Aurinia Submits IND Application to US Food & Drug Administration for AUR 200
20 Dezember 2023 - 12:00PM
Business Wire
- AUR200, a potent recombinant fusion protein, has a clinically
validated MOA with a high affinity for targeting both BAFF (B-cell
Activating Factor) and APRIL (A Proliferation-Inducing Ligand)
- Upon FDA clearance of the IND, AUR 200 will be studied in a
Phase 1 first-in-human trial in healthy volunteers, marking a
significant advancement for Aurinia’s emerging autoimmune
pipeline
Aurinia Pharmaceuticals Inc. (NASDAQ: AUPH) (Aurinia or the
Company) – Aurinia announced today the submission of its
Investigational New Drug application (IND) to the U.S. Food and
Drug Administration (FDA) for AUR200, a potential next generation
therapy for B-cell mediated autoimmune diseases. Upon receiving FDA
clearance to proceed with proposed research, Aurinia plans to
initiate a Phase 1 study in the first half of 2024 to evaluate the
safety, tolerability, pharmacokinetic and pharmacodynamic
properties of AUR200 in healthy volunteers.
AUR200 is a highly potent and specific Fc-fusion protein
containing a modified B cell maturation antigen (BCMA), for
enhanced binding to both BAFF (B-cell Activating Factor) and APRIL
(A Proliferation-Inducing Ligand). BAFF and APRIL play important
roles in regulating B-cell survival and differentiation.
“Our IND submission for AUR200 is an important step forward for
Aurinia’s pipeline and our mission to change the trajectory of
autoimmune disease. Dual inhibition of BAFF and APRIL is a
clinically validated mechanism which has demonstrated great
potential in the treatment of autoimmune diseases such as IgA
nephropathy and systemic lupus erythematosus. We are encouraged by
AUR200’s unique profile and pre-clinical activity and look forward
to sharing further data and updates once our clinical research
program begins,” said Volker Knappertz, M.D., Executive
Vice-President, Research & Development, Aurinia.
In animal data presented at the annual American College of
Rheumatology Convergence 2022, AUR200 dosed therapeutically reduced
several markers of disease activity and improved overall survival
in a mouse model of lupus. AUR200 was also well-tolerated in both
mice and cynomolgus monkeys, with no adverse effects. These data
highlight the potential utility for AUR200 in the treatment of
autoimmune diseases.
Aurinia acquired AUR200 as part of a strategy to diversify its
development pipeline and leverage existing R&D capabilities to
advance innovative therapeutic solutions to help people living with
autoimmune diseases.
About Aurinia
Aurinia Pharmaceuticals is a fully integrated biopharmaceutical
company focused on delivering therapies to treat targeted patient
populations with high unmet medical needs that are impacted by
autoimmune, kidney and rare diseases. In January 2021, the Company
introduced LUPKYNIS® (voclosporin), the first FDA-approved oral
therapy dedicated to the treatment of adult patients with active
lupus nephritis. The Company’s head office is in Edmonton, Alberta,
its U.S. commercial office is in Rockville, Maryland. The Company
focuses its development efforts globally.
Reference
Morales S, Cross J, Huizinga R. AUR200: An Improved BAFF/APRIL
Inhibitor with Increased Potency and Safety for the Treatment of B
Cell-Mediated Diseases [abstract]. Arthritis Rheumatol. 2022; 74
(suppl 9).
https://acrabstracts.org/abstract/aur200-an-improved-baff-april-inhibitor-with-increased-potency-and-safety-for-the-treatment-of-b-cell-mediated-diseases/.
Accessed December 6, 2023.
View source
version on businesswire.com: https://www.businesswire.com/news/home/20231220562044/en/
Media Inquiries: Andrea Christopher Corporate
Communications Director, Aurinia achristopher@auriniapharma.com
Investor Inquiries: ir@auriniapharma.com
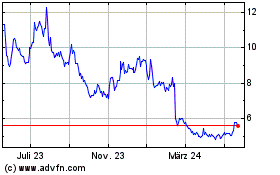
Aurinia Pharmaceuticals (NASDAQ:AUPH)
Historical Stock Chart
Von Okt 2024 bis Nov 2024
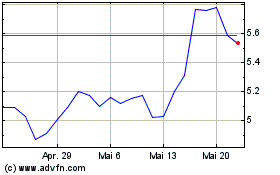
Aurinia Pharmaceuticals (NASDAQ:AUPH)
Historical Stock Chart
Von Nov 2023 bis Nov 2024