Report of Foreign Issuer Pursuant to Rule 13a-16 or 15d-16 (6-k)
01 November 2021 - 12:42PM
Edgar (US Regulatory)
FORM 6-K
SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
Report
of Foreign Issuer
Pursuant
to Rule 13a-16 or 15d-16 of
the
Securities Exchange Act of 1934
For the
month of November
2021
Commission
File Number: 001-11960
AstraZeneca PLC
1
Francis Crick Avenue
Cambridge
Biomedical Campus
Cambridge
CB2 0AA
United
Kingdom
Indicate
by check mark whether the registrant files or will file annual
reports under cover of Form 20-F or Form 40-F.
Form
20-F X Form 40-F __
Indicate
by check mark if the registrant is submitting the Form 6-K in paper
as permitted by Regulation S-T Rule 101(b)(1):
Indicate
by check mark if the registrant is submitting the Form 6-K in paper
as permitted by Regulation S-T Rule 101(b)(7): ______
Indicate
by check mark whether the registrant by furnishing the information
contained in this Form is also thereby furnishing the information
to the Commission pursuant to Rule 12g3-2(b) under the Securities
Exchange Act of 1934.
Yes __
No X
If
“Yes” is marked, indicate below the file number
assigned to the Registrant in connection with Rule 12g3-2(b):
82-_____________
AstraZeneca PLC
INDEX
TO EXHIBITS
1.
AstraZeneca to transfer rights to Eklira, Duaklir
1
November 2021 07:00 GMT
AstraZeneca to transfer global rights for Eklira and Duaklir to Covis Pharma
Agreement sharpens AstraZeneca’s focus on priority
medicines
in Respiratory & Immunology portfolio
AstraZeneca
has agreed to transfer its global rights to Eklira (aclidinium bromide), known as
Tudorza in the US, and
Duaklir (aclidinium
bromide/formoterol) to Covis Pharma Group (Covis
Pharma).
Both
medicines are delivered via the Genuair device and used for the
treatment of patients with chronic obstructive pulmonary disease
(COPD).
The
agreement will ensure continued patient access to these established
medicines.
Covis
Pharma previously
acquired the rights to the respiratory medicines Alvesco, Omnaris and Zetonna from AstraZeneca in
2018.
Financial considerations
Covis
Pharma will pay AstraZeneca $270m on completion. Covis Pharma will
also cover certain ongoing development costs related to the
medicines. The income arising from the upfront payment will be
fully offset by a charge for derecognition of the associated
intangible asset and therefore no Other Operating Income will be
recognised in AstraZeneca’s financial statements. In 2020,
Eklira and Duaklir generated AstraZeneca revenue
of $143m in the countries covered by this agreement.
The
transaction is expected to close in the fourth quarter of 2021,
subject to customary closing conditions and regulatory clearances.
The agreement will not impact the Company’s financial
guidance for 2021.
Eklira and Duaklir
Eklira (aclidinium bromide) and
Duaklir (aclidinium
bromide/formoterol) are inhaled respiratory medicines used for the
maintenance treatment of COPD. Eklira is a long-acting muscarinic
antagonist (LAMA), which is marketed in the US as Tudorza and in some countries as
Bretaris. Duaklir is a combination therapy that
contains both a LAMA and a long-acting beta2-agonist (LABA). It is
marketed in some countries as Brimica. Both medicines are presented
as a dry powder for inhalation and are delivered via a
breath-actuated multi-dose dry powder inhaler, Genuair (Pressair in the US). AstraZeneca licensed the global
rights to both products from Almirall S.A. in 2014.
AstraZeneca
AstraZeneca
(LSE/STO/Nasdaq: AZN) is a global, science-led biopharmaceutical
company that focuses on the discovery, development, and
commercialisation of prescription medicines in Oncology, Rare
Diseases, and BioPharmaceuticals, including Cardiovascular, Renal
& Metabolism, and Respiratory & Immunology. Based in
Cambridge, UK, AstraZeneca operates in over 100 countries and its
innovative medicines are used by millions of patients worldwide.
Please visit astrazeneca.com
and follow the Company on Twitter @AstraZeneca.
Contacts
For
details on how to contact the Investor Relations Team, please click
here. For Media
contacts, click here.
Adrian Kemp
Company Secretary
AstraZeneca PLC
SIGNATURES
Pursuant
to the requirements of the Securities Exchange Act of 1934, the
Registrant has duly caused this report to be signed on its behalf
by the undersigned, thereunto duly authorized.
Date:
1 November
2021
|
|
By: /s/
Adrian Kemp
|
|
|
Name:
Adrian Kemp
|
|
|
Title:
Company Secretary
|
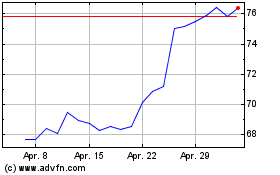
AstraZeneca (NASDAQ:AZN)
Historical Stock Chart
Von Jun 2024 bis Jul 2024
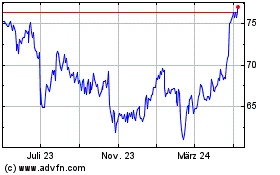
AstraZeneca (NASDAQ:AZN)
Historical Stock Chart
Von Jul 2023 bis Jul 2024