ACTICOR BIOTECH: Half-Year Report on the Liquidity Contract With the Brokerage Firm Kepler Cheuvreux
17 Januar 2024 - 6:00PM
Business Wire
Regulatory News:
ACTICOR BIOTECH (ISIN: FR0014005OJ5 – ALACT), a clinical stage
biopharmaceutical company developing glenzocimab, an innovative
drug for the treatment of cardiovascular emergencies, today
announces its half-year report on the liquidity contract with the
brokerage firm Kepler Cheuvreux.
Under the liquidity contract entered into between ACTICOR
BIOTECH and Kepler Cheuvreux, the following resources appeared on
the liquidity account on December 31th 2023:
- 65,122 shares - € 299,098.61 - Number of
executions on buy side on semester: 340 - Number of executions on
sell side on semester: 227 - Traded volume on buy side on semester:
39,590 shares for € 173,764.12 - Traded volume on sell side on
semester: 17,891 shares for € 85,132.33
As a reminder:
- the following resources appeared on the last half year
statement on 30 June 2023 on the liquidity account: - 43,423 shares
- € 382,720.54 - Number of executions on buy side on semester: 631
- Number of executions on sell side on semester: 505 - Traded
volume on buy side on semester: 59,726 shares for € 419,225.09 -
Traded volume on sell side on semester: 50,122 shares for €
385,042.61
- the following resources appeared on the liquidity account when
the activity started: - 0 shares - € 600,000.00
The implementation of this report is carried out in accordance
with AMF Decision N°2021-01 of June 22nd 2021 renewing the
implementation of liquidity contracts for shares as an accepted
market practice.
About ACTICOR BIOTECH
Acticor Biotech is a clinical stage biopharmaceutical company, a
spin-off from INSERM (the French National Institute of Health and
Medical Research), which is aiming to develop an innovative
treatment for cardiovascular emergencies, including ischemic
stroke.
The positive results of the phase 1b/2a study, ACTIMIS,
confirmed the safety profile of glenzocimab and showed a reduction
in mortality and intracerebral hemorrhage in the
glenzocimab-treated group of stroke patients. These results were
confirmed by a post-hoc analysis of brain imaging at 0 and 24 hours
using artificial intelligence (Brainomix, UK). This independent
analysis confirmed the reduction in the number and volume of
intracerebral lesions in patients treated with glenzocimab. The
efficacy of glenzocimab is now being analyzed in an international
Phase 2/3 study, ACTISAVE, with clinical results expected in Q2
2024.
In July 2022, Acticor Biotech was granted "PRIME" status by the
European Medicines Agency (EMA) for glenzocimab in the treatment of
stroke. This designation will allow the company to strengthen its
interactions and obtain early dialogues with regulatory
authorities.
Acticor Biotech is supported by a panel of European and
international investors (Mediolanum farmaceutici, Karista, Go
Capital, Newton Biocapital, CMS Medical Venture Investment (HK)
Limited, A&B (HK) Limited, Anaxago, and the Armesa foundation).
Acticor Biotech is listed on Euronext Growth Paris since November
2021 (ISIN: FR0014005OJ5 – ALACT).
For more information, visit: www.acticor-biotech.com
View source
version on businesswire.com: https://www.businesswire.com/news/home/20240117088932/en/
ACTICOR BIOTECH Gilles AVENARD, MD CEO and Founder
gilles.avenard@acticor-biotech.com T. : +33 (0)6 76 23 38
13
Sophie BINAY, PhD General Manager and CSO
Sophie.binay@acticor-biotech.com T. : +33 (0)6 76 23 38
13
NewCap Mathilde BOHIN Investor Relations
acticor@newcap.eu T. : +33 (0)1 44 71 94 95
NewCap Arthur ROUILLÉ Media Relations acticor@newcap.eu
T. : +33 (0)1 44 71 00 15
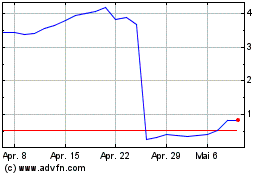
Acticor Biotech (EU:ALACT)
Historical Stock Chart
Von Jun 2024 bis Jul 2024
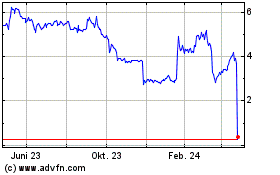
Acticor Biotech (EU:ALACT)
Historical Stock Chart
Von Jul 2023 bis Jul 2024