Board-certified dermatologist, Dr. Sandra Lee,
widely known as Dr. Pimple Popper, among others, details her
journey with mild-moderate atopic dermatitis, the most common form
of eczema, and her experience on Opzelura® (ruxolitinib) cream
1.5%
Incyte (Nasdaq:INCY) announced today the expansion of its
Moments of Clarity program, an educational initiative highlighting
stories of people living with mild to moderate atopic dermatitis
(AD) and their paths to finding relief. Building on last year’s
program launch in partnership with Mandy Moore, the Moments of
Clarity program will now feature several additional real-life
patient perspectives, including that of Dr. Sandra Lee and Emily, a
mom of three, who share their emotional experiences and the impact
AD has had on their lives.
This press release features multimedia. View
the full release here:
https://www.businesswire.com/news/home/20241009443934/en/
Dr. Sandra Lee partners with Incyte on
Moments of Clarity program to encourage those with eczema to see a
healthcare provider about treatment (Photo: Business Wire)
In the new program content, each patient shares defining moments
throughout their journeys including how they finally found relief
with Opzelura® (ruxolitinib) cream 1.5%. Opzelura is a
nonsteroidal, twice-daily cream that is indicated for the
short-term and non-continuous treatment of mild to moderate eczema
in certain people 12 and older whose disease is not well-controlled
with topical prescription therapies or when those therapies are not
recommended. Use of Opzelura in combination with biologics, other
JAK inhibitors or potent immunosuppressants is not recommended.
“While many people know me as a dermatologist from my TV show,
most probably don’t know that I live with eczema and have struggled
with symptoms like persistent itch and skin inflammation throughout
my life,” said Dr. Lee. “I started using Opzelura, a non-steroidal
topical option, to treat my eczema flares, which helped alleviate
some of my worst symptoms like itch. In the pivotal clinical trial
for mild to moderate AD, 54% of patients had clear to almost clear
skin at 8 weeks and 52% of patients had a significant reduction in
their itch, compared to 15% of patients who used a cream that did
not contain medication. I partnered with Incyte on their Moments of
Clarity program to share my story and to empower others to seek out
a treatment that is right for them.”
AD – the most common type of eczema – is a chronic condition
characterized by inflammation of the skin, which can manifest as
persistent itch, dry and scaly patches or red lesions on the body1.
AD affects more than 21 million people aged 12 years and older in
the U.S. Signs and symptoms include irritated and itchy skin that
can cause red lesions that may ooze and crust.
“We understand the impact eczema can have on daily life, which
furthers the need for patients to find a treatment to address their
individual symptoms,” said Matteo Trotta, Executive Vice President,
General Manager, U.S. Dermatology, Incyte. “We are proud to elevate
the patient voice and share the real, lived experience with
Opzelura as part of our commitment to patients and to providing a
treatment that can help address some of the most burdensome
symptoms of AD. Our hope is that the Moments of Clarity program
helps those living with eczema connect with others and inspires
them to have conversations with their doctor.” OPZELURA is not for
everyone. See below for IMPORTANT SAFETY INFORMATION including
boxed Warning for Serious Infections, Increased Risk of Death,
Lymphoma and other Cancers, Major Cardiovascular Events and Blood
Clots.
To hear more visit MyMomentsofClarity.com.
All individuals were compensated for their
participation.
About Opzelura® (ruxolitinib) Cream Opzelura®
(ruxolitinib) cream, a novel cream formulation of Incyte’s
selective JAK1/JAK2 inhibitor ruxolitinib, approved by the U.S.
Food & Drug Administration for the topical treatment of
nonsegmental vitiligo in patients 12 years of age and older, is the
first and only treatment for repigmentation approved for use in the
United States. Opzelura is also approved in the U.S. for the
topical short-term and non-continuous chronic treatment of mild to
moderate atopic dermatitis (AD) in non-immunocompromised patients
12 years of age and older whose disease is not adequately
controlled with topical prescription therapies, or when those
therapies are not advisable. Use of Opzelura in combination with
therapeutic biologics, other JAK inhibitors, or potent
immunosuppressants, such as azathioprine or cyclosporine, is not
recommended.
Incyte has worldwide rights for the development and
commercialization of ruxolitinib cream, marketed in the United
States as Opzelura. In April 2022, Incyte entered into a strategic
alliance agreement with Maruho Co., Ltd. for the development,
manufacturing and exclusive commercialization of ruxolitinib cream
for treatment of autoimmune and inflammatory dermatology
indications in Japan.
Opzelura is a registered trademark of Incyte.
IMPORTANT SAFETY INFORMATION
OPZELURA is for use on the skin only. Do not use OPZELURA in
your eyes, mouth, or vagina.
OPZELURA may cause serious side effects, including:
Serious Infections: OPZELURA contains ruxolitinib.
Ruxolitinib belongs to a class of medicines called Janus kinase
(JAK) inhibitors. JAK inhibitors are medicines that affect your
immune system. JAK inhibitors can lower the ability of your immune
system to fight infections. Some people have had serious infections
while taking JAK inhibitors by mouth, including tuberculosis (TB),
and infections caused by bacteria, fungi, or viruses that can
spread throughout the body. Some people have been hospitalized or
died from these infections. Some people have had serious infections
of their lungs while taking OPZELURA. Your healthcare provider
should watch you closely for signs and symptoms of TB during
treatment with OPZELURA.
OPZELURA should not be used in people with an active, serious
infection, including localized infections. You should not start
using OPZELURA if you have any kind of infection unless your
healthcare provider tells you it is okay. You may be at a higher
risk of developing shingles (herpes zoster) while using
OPZELURA.
Increased risk of death due to any reason (all causes):
Increased risk of death has happened in people 50 years of age and
older who have at least 1 heart disease (cardiovascular) risk
factor and are taking a medicine in the class of medicines called
JAK inhibitors by mouth.
Cancer and immune system problems: OPZELURA may increase
your risk of certain cancers by changing the way your immune system
works. Lymphoma and other cancers have happened in people taking a
medicine in the class of medicines called JAK inhibitors by mouth.
People taking JAK inhibitors by mouth have a higher risk of certain
cancers including lymphoma and lung cancer, especially if they are
a current or past smoker. Some people have had skin cancers while
using OPZELURA. Your healthcare provider will regularly check your
skin during your treatment with OPZELURA. Limit the amount of time
you spend in the sunlight. Wear protective clothing when you are in
the sun and use a broad-spectrum sunscreen.
Increased risk of major cardiovascular events: Increased
risk of major cardiovascular events such as heart attack, stroke,
or death have happened in people 50 years of age and older who have
at least 1 heart disease (cardiovascular) risk factor and taking a
medicine in the class of medicines called JAK inhibitors by mouth,
especially in current or past smokers.
Blood clots: Blood clots in the veins of your legs (deep
vein thrombosis, DVT) or lungs (pulmonary embolism, PE) can happen
in some people taking OPZELURA. This may be life-threatening. Blood
clots in the vein of the legs (deep vein thrombosis, DVT) and lungs
(pulmonary embolism, PE) have happened more often in people who are
50 years of age and older and with at least 1 heart disease
(cardiovascular) risk factor taking a medicine in the class of
medicines called JAK inhibitors by mouth.
Low blood cell counts: OPZELURA may cause low platelet
counts (thrombocytopenia), low red blood cell counts (anemia), and
low white blood cell counts (neutropenia). If needed, your
healthcare provider will do a blood test to check your blood cell
counts during your treatment with OPZELURA and may stop your
treatment if signs or symptoms of low blood cell counts happen.
Cholesterol increases: Cholesterol increase has happened
in people when ruxolitinib is taken by mouth. Tell your healthcare
provider if you have high cholesterol or triglycerides.
Before starting OPZELURA, tell your healthcare provider if
you:
- have an infection, are being treated for one, or have had an
infection that does not go away or keeps coming back
- have diabetes, chronic lung disease, HIV, or a weak immune
system
- have TB or have been in close contact with someone with TB
- have had shingles (herpes zoster)
- have or have had hepatitis B or C
- live, have lived in, or have traveled to certain parts of the
country (such as the Ohio and Mississippi River valleys and the
Southwest) where there is an increased chance for getting certain
kinds of fungal infections. These infections may happen or become
more severe if you use OPZELURA. Ask your healthcare provider if
you do not know if you have lived in an area where these infections
are common.
- think you have an infection or have symptoms of an infection
such as: fever, sweating, or chills, muscle aches, cough or
shortness of breath, blood in your phlegm, weight loss, warm, red,
or painful skin or sores on your body, diarrhea or stomach pain,
burning when you urinate or urinating more often than usual,
feeling very tired.
- have ever had any type of cancer, or are a current or past
smoker
- have had a heart attack, other heart problems, or a stroke
- have had blood clots in the veins of your legs or lungs in the
past
- have high cholesterol or triglycerides
- have or have had low white or red blood cell counts
- are pregnant or plan to become pregnant. It is not known if
OPZELURA will harm your unborn baby. There is a pregnancy exposure
registry for individuals who use OPZELURA during pregnancy. The
purpose of this registry is to collect information about the health
of you and your baby. If you become exposed to OPZELURA during
pregnancy, you and your healthcare provider should report exposure
to Incyte Corporation at 1-855-463-3463.
- are breastfeeding or plan to breastfeed. It is not known if
OPZELURA passes into your breast milk. Do not breastfeed during
treatment with OPZELURA and for about 4 weeks after the last
dose.
After starting OPZELURA:
- Call your healthcare provider right away if you have any
symptoms of an infection. OPZELURA can make you more likely to get
infections or make worse any infections that you have.
- Get emergency help right away if you have any symptoms of a
heart attack or stroke while using OPZELURA, including:
- discomfort in the center of your chest that lasts for more than
a few minutes, or that goes away and comes back
- severe tightness, pain, pressure, or heaviness in your chest,
throat, neck, or jaw
- pain or discomfort in your arms, back, neck, jaw, or
stomach
- shortness of breath with or without chest discomfort
- breaking out in a cold sweat
- nausea or vomiting
- feeling lightheaded
- weakness in one part or on one side of your body
- slurred speech
- Tell your healthcare provider right away if you have any signs
and symptoms of blood clots during treatment with OPZELURA,
including: swelling, pain, or tenderness in one or both legs,
sudden, unexplained chest or upper back pain, or shortness of
breath or difficulty breathing.
- Tell your healthcare provider right away if you develop or have
worsening of any symptoms of low blood cell counts, such as:
unusual bleeding, bruising, tiredness, shortness of breath, or
fever.
Tell your healthcare provider about all the medicines you take,
including prescription and over-the-counter medicines, vitamins,
and herbal supplements.
The most common side effects of OPZELURA in people treated
for atopic dermatitis include: common cold (nasopharyngitis),
diarrhea, bronchitis, ear infection, increase in a type of white
blood cell (eosinophil) count, hives, inflamed hair pores
(folliculitis), swelling of the tonsils (tonsillitis), and runny
nose (rhinorrhea).
The most common side effects of OPZELURA in people treated
for nonsegmental vitiligo include: acne at the application
site, itching at the application site, common cold
(nasopharyngitis), headache, urinary tract infection, redness at
the application site, and fever.
These are not all of the possible side effects of OPZELURA. Call
your doctor for medical advice about side effects. You may report
side effects to FDA at 1-800-FDA-1088. You may also report side
effects to Incyte Corporation at 1-855-463-3463.
INDICATIONS AND USAGE
OPZELURA is a prescription medicine used on the skin (topical)
for:
- short-term and non-continuous chronic treatment of mild to
moderate eczema (atopic dermatitis) in non-immunocompromised adults
and children 12 years of age and older whose disease is not well
controlled with topical prescription therapies or when those
therapies are not recommended
- the treatment of a type of vitiligo called nonsegmental
vitiligo in adults and children 12 years of age and older
The use of OPZELURA along with therapeutic biologics, other JAK
inhibitors, or strong immunosuppressants such as azathioprine or
cyclosporine is not recommended.
It is not known if OPZELURA is safe and effective in children
less than 12 years of age with atopic dermatitis or nonsegmental
vitiligo.
Please see the Full Prescribing Information,
including Boxed Warning, and Medication Guide for
OPZELURA.
About Incyte Dermatology Incyte’s science-first approach
and expertise in immunology has formed the foundation of the
company. Today, we are building on this legacy as we discover and
develop innovative dermatology treatments to bring solutions to
patients in need.
We strive to identify and develop therapies to modulate immune
pathways driving uncontrolled inflammation to help restore normal
immune function and bring the body closer to homeostasis.
Specifically, our efforts in dermatology are focused on a number of
immune-mediated dermatologic conditions with a high unmet medical
need, including atopic dermatitis, vitiligo, hidradenitis
suppurativa, lichen planus, lichen sclerosus and prurigo
nodularis
To learn more, visit the Dermatology section of Incyte.com.
About Incyte A global biopharmaceutical company on a
mission to Solve On., Incyte follows the science to find solutions
for patients with unmet medical needs. Through the discovery,
development and commercialization of proprietary therapeutics,
Incyte has established a portfolio of first-in-class medicines for
patients and a strong pipeline of products in Oncology and
Inflammation & Autoimmunity. Headquartered in Wilmington,
Delaware, Incyte has operations in North America, Europe and
Asia.
For additional information on Incyte, please visit Incyte.com or
follow us on social media: LinkedIn, X, Instagram, Facebook,
YouTube.
Forward-Looking Statements Except for the historical
information set forth herein, the matters set forth in this press
release, including statements regarding the partnership between
Incyte and Dr. Sandra Lee Incyte’s goal of improving the lives of
patients, whether and when Opzelura might provide a successful
treatment option for patients with atopic dermatitis, and Incyte’s
dermatology program generally, contain predictions, estimates and
other forward-looking statements.
These forward-looking statements are based on Incyte’s current
expectations and subject to risks and uncertainties that may cause
actual results to differ materially, including unanticipated
developments in and risks related to: unanticipated delays; further
research and development and the results of clinical trials
possibly being unsuccessful or insufficient to meet applicable
regulatory standards or warrant continued development; the ability
to enroll sufficient numbers of subjects in clinical trials;
determinations made by the FDA and other regulatory authorities
outside of the United States; the efficacy or safety of Incyte’s
products; the acceptance of Incyte’s products in the marketplace;
market competition; sales, marketing, manufacturing and
distribution requirements; and other risks detailed from time to
time in Incyte’s reports filed with the Securities and Exchange
Commission, including its annual report and its quarterly report on
Form 10-Q for the quarter ended June 30, 2024. Incyte disclaims any
intent or obligation to update these forward-looking
statements.
1 National Eczema Association. What is atopic dermatitis?
https://nationaleczema.org/eczema/types-of-eczema/atopic-dermatitis/
View source
version on businesswire.com: https://www.businesswire.com/news/home/20241009443934/en/
Media media@incyte.com Investors ir@incyte.com
Incyte (NASDAQ:INCY)
Historical Stock Chart
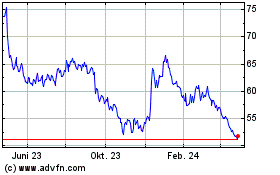
Von Nov 2024 bis Dez 2024
Incyte (NASDAQ:INCY)
Historical Stock Chart
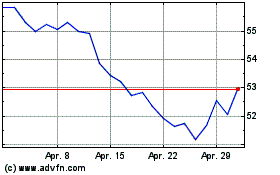
Von Dez 2023 bis Dez 2024