:
| |
· |
|
the designation and terms of the units and of the securities comprising the units, including whether and under what circumstances those securities may be held or transferred separately; |
| |
· |
|
any provisions of the governing unit agreement; |
| |
· |
|
the price or prices at which such units will be issued; |
| |
· |
|
the applicable United States federal income tax considerations relating to the units; |
| |
· |
|
any provisions for the issuance, payment, settlement, transfer or exchange of the units or of the securities comprising the units; and |
| |
· |
|
any other terms of the units and of the securities comprising the units. |
The provisions described in this subsection, as well as those described
under “Description of Securities” will apply to the securities included in each unit, to the extent relevant and as may be
updated in any prospectus supplements.
USE OF PROCEEDS
We will retain broad discretion over the use of the net proceeds from
the sale of our securities offered hereby. Except as described in any prospectus supplement, we currently anticipate using the net proceeds
from the sale of our securities offered hereby primarily for general corporate purposes, which include, but are not limited to, funding
our preclinical and clinical development, other research and development activities and for general and administrative expenses. We may
also use a portion of the net proceeds to pay off outstanding indebtedness, if any, and/or acquire or invest in complementary businesses,
products and technologies. Further, from time to time we may evaluate acquisition opportunities and engage in related discussions with
other companies.
Pending the use of the net proceeds, we intend to invest the net proceeds
in short-term, interest-bearing, investment-grade securities.
RATIO OF EARNINGS TO FIXED CHARGES
If we offer debt securities and/or preferred equity securities under
this prospectus, then we will, if required at that time, provide a ratio of earnings to fixed charges and/or ratio of combined fixed charges
and preference dividends to earnings, respectively, in the applicable prospectus supplement for such offering.
PLAN OF DISTRIBUTION
We may sell the securities covered by this prospectus from time to
time in one or more offerings. Registration of the securities covered by this prospectus does not mean, however, that those securities
will necessarily be offered or sold.
We may sell the securities separately or together:
| |
· |
|
through one or more underwriters or dealers in a public offering and sale by them; |
| |
· |
|
directly to investors; or |
We may sell the securities from time to time:
| |
· |
|
in one or more transactions at a fixed price or prices, which may be changed from time to time; |
| |
· |
|
at market prices prevailing at the times of sale; |
| |
· |
|
at prices related to such prevailing market prices; or |
We will describe the method of distribution of the securities and the
terms of the offering in the prospectus supplement. Any discounts or concessions allowed or re-allowed or paid to dealers may be changed
from time to time.
If underwriters are used in the sale of any securities, the securities
will be acquired by the underwriters for their own account and may be resold from time to time in one or more transactions described above.
The securities may be either offered to the public through underwriting syndicates represented by managing underwriters, or directly by
underwriters. Generally, the underwriters’ obligations to purchase the securities will be subject to conditions precedent and the
underwriters will be obligated to purchase all of the securities if they purchase any of the securities. We may use underwriters with
whom we have a material relationship. We will describe in the prospectus supplement, naming the underwriter, the nature of any such relationship.
We may authorize underwriters, dealers or agents to solicit offers
by certain purchasers to purchase the securities from us at the public offering price set forth in the prospectus supplement pursuant
to delayed delivery contracts providing for payment and delivery on a specified date in the future. The contracts will be subject only
to those conditions set forth in the prospectus supplement, and the prospectus supplement will set forth any commissions we pay for solicitation
of these contracts.
We may enter into derivative transactions with third parties, or sell
securities not covered by this prospectus to third parties in privately negotiated transactions. If the applicable prospectus supplement
indicates, in connection with those derivatives, the third parties may sell securities covered by this prospectus and the applicable prospectus
supplement, including in short sale transactions. If so, the third party may use securities pledged by us or borrowed from us or others
to settle those sales or to close out any related open borrowings of stock, and may use securities received from us in settlement of those
derivatives to close out any related open borrowings of stock. The third party in such sale transactions will be an underwriter and will
be identified in the applicable prospectus supplement or in a post-effective amendment.
Underwriters, dealers and agents may be entitled to indemnification
by us against certain civil liabilities, including liabilities under the Securities Act, or to contribution with respect to payments made
by the underwriters, dealers or agents, under agreements between us and the underwriters, dealers and agents.
We may grant underwriters who participate in the distribution of securities
an option to purchase additional securities to cover over-allotments, if any, in connection with the distribution.
Underwriters, dealers or agents may receive compensation in the form
of discounts, concessions or commissions from us or our purchasers, as their agents in connection with the sale of securities. These underwriters,
dealers or agents may be considered to be underwriters under the Securities Act. As a result, discounts, commissions or profits on resale
received by the underwriters, dealers or agents may be treated as underwriting discounts and commissions. The prospectus supplement will
identify any such underwriter, dealer or agent and describe any compensation received by them from us. Any initial public offering price
and any discounts or concessions allowed or re-allowed or paid to dealers may be changed from time to time.
Unless otherwise specified in the related prospectus supplement, all
securities we offer, other than common stock, will be new issues of securities with no established trading market. Any underwriters may
make a market in these securities, but will not be obligated to do so and may discontinue any market making at any time without notice.
Any common stock sold pursuant to a prospectus supplement will be listed for trading on the NASDAQ Global Market or other principal market
for our common stock. We may apply to list any series of debt securities, preferred stock, warrants or units on an exchange, but we are
not obligated to do so. Therefore, there may not be liquidity or a trading market for any series of securities.
Any underwriter may engage in over-allotment transactions, stabilizing
transactions, short-covering transactions and penalty bids in accordance with Regulation M under the Exchange Act. Over-allotment involves
sales in excess of the offering size, which create a short position. Stabilizing transactions permit bids to purchase the underlying security
so long as the stabilizing bids do not exceed a specified maximum. Short covering transactions involve purchases of the securities in
the open market after the distribution is completed to cover short positions. Penalty bids permit the underwriters to reclaim a selling
concession from a dealer when the securities originally sold by the dealer are purchased in a covering transaction to cover short positions.
Those activities may cause the price of the securities to be higher than it would otherwise be. If commenced, the underwriters may discontinue
any of the activities at any time. We make no representation or prediction as to the direction or magnitude of any effect that such transactions
may have on the price of the securities. For a description of these activities, see the information under the heading “Underwriting”
or “Plan of Distribution” in the applicable prospectus supplement.
Underwriters, broker-dealers or agents who may become involved in the
sale of the common stock may engage in transactions with and perform other services for us in the ordinary course of their business for
which they receive compensation.
LEGAL MATTERS
The legality of the issuance of the securities being offered hereby
and the binding nature of any debt securities, warrants or units being offered hereby is being passed upon by Gibson, Dunn & Crutcher
LLP, San Francisco, California. The legality of the securities for any underwriters, dealers or agents will be passed upon by counsel
as may be specified in the applicable prospectus supplement.
EXPERTS
The consolidated financial statements as of December 31, 2020 and 2019
and for each of the two years in the period ended December 31, 2020 incorporated by reference in this prospectus have been so incorporated
in reliance on the report of BDO USA, LLP, an independent registered public accounting firm, incorporated herein by reference, given on
the authority of said firm as experts in auditing and accounting.
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The SEC allows us to incorporate by reference into this prospectus
the information contained in other documents we file with the SEC, which means that we can disclose important information to you by referring
you to those documents. Any statement contained in any document incorporated or deemed to be incorporated by reference herein shall be
deemed to be modified or superseded, for purposes of this prospectus, to the extent that a statement contained in or omitted from this
prospectus, or in any other subsequently filed document that also is or is deemed to be incorporated by reference herein, modifies or
supersedes such statement. Any such statement so modified or superseded shall not be deemed, except as so modified or superseded, to constitute
a part of this prospectus. We incorporate by reference the documents listed below which have been filed by us:
|
· |
|
Our Annual Report on Form 10-K for the year ended December 31,
2020, filed with the SEC on March 25, 2021, including those portions of the Form 10-K incorporated by reference from our definitive proxy statement filed on April 30, 2021;
|
| |
· |
|
The description of our common stock contained in our registration on Form 8-A12B (File No. 001-36304) filed with the SEC on February 7, 2014, including any amendment or report filed for the purpose of updating such description. |
All documents we file with the SEC pursuant to Sections 13(a), 13(c),
14 or 15(d) of the Exchange Act, except as to any portion of any report or documents that is not deemed filed under such provisions, (1)
on or after the date of filing of the registration statement containing this prospectus and prior to the effectiveness of the registration
statement and (2) on or after the date of this prospectus until the earlier of the date on which all of the securities registered hereunder
have been sold or the registration statement of which this prospectus is a part has been withdrawn, shall be deemed incorporated by reference
in this prospectus and to be a part of this prospectus from the date of filing of those documents and will be automatically updated and,
to the extent described above, supersede information contained or incorporated by reference in this prospectus and previously filed documents
that are incorporated by reference in this prospectus.
Nothing in this prospectus shall be deemed to incorporate information
furnished but not filed with the SEC pursuant to Item 2.02, 7.01 or 9.01 of Form 8-K.
Upon written or oral request, we will provide without charge to each
person, including any beneficial owner, to whom a copy of the prospectus is delivered a copy of any or all of the reports or documents
incorporated by reference herein (other than exhibits to such documents, unless such exhibits are specifically incorporated by reference
herein). You may request a copy of these filings, at no cost, by writing or telephoning us at the following address: Phio Pharmaceuticals
Corp., 257 Simarano Drive, Suite 101, Marlborough, Massachusetts 01752 Attention: Investor Relations, telephone: (508) 767-3861. We maintain
a website at www.phiopharma.com. You may access our definitive proxy statements on Schedule 14A, annual reports on Form 10-K, quarterly
reports on Form 10-Q, current reports on Form 8-K and periodic amendments to those reports filed or furnished pursuant to Section 13(a)
or 15(d) of the Exchange Act with the SEC free of charge at our website as soon as reasonably practicable after such material is electronically
filed with, or furnished to, the SEC. The information contained in, or that can be accessed through, our website is not incorporated by
reference in, and is not part of, this prospectus. We have not authorized any one to provide you with any information that differs from
that contained in this prospectus. Accordingly, you should not rely on any information that is not contained in this prospectus. You should
not assume that the information in this prospectus is accurate as of any date other than the date of the front cover of this prospectus.
WHERE YOU CAN FIND MORE INFORMATION
We are required to file annual, quarterly and current reports, proxy
statements and other information with the SEC. The SEC maintains an Internet site that contains all reports and other information that
we file electronically with the SEC. The address of that website is www.sec.gov. Copies of certain information filed by us with the SEC
are also available on our website at www.phiopharma.com. Our website is not a part of this prospectus and is not incorporated by reference
in this prospectus, and you should not consider the contents of our website in making an investment decision with respect to our common
stock.
We have filed a registration statement, of which this prospectus is
a part, covering the securities offered hereby. As allowed by SEC rules, this prospectus does not include all of the information contained
in the Registration Statement and the included exhibits, financial statements and schedules. You are referred to the Registration Statement,
the included exhibits, financial statements and schedules for further information. Statements contained in this prospectus as to the contents
of any contract or other document are not necessarily complete, and in each instance we refer you to the copy of the contract or document
filed as an exhibit to the registration statement, each such statement being qualified in all respects by such reference. This prospectus
is qualified in its entirety by such other information.
* * *
Phio Pharmaceuticals Corp.

862,500 Shares of Common Stock
Common Stock
PROSPECTUS SUPPLEMENT
May 16, 2024
Phio Pharmaceuticals (NASDAQ:PHIO)
Historical Stock Chart
Von Dez 2024 bis Jan 2025
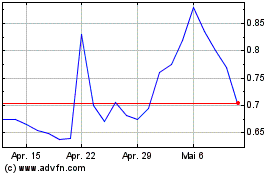
Phio Pharmaceuticals (NASDAQ:PHIO)
Historical Stock Chart
Von Jan 2024 bis Jan 2025