Current Report Filing (8-k)
29 Dezember 2022 - 1:06PM
Edgar (US Regulatory)
0001437402false00014374022022-12-272022-12-27
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): December 27, 2022
ARDELYX, INC.
(Exact name of registrant as specified in its charter)
| | | | | | | | |
| Delaware | 001-36485 | 26-1303944 |
(State or other jurisdiction
of incorporation) | (Commission
File Number) | (IRS Employer
Identification Number) |
400 FIFTH AVE., SUITE 210, WALTHAM, MASSACHUSETTS 02451
(Address of principal executive offices, including Zip Code)
Registrant’s telephone number, including area code: (510) 745-1700
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| | | | | |
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| | | | | | | | | | | | | | |
| Title of each class | | Trading Symbol(s) | | Name of each exchange on which registered |
| Common Stock, par value $0.0001 | | ARDX | | The Nasdaq Global Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. o
Item 8.01 Other Events
On December 27, 2022, Ardelyx, Inc. (the “Company”) received an Appeal Granted Letter (“AGL”) from the Office of New Drugs (“OND”), Center for Drug Evaluation and Research of Cardiology, Hematology, Endocrinology and Nephrology (“OCHEN”) of the U.S. Food and Drug Administration (the “FDA”) granting the Company’s appeal of the July 28, 2021 issuance of a Complete Response Letter (“CRL”) for the Company’s New Drug Application for XPHOZAH (tenapanor). The OND’s response follows a favorable vote at a Cardiovascular and Renal Drugs Advisory Committee (“CRDAC”) meeting held on November 16, 2022. In the AGL, OND directs the FDA’s Division of Cardiology and Nephrology (“DCN”) to work with the Company to develop an appropriate label. The Company believes that a label could reflect an indication for patients whose hyperphosphatemia is insufficiently managed on binder therapy. OND has requested that the Company request a meeting with the DCN to discuss what will be required in the Company’s resubmission of the NDA, and the Company currently intends to request such a meeting as soon as possible. The Company currently expects to resubmit the NDA in the first half of 2023. Within thirty (30) days of resubmitting the NDA, the Company expects it will receive notification from the FDA as to the classification of the resubmission (Class 1 or Class 2) at which point the timing for review will also be known (2-months for a Class 1 and 6-months for a Class 2), as well as a goal review date.
This item 8.01 contains forward-looking statements, including, but not limited to, statements related to the Company’s expectations regarding the potential indication for XPHOZAH, and the Company’s expectations regarding the timing to resubmit the NDA for XPHOZAH and the potential timing of review of the resubmission. The Company’s actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of these risks and uncertainties, which include, without limitation, risks related to the regulatory process. A further description of the risks and uncertainties relating to the business of the Company is contained in the Company’s Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2022, filed with the Securities and Exchange Commission (the “SEC”), and the Company’s subsequent current reports filed with the SEC. The Company undertakes no duty or obligation to update any forward-looking statements contained in this Item 8.01 as a result of new information, future events, or changes in its expectations.
| | | | | | | | |
Exhibit
No. | | Description |
| 104 | | Cover Page Interactive Data File (embedded within the Inline XBRL document). |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| | | | | | | | |
| Date: December 29, 2022 | ARDELYX, INC. |
| | |
| By: | /s/ Elizabeth Grammer |
| | Elizabeth Grammer |
| | Chief Legal and Administrative Officer |
Ardelyx (NASDAQ:ARDX)
Historical Stock Chart
Von Jun 2024 bis Jul 2024
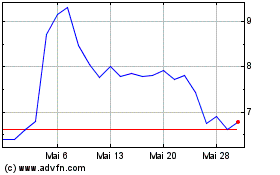
Ardelyx (NASDAQ:ARDX)
Historical Stock Chart
Von Jul 2023 bis Jul 2024