As filed with the U.S. Securities and Exchange
Commission on July 19, 2024.
Registration
No. 333-280806
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
AMENDMENT NO.
1
TO
FORM
S-1
REGISTRATION
STATEMENT
UNDER
THE
SECURITIES ACT OF 1933
AZITRA,
INC.
(Exact
name of registrant as specified in its charter)
| Delaware |
|
2834 |
|
46-4478536 |
(State
or other jurisdiction of
incorporation
or organization) |
|
(Primary
Standard Industrial
Classification
Code Number) |
|
(I.R.S.
Employer
Identification
Number) |
21
Business Park Drive
Branford,
CT 06405
(203)
646-6446 |
(Address,
including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Francisco
D. Salva
21
Business Park Drive
Branford,
CT 06405
(203)
646-6446
(Name,
address, including zip code, and telephone number, including area code, of agent for service)
Copies
to:
|
Daniel K. Donahue, Esq.
Greenberg Traurig, LLP
18565 Jamboree Road, Suite 500
Irvine, California 92612
(949) 732-6557 |
Barry Grossman, Esq.
Matthew Bernstein, Esq.
Justin Grossman, Esq.
Ellenoff Grossman & Schole LLP
1345 Avenue of the Americas
New
York, New York 10105
(212) 370-1300 |
Approximate
date of commencement of proposed sale to the public: As soon as practicable after this Registration Statement becomes effective.
If
any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the
Securities Act of 1933, check the following box. ☒
If
this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the
following box and list the Securities Act registration statement number of the earlier effective registration statement for the same
offering. ☐
If
this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the
Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If
this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the
Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate
by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company,
or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller
reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act:
| Large
accelerated filer ☐ |
Accelerated
filer ☐ |
Non-accelerated
filer ☒ |
Smaller
reporting company ☒ |
| |
|
|
Emerging
Growth Company ☒ |
| |
|
|
|
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided to Section 7(a)(2)(B) of the Securities Act. ☐
The
Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the
Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective
in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the Registration Statement shall become effective
on such date as the Commission, acting pursuant to said Section 8(a), may determine.
The
information contained in this preliminary prospectus is not complete and may be changed. These securities may not be sold until the registration
statement filed with the U.S. Securities and Exchange Commission is declared effective. This preliminary prospectus is not an offer to
sell these securities and it is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
| PRELIMINARY PROSPECTUS |
SUBJECT TO COMPLETION |
DATED JULY 19,
2024 |
Up
to 3,115,265 Units
Each
Unit consisting of one Share of Common Stock or one Pre-Funded Warrant to Purchase one share of Common Stock and Two Class A Warrants
each to Purchase One Share of Common Stock
Up
to 6,230,530 Shares of
Common Stock Underlying the Class A Warrants
Up
to 3,115,265 Shares of Common Stock Underlying Pre-Funded Warrants
Up
to 124,610 Placement Agent Warrants to Purchase Shares of Common Stock
Up
to 124,610 Shares of Common Stock Underlying the Placement Agent Warrants

We are offering on a best
efforts basis up to 3,115,265 units
(“Units”), each Unit consisting of one share of our common stock, par value $0.0001 per share (the “common
stock”) and two Class A Warrants each to purchase one share of our common stock (each, a “Class A Warrant”) The assumed public
offering price for each Unit is $3.21, which was the last reported sale price of our common stock on the NYSE American on July 11,
2024.
The
Units have no stand-alone rights and will not be certificated or issued as stand-alone securities. Each Class A Warrant
offered hereby is exercisable upon issuance at an initial exercise price of
3.21 (assuming an offering price of $3.21 per Unit) per share of
common stock, and will expire five (5) years from the date of issuance. issuance
On
the date that is 30 calendar days immediately following the date of issuance of the Class A Warrants (the “Reset Date”),
if the Reset Price, as defined below, is less than the exercise price at such time, the exercise price will be decreased to the
Reset Price. “Reset Price” shall mean 100% of the trailing five day VWAP immediately preceding the Reset Date, provided,
that in no event shall the Reset Price be less than 20% of the most recent closing price at the time of execution of the placement agency agreement (subject to adjustment for reverse and forward stock splits, recapitalizations and similar transactions following
the date of the securities purchase agreement). The number of shares of common stock issuable upon exercise of the Class A Warrants
will not be proportionately adjusted in the event of a reset of the exercise price.
We
are also offering to each purchaser of Units whose purchase of shares of our common stock in this offering would otherwise
result in the purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the
election of the holder, 9.99%) of our outstanding shares of common stock immediately following consummation of this offering, the
opportunity to purchase, if the purchaser so chooses, Units consisting of one Pre-Funded Warrant to purchase one share
of common stock, or the Pre-Funded Warrants, and two Class A Warrants. Each Pre-Funded Warrant will
be exercisable for one share of our common stock. The purchase price of each Unit including a Pre-Funded Warrant will equal
the price per Unit of the Units including one share of common stock being sold to the public in this offering, minus $0.0001,
and the exercise price of each Pre-Funded Warrant will be $0.0001 per share. For each Unit including a Pre-Funded Warrant
that we sell, the number of Units including one share of our common stock that we are offering will be decreased on a
one-for-one basis.
The
common stock and Prefunded Warrants can each be purchased in this offering only with the accompanying Class A Warrants that are part of a Unit, but the components of the Units will be immediately separable and will be issued separately in this
offering. See “Description of Securities” in this prospectus for more information.
The
Units, Class A Warrants and the Pre-Funded Warrants will not be listed on the NYSE American and are not
expected to trade in any market; however, we anticipate that the shares of our common stock included in the Units and to be issued
upon exercise of the Class A Warrants and Pre-Funded Warrants will trade on the NYSE American. We
are also registering the shares of common stock issuable upon exercise of the Class A Warrants, Pre-Funded
Warrants and placement agent warrants pursuant to this prospectus.
This offering will terminate
on the earlier of August 9, 2024 and two trading days following the effective date of the registration statement that this prospectus
forms a part, unless completed sooner or we decide to terminate the offering (which we may do at any time in our discretion) prior
to that date. We will deliver all securities to
be issued in connection with this offering delivery versus payment/receipt versus payment upon receipt by us of investor funds. We will
have one closing for all the securities purchased in this offering. Accordingly, neither we nor the placement agent (as defined below)
have made any arrangements to place investor funds in an escrow account or trust account since the placement agent will not receive investor
funds in connection with the sale of the securities offered hereunder.
Our common stock is listed on
the NYSE American under the symbol “AZTR.” The last reported sale price of our common stock on the NYSE American on
July 11, 2024, was $3.21 per share. The final public offering price per Unit including one share of common stock and
per Unit including one Pre-Funded Warrant will be determined between us and investors based on market conditions at the time of
pricing. The recent market price used throughout this prospectus may not be indicative of the actual public offering price. The actual
public offering price may be based upon a number of factors, including our history and our prospects, the industry in which we operate,
our past and present operating results, the previous experience of our executive officers and the general condition of the securities
markets at the time of this offering. There is no established public trading market for the Units, Class A Warrants or Pre-Funded Warrants and we do not expect a market for any of such securities to develop. We do not intend to
list the Class A Warrants or Pre-Funded Warrants on the NYSE American, any other national securities
exchange or any other trading system. Without an active trading market, the liquidity of the Class A Warrants
and the Pre-Funded Warrants will be limited.
We have engaged Maxim Group LLC,
or the placement agent, to act as our exclusive placement agent in connection with this offering. The placement agent has agreed to use
its reasonable best-efforts to arrange for the sale of the securities offered by this prospectus. The placement agent is not purchasing
or selling any of the securities we are offering and the placement agent is not required to arrange the purchase or sale of any specific
number or dollar amount of securities. We have agreed to pay to the placement agent the placement agent fees set forth in the table below,
which assumes that we sell all of the securities offered by this prospectus. There is no arrangement for funds to be received in escrow,
trust or similar arrangement. There is no minimum offering requirement as a condition of closing of this offering. Because there is no
minimum offering amount required as a condition to closing this offering, we may sell fewer than all of the securities offered hereby,
which may significantly reduce the amount of proceeds received by us, and investors in this offering will not receive a refund in the
event that we do not sell an amount of securities sufficient to pursue our business goals described in this prospectus. We will bear
all costs associated with the offering. See “Plan of Distribution” on page 27 of this prospectus for more information
regarding these arrangements.
We
are an “emerging growth company” under the U.S. federal securities laws and have elected to comply with certain reduced public
company reporting requirements.
Investing
in our securities involves a high degree of risk. See the section titled “Risk Factors” beginning on page 10. Neither
the Securities and Exchange Commission, or the SEC, nor any state securities commission has approved or disapproved of these securities
or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
| | |
Per Unit | | |
Total | |
| Public offering price(1) | |
$ | | | |
$ | | |
| Placement agent fees(2) | |
$ | | | |
$ | | |
| Proceeds to us, before expenses(3) | |
$ | | | |
$ | | |
| (1) | Assumes
sale of Units including one share of common stock. The public offering price of Units including
one Pre-Funded Warrant will be $3,2099. |
| (2) | We
have agreed to pay the placement agent a cash fee equal to 7.0% of the gross proceeds raised
in this offering. We have also agreed to reimburse the placement agent for certain of its
offering-related expenses, including legal fees and other out-of-pocket expenses, up to $100,000.
In addition, we have agreed to issue to the placement agent or its designees warrants to
purchase a number of shares of common stock equal to 4.0% of the shares of common stock made
part of the Units sold in this offering (including the shares of common stock issuable
upon exercise of the Pre-Funded Warrants), at an exercise price of $4.0125 per share,
which represents 125% of the assumed public offering price per share. For more information
about the compensation to be received by the placement agent, see “Plan of Distribution.” |
| (3) | Because
there is no minimum number of securities or amount of proceeds required as a condition to
closing in this offering, the actual public offering amount, placement agent fees, and proceeds
to us, if any, are not presently determinable and may be substantially less than the total
maximum offering amounts set forth above. For more information, see “Plan of Distribution.” |
The
delivery to purchasers of the shares of common stock, Pre-Funded Warrants and Class A Warrants in
this offering is expected to be made on or
about , 2024,
subject to satisfaction of certain customary closing conditions.
Maxim
Group LLC
The
date of this prospectus is , 2024.
Table
of Contents
You should rely only
on the information contained in this prospectus or incorporated by reference. We have not authorized any other person to provide
you with information different from or in addition to that contained in this prospectus, and we take no responsibility for any other
information others may give you. If anyone provides you with different or inconsistent information, you should not rely on it. We are
not making an offer to sell these securities in any jurisdiction where an offer or sale is not permitted. Except as otherwise stated,
you should assume that the information appearing in this prospectus is accurate only as of the date on the front cover of this prospectus
and that the information in any report incorporated by reference is accurate only as of the date of such report. Our business,
financial condition, results of operations and prospects may have changed since such dates.
No
action is being taken in any jurisdiction outside the United States to permit a public offering of our securities or possession
or distribution of this prospectus in that jurisdiction. Persons who come into possession of this prospectus in jurisdictions outside
the United States are required to inform themselves about and to observe any restrictions as to this offering and the distribution of
this prospectus applicable to that jurisdiction.
As
used in this prospectus, unless the context indicates or otherwise requires, “the Company,” “our Company,” “we,”
“us,” and “our” refer to Azitra, Inc., a Delaware corporation.
INDUSTRY
AND MARKET DATA
This prospectus contains or
incorporates by reference observations, statistical data, estimates, and forecasts that are based on independent industry, government
and non-government organization publications or other publicly available information, as well as other information based on our internal
sources. Although we believe that the third-party sources referred to in this prospectus or incorporated by reference are reliable,
estimates as they relate to projections involve numerous assumptions, are subject to risks and uncertainties, and are subject to change
based on various factors, including those discussed under the section titled “Risk Factors” and elsewhere in this
prospectus. These and other factors could cause results to differ materially from those expressed in the estimates made by the independent
parties and by us.
Certain
information in the text of this prospectus or incorporated by reference is contained in independent industry government and non-governmental
organizational publications. The sources of these publications are provided below:
| |
● |
Stacy
and Belkaid Study, Apollo Stacy and Yasmine Belkaid, Microbial Guardians of Skin Health. Science, 2019 Jan 18;363(6424):227-228.
Doi: 10.1126/science.aat4326. PMID: 30655428 |
| |
● |
Oh
Study, Zhou W, Spoto M, Hardy R, Guan C, Fleming E, Larson PJ, Brown JS, Oh J. Host-Specific Evolutionary and Transmission Dynamics
Shape the Functional Diversification of Staphylococcus epidermidis in Human Skin. Cell. 2020 Feb 6;180(3):454-470.e18. doi: 10.1016/j.cell.2020.01.006.
Epub 2020 Jan 30. PMID: 32004459; PMCID |
| |
● |
Satoh
Study, Satoh TK, Mellett M, Meier-Schiesser B, Fenini G, Otsuka A, Beer HD, Rordorf T, Maul JT, Hafner J, Navarini AA, Contassot
E, French LE. IL-36γ drives skin toxicity induced by EGFR/MEK inhibition and commensal Cutibacterium acnes. J Clin Invest.
2020 Mar 2;130(3):1417-1430. Doi: 10.1172/JCI128678. PMID: 31805013; PMCID: PMC7269569 |
| |
● |
Barbati
Study, Netherton Syndrome in Children: Management and Future Perspectives, Federica Barbati, Mattia Giovannini Teresa Oranges, Lorenzo
Lodi, Simona Barni, Elio Novembre, Ermanno Baldo, Mario Cristofolini, Stefano Stagi, Silvia Ricci, Francesca Mori, Cesare Filippeschi,
Chiara Azzari and Giuseppe Indol; Frontiers in Pediatrics, May 2021 |
| |
● |
Sun
Study, Netherton syndrome: A case report and review of the literature, Joannie D. Sun, MD, and Kenneth G. Linden, PhD, MD, International
Journal of Dermatology 2006 |
| |
● |
Orphanet,
Netherton Syndrome, Orphanet: Netherton syndrome |
PROSPECTUS
SUMMARY
This
summary highlights certain information appearing elsewhere in this prospectus. Investing in our common stock involves a high degree of
risk. Because it is only a summary, it does not contain all of the information that you should consider before investing in our common
stock and it is qualified in its entirety by, and should be read in conjunction with, the more detailed information appearing elsewhere
in this prospectus. Before you decide to invest in our common stock, you should read the entire prospectus carefully, including “Risk
Factors” beginning on page 10 and the financial statements and related notes included in this prospectus or incorporated by
reference.
On
July 1, 2024, we effected a 1-for-30 reverse stock split of our issued and outstanding shares of common stock. All historical share
and per share amounts reflected in this prospectus have been adjusted to reflect the reverse stock split.
Our
Company
We
are an early-stage clinical biopharmaceutical company focused on developing innovative therapies for precision dermatology using engineered
proteins and topical live biotherapeutic products. We have built a proprietary platform that includes a microbial library comprised of
approximately 1,500 unique bacterial strains that can be screened for unique therapeutic characteristics. The platform is augmented by
an artificial intelligence and machine learning technology that analyzes, predicts and helps screen our library of strains for drug like
molecules. The platform also utilizes a licensed genetic engineering technology, which can enable the transformation of previously genetically
intractable strains. Our initial focus is on the development of genetically engineered strains of Staphylococcus epidermidis, or S.
epidermidis, which we consider to be an optimal therapeutic candidate species for engineering of dermatologic therapies. The particular
species demonstrates a number of well-described properties in the skin. As of the date of this prospectus, we have identified among our
microbial library over 60 distinct bacterial species that we believe are capable of being engineered to create living organisms or engineered
proteins with significant therapeutic effect.
We
are a pioneer in genetically engineering bacteria for therapeutic use in dermatology. Our goal is to leverage our platforms and internal
microbial library bacterial strains to create new therapeutics that are either engineered living organisms or engineered proteins or
peptides to treat skin diseases. Our initial focus is on the development of our current product candidates, including:
| |
● |
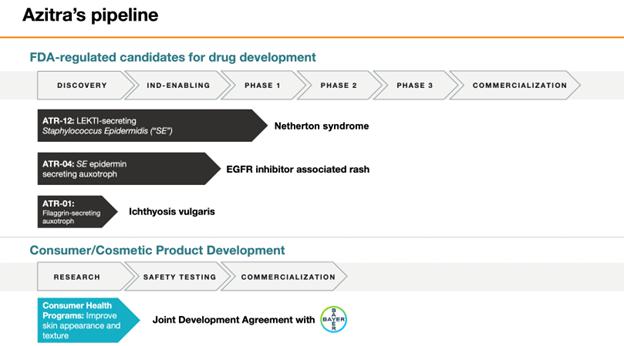
ATR-12,
a genetically modified strain of S. epidermidis for treating the orphan disease, Netherton syndrome, a chronic and sometimes
fatal disease of the skin estimated to affect approximately one to nine in every 100,000, but its prevalence may be underestimated
due to misdiagnosis caused by similarities to other skin diseases. We received Pediatric Rare Disease Designation for ATR-12 by the
United States Food and Drug Administration, or FDA, in 2019. In December 2022, we submitted an investigational new drug application,
or IND, for a Phase 1b clinical trial of ATR-12 in Netherton syndrome patients, and on January 27, 2023, we received notification
from the FDA that the “study may proceed” with respect to the proposed Phase 1b clinical trial. We commenced our Phase
1b clinical trial in December 2023 and expect to report initial safety results in the second half of 2024. |
| |
● |
ATR-04,
a genetically modified strain of S. epidermidis for treating the papulopustular rash experienced by cancer patients undergoing
epidermal growth factor receptor inhibitor, or EGFRi, targeted therapy. We intend to submit an IND for a Phase 1b clinical trial
in certain cancer patients undergoing EGFRi targeted therapy by mid-2024. Subject to FDA clearance of our IND, we expect to
commence our Phase 1b clinical trial in the fourth quarter of 2024. |
| |
● |
ATR-01,
a genetically modified strain of S. epidermidis that expresses an engineered recombinant human filaggrin protein for
treating ichthyosis vulgaris, a chronic, xerotic (abnormally dry), scaly skin disease with an estimated incidence and prevalence
of 1 in 250, which suggests a total patient population of 1.3 million in the United States. We are planning to complete lead optimization
and IND-enabling studies in 2024 to support an IND filing target in the second half of 2025. |
| |
● |
Two
separate strains of bacterial microbes are being investigated and developed by us and Bayer Consumer Care AG, the consumer products
division of Bayer AG, or Bayer, the international life science company pursuant to a Joint Development Agreement, or JDA, entered
into between the parties in December 2019. Under the terms of the JDA, we are responsible for testing our library of bacterial strains
and their natural products for key preclinical properties. After screening through hundreds of strains, we and Bayer have selected
two particular strains to move forward into further development. Bayer holds the exclusive option to license the patent rights to
these strains. In December 2020, Bayer purchased $8 million of our Series B preferred stock, which converted into 48,324 shares
of our common stock. |
We
also have established partnerships with teams from Carnegie Mellon University and the Fred Hutchinson Cancer Center, or Fred Hutch, two
of the premier academic centers in the United States. Our collaboration with the Carnegie Mellon based team takes advantage of the power
of whole genome sequencing. This partnership is mining our proprietary library of bacterial strains for novel, drug-like peptides and
proteins. The artificial intelligence/machine learning technology developed by this team predicts the molecules made by microbes from
their genetic sequences. The system then compares the predictions to the products actually made through tandem mass spectroscopy and/or
nuclear magnetic resonance imaging to refine future predictions. The predictions can be compared to publicly available 2D and 3D protein
databases to select drug like structures.
We
hold an exclusive, worldwide license from Fred Hutch regarding the use of its patented SyngenicDNA Minicircle Plasmid, or SyMPL, technologies
for all fields of genetic engineering, including to discover, develop and commercialize engineered microbial therapies and microbial-derived
peptides and proteins for skin diseases. We are utilizing our licensed patent rights to build plasmids in order to make genetic transformations
that have never been previously achieved. Our collaboration with Fred Hutch is led by Dr. Christopher Johnston, an expert in microbial
engineering, and the innovator behind the SyMPL technology.
Bayer
Partnership
In
December 2019, we entered into a Joint Development Agreement, or JDA, with Bayer pursuant to which we agreed to the joint development
of certain strains selected from our proprietary microbial library. We and Bayer have agreed to cooperate in the identification and in
vitro and ex vivo characterization of microbial strains for topical formulations, which we intend to develop as potential
over-the-counter cosmetic products. Bayer paid us a one-time payment upon execution of the JDA and has agreed to reimburse us for our
development costs. In October 2021, Bayer expanded the option agreement and paid us a second fee for additional characterization work.
We have granted Bayer an option to acquire an exclusive royalty bearing license for up to six strains subject to development activities
under the JDA, including an exclusive royalty bearing license to any related patent rights. Bayer has an option to acquire the exclusive
license rights for a period of six months following our delivery of the results of the JDA development activities to Bayer. After screening
through hundreds of strains, we and Bayer have selected two particular strains we characterized with in vitro and ex
vivo studies.
In
September 2020, Bayer’s venture capital group, LEAPS by Bayer, purchased $8 million of our Series B preferred stock, which converted
into 48,324 shares of our common stock.
Our
Strategy
Beyond
our three lead product candidates and collaboration with Bayer, our goal is to develop a broad portfolio of product candidates focused
on expanding the application of our platforms for precision dermatology. We believe that we have established a unique position in advancing
the development of biologics for precision dermatology.
We
intend to create a broad portfolio of product candidates for precision dermatology through our development of genetically engineered
proteins selected from our proprietary microbial library of approximately 1,500 unique bacterial strains. Our strategy is as follows:
| |
● |
Build
a sustainable precision dermatology company. Our goal is to build a leading precision dermatology company with a sustainable
pipeline of product candidates. To that end, we are focused on rapidly advancing our current pipeline of live biotherapeutic candidates
while actively developing additional product candidates. Each of our current product candidates are proprietary and subject to pending
patent applications. We expect that most of our genetically engineered product candidates we develop will be eligible for patent
protection. |
| |
● |
Advance
our lead product candidates, ATR-12 and ATR-04, through clinical trials. We expect to report initial safety results of our
Phase 1b clinical trial for our ATR-12 in Netherton syndrome patients in the second half of 2024 and are currently planning to commence
a Phase 1b trial of our ATR-04 in certain cancer patients undergoing EGFRi therapy in the fourth quarter of 2024. Our Phase 1b
clinical trial for ATR-12 is currently enrolling and we expect to file an IND for ATR-04 by mid- 2024. |
| |
● |
Broaden
our platform by selectively exploring strategic partnerships that maximize the potential of our precision dermatology programs.
We intend to maintain significant rights to all of our core technologies and product candidates. However, we will continue to evaluate
partnering opportunities in which a strategic partner could help us to accelerate development of our technologies and product candidates,
provide access to synergistic combinations, or provide expertise that could allow us to expand into the treatment of different types
of skin diseases. We may also broaden the reach of our platform by selectively in-licensing technologies or product candidates. In
addition, we will consider potentially out-licensing certain of our proprietary technologies for indications and industries that
we are not pursuing. We believe our genetic engineering techniques and technologies have applicability outside of the field of medicine,
including cosmetics and in the generation of clean fuels and bioremediation. |
| |
● |
Leverage
our academic partnerships. We currently have partnerships with investigators at the Fred Hutchinson Cancer Center, Yale University,
Jackson Laboratory for Genomic Medicine, and Carnegie Mellon University. We expect to leverage these partnerships and potentially
expand them or form other academic partnerships to bolster our engineering platforms and expand our research and development pipeline. |
| |
● |
Expand
on our other potential product candidates. Beyond our three lead product candidates, our goal is to develop a broad portfolio
of product candidates focused on expanding the application of our platforms for precision dermatology. We have a proprietary platform
for discovering and developing therapeutic products for precision dermatology. Our platform is built around a microbial library comprised
of approximately 1,500 unique bacterial strains to allow screening for unique therapeutic characteristics and utilizes a microbial
genetic technology that analyzes, predicts and engineers the proteins, peptides and molecules made by skin microbes. Our ability
to genetically engineer intractable microbial species is uniquely leveraged by our exclusive license to the SyMPL technology. |
Our
Intellectual Property
As
of the date of this prospectus, we own three issued U.S. patents, ten pending U.S. patent applications and 60 other
foreign patents and patent applications that are important to the development of our business.
Our
Leadership Team
We
are led by Francisco D. Salva, our chief executive officer, and Travis Whitfill, our co-founder and chief operating officer, who have
more than 35 years of combined experience in the management of biotechnology companies and healthcare investing. Mr. Salva was previously
a co-founder of Acerta Pharma, which was sold to AstraZeneca for approximately $6.3 billion in a staged acquisition in 2016. He also
worked on the turnaround of Pharmacyclics, which subsequently sold to Abbvie for approximately $21 billion in 2015. Before that, Mr.
Salva spent almost a decade in life sciences venture capital. Mr. Whitfill served as associate research scientist and serves as assistant
professor adjunct at Yale University with appointments in the Departments of Pediatrics and Emergency Medicine. He spent nearly a decade
in venture capital as a partner in a biotech-focused venture capital fund, Bios Partners. He has led numerous grant-funded projects,
holds nearly a dozen patents and has co-authored over 60 publications. Our board of directors, or Board, is comprised of renowned group
of senior executives, scientists and investors in the biotechnology industry.
Our
Competitive Strengths
We
are a pioneer in genetically engineering bacteria for therapeutic use in dermatology clinical trials. We have built a proprietary platform
that includes a microbial library comprised of approximately 1,500 unique bacterial strains that are screened for therapeutic characteristics
as well as lead drug candidates. Furthermore, we have exclusively licensed a novel technology, which potentially enables the genetic
transformation of previously intractable bacterial microbes. The history of recombinant protein engineering in biotech has traditionally
been limited to less than 20 species. Our licensed technology opens up the potential to genetically engineer thousands of microbial species
to build proteins and peptides that have never been previously built. Our management team has significant experience in discovering,
developing, manufacturing and commercializing therapeutics. The members of our leadership team have specialized expertise developed at
companies including Pharmacyclics, Acerta Pharma, Castle Creek Biosciences, VYNE Therapeutics (fka Menlo Therapeutics), Revance Therapeutics,
Biogen, Novartis and Connetics Corp.
Our
Market Opportunity
We
believe there are significant market opportunities to capture in each of our addressable markets. The dermatology market itself has shown
considerable growth over the last decade and is predicted to continue to grow. According to Vision Research Reports, the dermatology
drug market surpassed $17 billion in 2021 and is expected to grow at a compound annual growth rate of 8.8% through 2030. Our first product
candidate to emerge from our platform focuses on the orphan indication of Netherton syndrome. Based on the Barbati and Sun Studies, we
believe that this product candidate represents a potential $250 million global sales opportunity by mid-2030. Our second product candidate
focuses on papulopustular rash due to EGFR inhibitors. We believe this product candidate represents a potential $1 billion global sales
opportunity by 2030. The diseases we intend to target are well characterized, often by a monogenic genetic mutation. Additionally, the
era of genomic sequencing has ushered in unprecedented progress in genetic testing. The defined molecular pathophysiology of over 100
rare skin diseases has now been defined.
Our
Corporate Information
We
were incorporated under the laws of the state of Delaware on January 2, 2014. Our principal executive offices are located at 21 Business
Park Drive, Branford, Connecticut 06405, and our telephone number is (203) 646-6446. Our website address is www.azitrainc.com. The information
contained in, or accessible through, our website is not incorporated by reference into this prospectus, and you should not consider any
information contained in, or that can be accessed through, our website as part of this prospectus or in deciding whether to purchase
our common stock or Pre-Funded Warrants.
We
own U.S. and foreign registered trademarks, including our company name. All other trademarks or trade names referred to in this prospectus
are the property of their respective owners. Solely for convenience, the trademarks and trade names in this prospectus are referred to
without the symbols ® and ™, but such references should not be construed as any indication that their respective owners will
not assert, to the fullest extent under applicable law, their rights thereto.
Additional Information
For additional
information related to our business and operations, please refer to the reports incorporated herein by reference, including our Annual
Report on Form 10-K for the year ended December 31, 2023 as filed with the SEC on March 15, 2024 and the amendment thereto filed with
the SEC on April 29, 2024, which we collectively refer to as the 2023 Form 10-K, as described in the section entitled “Incorporation
of Certain Documents by Reference” in this prospectus.
Implications
of Being an Emerging Growth Company
The
Jumpstart Our Business Startups Act, or the JOBS Act, was enacted in April 2012 with the intention of encouraging capital formation in
the United States and reducing the regulatory burden on newly public companies that qualify as “emerging growth companies.”
We are an emerging growth company within the meaning of the JOBS Act. As an emerging growth company, we may take advantage of certain
exemptions from various public reporting requirements, including:
| |
● |
the
requirement that our internal control over financial reporting be attested to by our independent registered public accounting firm
pursuant to Section 404 of the Sarbanes-Oxley Act of 2002; |
| |
● |
certain
requirements related to the disclosure of executive compensation in this prospectus and in our periodic reports and proxy statements; |
| |
● |
the
requirement that we hold a nonbinding advisory vote on executive compensation and any golden parachute payments; and |
| |
● |
the
ability to delay compliance with new or revised financial accounting standards until private companies are required to comply with
the new or revised financial accounting standard. |
We
may take advantage of the exemptions under the JOBS Act discussed above until we are no longer an emerging growth company. We will remain
an emerging growth company until the earliest to occur of (1) the last day of the fiscal year in which we have $1.235 billion or more
in annual revenue; (2) the date we qualify as a “large accelerated filer,” with at least $700 million of equity securities
held by non-affiliates; (3) the date on which we have issued, in any three-year period, more than $1.0 billion in non-convertible debt
securities; or (4) the last day of the fiscal year ending after the fifth anniversary of the IPO.
We
may choose to take advantage of some, but not all, of the available benefits under the JOBS Act. We have chosen to take advantage of
all of the other exemptions discussed above. Accordingly, the information contained herein and in our subsequent filing with the SEC
may be different than the information you receive from other public companies in which you hold stock.
For
certain risks related to our status as an emerging growth company, see the disclosure elsewhere in this prospectus under “Risk
Factors—Risks Related to this Offering and Owning Our Common Stock—We are an ‘emerging growth company’ under
the JOBS Act and we cannot be certain if the reduced disclosure requirements applicable to emerging growth companies will make our common
stock less attractive to investors.”
Implications
of Being a Smaller Reporting Company
Additionally,
we are a “smaller reporting company” as defined in Rule 10(f)(1) of Regulation S-K. Smaller reporting companies may take
advantage of certain reduced disclosure obligations, including, among other things, providing only two years of audited financial statements.
We will remain a smaller reporting company until the last day of the fiscal year in which (1) the market value of our common stock held
by non-affiliates equals or exceeds $250 million as of the end of that year’s second fiscal quarter, or (2) our annual revenues
equaled or exceeded $100 million during such completed fiscal year and the market value of our common stock held by non-affiliates equals
or exceeds $700 million as of the end of that year’s second fiscal quarter.
THE
OFFERING
| Issuer |
Azitra,
Inc. |
| |
|
| Units to be Offered |
3,115,265 Units based
on assumed public offering price of $3.21 per Unit. Each Unit will consist of one share of common stock (or Pre-Funded Warrant
to purchase one share of our common stock in lieu thereof), two Class A Warrants each to purchase one share of common stock. The Units have no stand-alone rights and will not be certificated or issued as stand-alone securities.
The shares of common stock and Pre-Funded Warrants, if any, can each be purchased in this offering only with the accompanying
Class A Warrants as part of Units, but the components of the Units will be immediately separable
and will be issued separately in this offering. |
| |
|
| Pre-Funded Units to be Offered |
We are also offering
to certain purchasers whose purchase of Units in this offering would otherwise result in the purchaser, together with its affiliates
and certain related parties, beneficially owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding
common stock immediately following the consummation of this offering, the opportunity to purchase, if such purchasers so choose,
Units including Pre-Funded Warrants to purchase shares of common stock, in lieu of Units including shares of common stock that would
otherwise result in any such purchaser’s beneficial ownership exceeding 4.99% (or, at the election of the purchaser, 9.99%)
of our outstanding common stock. The purchase price of each Unit including a Pre-Funded Warrant will be equal to the price at which
a Unit is sold to the public in this offering, minus $0.0001, and the exercise price of each Pre-Funded Warrant will be $0.0001 per
share. Each Pre-Funded Warrant will be exercisable for one share of our common stock and will be exercisable at any time after its
original issuance until exercised in full, provided that the purchaser will be prohibited from exercising Pre-Funded Warrants for
shares of our common stock if, as a result of such exercise, the purchaser, together with its affiliates and certain related parties,
would own more than 4.99% of the total number of shares of our common stock then issued and outstanding. However, any holder may
increase such percentage to any other percentage not in excess of 9.99%, provided that any increase in such percentage shall not
be effective until 61 days after such notice to us. |
| |
|
| Class A Warrants to be Offered |
6,230,530 Class A
Warrants. Each Unit includes one share of common stock and two Class A Warrants. Each Class A Warrant is initially exercisable at a
price of $3.21 per share (assuming an offering price of $3.211 per Unit), however, if on the Reset Date, the
Reset Price is less than the exercise price at such time, the exercise price shall be decreased to the Reset Price. The Class A
Warrants will be exercisable upon issuance and will expire five (5) years from the date of issuance. See “Description of Securities—Class A Warrants.” |
| |
|
| Common
stock to be outstanding after this offering |
4,075,411 shares of common stock, in each case,
assuming no sales of Pre-Funded Warrants and no exercise of the Class A Warrants being offered
in this offering. To the extent Pre-Funded Warrants are sold, it will reduce the number of shares of common stock that we are offering
on a one-for-one basis. |
| |
|
| Use
of proceeds |
If we sell all
of the securities offered hereby, we estimate that the net proceeds of this offering based
upon an assumed public offering price of $3.21 per Unit, and after deducting
placement agent fees and estimated offering expenses, will be approximately $8.95
million, assuming no exercise of the Class A Warrants.
However, because this offering is being made on a best-efforts basis, and there is no minimum
offering amount required as a condition to the closing of this offering, we
may sell fewer than all of the securities offered hereby and may receive significantly less
in net proceeds from this offering.
Assuming
that we receive only $5.23 million of net proceeds from this offering (based on
gross offering proceeds of $6 million), we believe that the net proceeds from this offering,
together with our cash on hand, will satisfy our capital needs until November 2024
under our current business plan and assuming that we receive $3.37 million of
net proceeds from this offering (based on gross offering proceeds of $4 million),
we believe that the net proceeds from this offering, together with our cash on hand, will
satisfy our capital needs until October 2024 under our current business plan.
We intend to use the net proceeds from this offering, along with our
existing cash and cash equivalents, for working capital and other general corporate purposes. Following this offering, we will need to
raise additional capital to fund our operations and continue to support our planned development and commercialization activities.
See the section titled “Use of Proceeds” in this prospectus for a more complete description of the intended use of
processed from this offering.
|
| |
|
| Best Efforts Offering |
We have agreed to issue and sell the securities offered
hereby to the purchasers through the placement agent. The placement agent is not required to buy or sell any specific number or dollar
amount of the securities offered hereby, but will use their reasonable best-efforts to solicit offers to purchase the securities
offered by this prospectus. See “Plan of Distribution” in this prospectus. |
| |
|
| Trading
market and symbol |
Our
common stock is listed on the NYSE American under the symbol “AZTR.”
We do not intend to list the Pre-Funded Warrants or
the Class A Warrants on the NYSE American or any other national securities exchange or nationally recognized trading
system. Without an active trading market, the liquidity of the Pre-Funded Warrants and Class A Warrants will be limited. |
| Risk
factors |
Investing
in our securities involves a high degree of risk. See the section titled “Risk Factors” beginning on page 10 and
the other information in this prospectus for a discussion of the factors you should consider carefully before you decide to invest
in our securities. |
| |
|
| Lock-up |
We
have agreed, subject to certain exceptions, not to sell, offer, agree to sell, contract to sell, hypothecate, pledge, grant any
option to purchase, make any short sale of, or otherwise dispose of or hedge, directly or indirectly, any shares of our capital
stock or any securities convertible into or exercisable or exchangeable for shares of capital stock, for a period of up to three
months from the close of this offering, without the prior written consent of the placement agent. In addition, our officers and
directors agreed not to sell, offer, agree to sell, contract to sell, hypothecate, pledge, grant any option to purchase, make any
short sale of, or otherwise dispose of or hedge, directly or indirectly, any shares of our capital stock or any securities
convertible into or exercisable or exchangeable for a period of up to two months from the close of this offering, without the
prior written consent of the placement agent. See the section of this prospectus entitled “Plan of Distribution” for
additional information. |
The number of shares of our common
stock to be outstanding after this offering is based on approximately 960,146 shares of our common stock outstanding as of June
30, 2024, and excludes:
| |
● |
41,608 shares of our common
stock issuable upon exercise of outstanding options, with a weighted average exercise price of $41.7 per share, granted pursuant
to our 2016 Stock Incentive Plan, or the 2016 Plan, and our 2023 Stock Incentive Plan, or the 2023 Plan; |
| |
● |
approximately 33,013 shares of our common stock issuable upon
exercise of outstanding warrants, with a weighted average exercise price of $54.00 per share; |
| |
● |
7,456 shares of our common
stock reserved for future grants under our 2016 Plan and 65,333 shares of our common stock reserved for future grants under
our 2023 Plan; |
| |
● |
up to 6,230,530 shares
of common stock issuable upon exercise of the Class A Warrants; |
| |
● |
up to 3,115,265 shares
of common stock issuable upon exercise of the Pre-Funded Warrants; and |
| |
● |
up to 124,610 shares of
common stock issuable upon exercise of the placement agent warrants. |
Unless
we indicate otherwise or unless the context otherwise requires, all information in this prospectus assumes the following:
| |
● |
no
exercise of outstanding warrants or options described above; and |
| |
● |
No exercise of the Class A Warrants
or Pre-Funded Warrants or placement agent warrants. |
SUMMARY
RISK FACTORS
Our
business is subject to numerous risks, including risks that may prevent us from achieving our business objectives or adversely affect
our business, results of operations, cash flows, and prospects. You should carefully consider the risks discussed below and further discussed
in the section “Risk Factors” immediately following this prospectus summary before investing in our securities.
| |
● |
We
are an early-stage clinical biopharmaceutical company with limited operating history; |
| |
● |
We
have a history of significant operating losses and anticipate continued operating losses for the foreseeable future; |
| |
● |
This offering
is being made on a best-efforts basis and we may sell fewer than all of the securities offered hereby and may receive significantly
less in net proceeds from this offering, which will provide us only limited working capital; |
| |
● |
We
expect we will need additional financing to execute our business plan and fund operations, which additional financing may not be
available on reasonable terms, or at all; |
| |
● |
The
clinical and commercial utility of our microbial library and genetic engineering platform is uncertain and may never be realized; |
| |
● |
Our
product candidates are in early stages of development, and therefore they will require extensive additional preclinical and clinical
testing; |
| |
● |
We
will need to grow the size of our organization, and we may experience difficulties in managing this growth; |
| |
● |
We
currently have no sales and marketing organization; |
| |
● |
We
will be completely dependent for the foreseeable future on third parties to manufacture our product candidates for commercial sale; |
| |
● |
Our
business model includes the potential out-licensing of strains from our proprietary microbial library or our product candidates to
other pharmaceutical companies; however, technology licensing in the pharmaceutical industry is a lengthy process and subject to
several risks and factors outside of our control; |
| |
● |
Our
business may suffer with the loss of key personnel; |
| |
● |
If
product liability lawsuits are brought against us, we may incur substantial liabilities and may be required to limit commercialization
of our product candidates; |
| |
● |
Our
business operations could suffer in the event of information technology systems’ failures or security breaches; |
| |
● |
We
face significant competition from other biotechnology and pharmaceutical companies targeting medical dermatological indications; |
| |
● |
Our
success is entirely dependent on our ability to obtain the marketing approval for our product candidates by the FDA and the regulatory
authorities in foreign jurisdictions in which we intend to market our product candidates, of which there can be no assurance; |
| |
● |
Our
clinical trials may fail to demonstrate substantial evidence of the safety and efficacy of our product candidates or any future product
candidates; |
| |
● |
Results
of preclinical studies of our product candidates may not be predictive of the results of future preclinical studies or clinical trials; |
| |
● |
Even
if we receive regulatory approval for any of our product candidates, we may not be able to successfully commercialize the product
and the revenue that we generate from its sales, if any, may be limited; |
| |
● |
Current
and future legislation may increase the difficulty and cost for us to obtain marketing approval of and commercialize our product
candidates and affect the prices we may obtain; |
| |
● |
It
is difficult and costly to protect our intellectual property rights, and we cannot ensure the protection of these rights; |
| |
● |
Our
product candidates may infringe the intellectual property rights of others, which could increase our costs and delay or prevent our
development and commercialization efforts; |
| |
● |
An
active, liquid and orderly trading market for our shares may not develop; |
| |
● |
Future
capital raises may dilute your ownership and have other adverse effects on our operations; |
| |
● |
The
market price of our shares may be subject to fluctuation and volatility; |
| |
● |
If
we fail to maintain an effective system of internal control over financial reporting, we may not be able to accurately report our
financial results or prevent fraud; |
| |
● |
There is no market for Class A Warrants or Pre-Funded Warrants and one is not expected to develop; |
| |
● |
Holders of the Class A Warrants
and Pre-Funded Warrants purchased in this offering will have no rights as common stockholders until such holders exercise such
warrants and acquire our common stock; |
| |
● |
We
ratified certain corporate actions pursuant to Section 204 of the Delaware General Corporate Law, or DGCL; however, there can be
no assurance that claims will not be made to challenge the validity of the ratification or the related corporate actions; and |
| |
● |
Our
charter documents and Delaware law may inhibit a takeover that stockholders consider favorable. |
SUMMARY
FINANCIAL DATA
The following tables summarize
our financial data. You should read this summary financial data together with the section entitled “Management’s Discussion
and Analysis of Financial Condition and Results of Operations” and our financial statements and related notes that are included
our 2023 Form 10-K and our Quarterly Report on Form 10-Q filed with the SEC on May 9, 2024, or our 2024 Form 10-Q, both of which are
incorporated herein by reference. The financial information as of and for the fiscal years ended December 31, 2023 and 2022
is derived from the audited financial statements that are included in our 2023 Form 10-K. The financial information as of
and for the three months ended March 31, 2024 and 2023 are derived from our unaudited financial statements included in
our 2024 Form 10-Q. The unaudited financial statements were prepared on the same basis as the audited financial statements. Our management
believes that the unaudited financial statements reflect all adjustments necessary for the fair presentation of the financial condition
and results of operations for such periods. Our historical
results are not necessarily indicative of the results that may be expected in the future.
| | |
Three
Months Ended March
31, | | |
Years Ended
December 31, | |
| (in thousands, except share amounts) | |
2024 | | |
2023 | | |
2023 | | |
2022 | |
| Statement of Operations Data | |
(unaudited) | | |
(unaudited) | | |
| | |
| |
| Revenues | |
$ | — | | |
$ | 113 | | |
$ | 686 | | |
$ | 284 | |
| Net loss | |
$ | (2,933 | ) | |
$ | (2,457 | ) | |
$ | (11,284 | ) | |
$ | (10,680 | ) |
| Net loss per share, basic and diluted | |
$ | (4.50 | ) | |
| (90.00 | ) | |
$ | (54.90 | ) | |
$ | (38.22 | ) |
| | |
March 31, | |
| (in thousands) | |
2024 |
|
| Balance Sheet Data: | |
(unaudited) |
|
| Cash and cash equivalents | |
$ | 3,001 |
|
| Working capital | |
$ | 2,268 |
|
| Total assets | |
$ | 6,068 |
|
| Additional paid-in capital | |
$ | 55,853 |
|
| Total stockholders’ equity | |
$ | 4,324 |
|
RISK
FACTORS
Any
investment in our securities involves a high degree of risk. You should carefully consider the risks described below and set
forth in the section “Risk Factors” in our 2023 Form 10-K, as well as any amendment or update to our risk factors reflected
in subsequent filings with the SEC, which are incorporated by reference in this prospectus, and all other information contained in this
prospectus and incorporated by reference in this prospectus, before you make a decision to invest in our securities. Please
note that the risks highlighted here are not the only ones that we may face. For example, additional risks presently unknown to us or
that we currently consider immaterial or unlikely to occur could also impair our operations. If any of the following events occur or
any additional risks presently unknown to us actually occur, our business, financial condition and operating results may be materially
adversely affected. In that event, the trading price of our common stock could decline and you could lose all or part of your investment.
Risks
Relating to this Offering and Our Units
As
an investor, you may lose all of your investment.
Investing
in our securities involves a high degree of risk. As an investor, you may never recoup all, or even part, of your investment and you
may never realize any return on your investment. You must be prepared to lose all of your investment.
We
expect we will need additional financing to execute our business plan and fund operations, which additional financing may not be available
on reasonable terms or at all.
As
of March 31, 2024, we had total assets of $6.1 million and working capital of $2.3 million. We believe that net
proceeds of this offering, along with our cash on hand as of the date of this prospectus, will not be sufficient to cover our
proposed plan of operations over, at least, the next 12 months. We intend to seek additional funds through various financing sources,
including the sale of our equity, licensing fees for our technology and joint ventures with industry partners. In addition, we will consider
alternatives to our current business plan that may enable to us to achieve revenue producing operations and meaningful commercial success
with a smaller amount of capital. However, there can be no guarantees that such funds will be available on commercially reasonable terms,
if at all. If such financing is not available on satisfactory terms, we may be unable to further pursue our business plan and we may
be unable to continue operations, in which case you may lose your entire investment.
The
report of our independent registered public accounting firm for the year ended December 31, 2023 states that due to our accumulated deficit,
recurring and negative cash flow from operations there is substantial doubt about our ability to continue as a going concern.
This
offering is being made on a best-efforts basis and we may sell fewer than all of the securities offered hereby and may receive significantly
less in net proceeds from this offering, which will provide us only limited working capital.
This offering is being made on
a best-efforts basis and we may sell fewer than all of the securities offered hereby and may receive significantly less in net proceeds
from this offering. Assuming that we receive net proceeds of approximately $8.95 million from this offering (assuming an offering
with gross proceeds of $10 million), we believe that the net proceeds from this offering, together with our existing cash and
cash equivalents, will meet our capital needs until February 2025 under our current business plan. Assuming that we receive net
proceeds of approximately $5.23 million from this offering (assuming an offering with gross proceeds of $6 million), we
believe that the net proceeds from this offering, together with our existing cash and cash equivalents, will satisfy our capital needs
until November 2024 under our current business plan. Assuming that we receive net proceeds of approximately $3.37 million
from this offering (assuming an offering with gross proceeds of $4 million), we believe that the net proceeds from this offering,
together with our existing cash and cash equivalents, will satisfy our capital needs until October 2024 under our current business
plan.
Because
we will have broad discretion and flexibility in how the net proceeds from this offering are used, we may use the net proceeds in ways
in which you disagree.
We
intend to use the net proceeds from this offering for working capital and general corporate purposes. See “Use of Proceeds”
for additional information. Accordingly, our management will have significant discretion and flexibility in applying the net proceeds
of this offering. You will be relying on the judgment of our management with regard to the use of these net proceeds, and you will not
have the opportunity, as part of your investment decision, to assess whether the net proceeds are being used appropriately. It is possible
that the net proceeds will be invested in a way that does not yield a favorable, or any, return for us. The failure of our management
to use such funds effectively could have a material adverse effect on our business, financial condition, operating results and cash flow.
We
may not be able to conclude a licensing arrangement with Bayer or, if we are able to do so, realize the expected benefits.
In
December 2019, we entered into a Joint Development Agreement, or JDA, with Bayer pursuant to which we agreed to the joint development
of certain strains selected from our proprietary microbial library. We have granted Bayer an option to acquire an exclusive royalty bearing
license for up to six strains subject to development activities under the JDA, including an exclusive royalty bearing license to any
related patent rights. We continue to conduct joint development work with Bayer and discuss the proposed royalty bearing license. While
we remain optimistic concerning the successful completion of a royalty bearing license agreement with Bayer, there can be no assurance
we will be able to conclude a licensing agreement or, if we are, that the terms of such license agreement will be favorable to us. Assuming
our joint development work is successful, our ability to convert the successful development work into a commercial license with Bayer
is dependent on a number of risks and factors, many of which are outside our control, including:
| |
● |
Bayer’s
internal evaluation of the economic benefits of marketing a dermatological product that may be competitive with other products currently
in development or commercial sale by Bayer regardless of the perceived benefits or advantages of the microbial strain; |
| |
● |
Pharmaceutical regulations vary by country and, depending on Bayer’s marketing plans for the product based on our microbial strain, the pharmaceutical regulations in countries to be targeted by Bayer may render the clinical development of the product to be too expensive, time consuming or at risk for regulatory approval;
|
| |
● |
Bayer’s internal budgetary and product development
issues, including their ability to commit the capital and human resources towards the development and commercialization of our microbial
strain; and |
| |
● |
Bayer’s
willingness to accept our requirements for upfront fees and ongoing royalties. |
In
addition, we believe that in many cases our potential partners or licensee, including Bayer, may engage with us in the early-stage feasibility
testing as part of their evaluation of multiple drug and drug delivery options and prior to making any decision or commitment to the
development of a new drug product. Consequently, even if our joint development activities with Bayer are successful, for reasons unrelated
to the performance of our technology, we may not be able to conclude a licensing agreement with Bayer.
We
are subject to a prohibition against engaging in variable transactions which might be applicable to this offering.
In
connection with our February 2024 follow-on offering, we entered into an underwriting agreement with the underwriter that included our
covenant not to engage in a variable transaction until August 13, 2024. That covenant was also disclosed to investors in that offering
by way of the offering prospectus. The provision in our Series A Warrant that provides for the potential reset of the exercise
price could cause this offering to be viewed as a variable transaction even though the reset
made part of the Series A Warrant will not become effective until after August 13, 2024. In the event the underwriter
or any investor from our February 2024 offering brings a claim that this offering breaches our variable transaction covenant, we
could become subject to an action for a court ordered injunction against the completion of this offering as well as a claim for damages.
There can be no assurance that we will not be subject to such a legal action claiming our breach of the variable prohibition or,
if so, that we will be successful in defending such action.
The
market price of our shares may be subject to fluctuation and volatility. You could lose all or part of your investment.
The market price of our common
stock is subject to wide fluctuations in response to various factors, some of which are beyond our control. Since shares of our common
stock were sold in our initial public offering, or IPO, in June 2023 at a price of $150.00 per share, the reported high and low
sales prices of our common stock have ranged from $155.40 to $2.22 through July 3, 2024. The market price of our
shares on the NYSE American may fluctuate as a result of a number of factors, some of which are beyond our control, including, but not
limited to:
| |
● |
actual
or anticipated variations in our and our competitors’ results of operations and financial condition; |
| |
● |
changes
in earnings estimates or recommendations by securities analysts, if our shares are covered by analysts; |
| |
● |
market
acceptance of our product candidates; |
| |
● |
development
of technological innovations or new competitive products by others; |
| |
● |
announcements
of technological innovations or new products by us; |
| |
● |
publication
of the results of preclinical or clinical trials for our product candidates; |
| |
● |
failure
by us to achieve a publicly announced milestone; |
| |
● |
delays
between our expenditures to develop and market new or enhanced products and the generation of sales from those products; |
| |
● |
developments
concerning intellectual property rights, including our involvement in litigation brought by or against us; |
| |
● |
regulatory
developments and the decisions of regulatory authorities as to the approval or rejection of new or modified products; |
| |
● |
changes
in the amounts that we spend to develop, acquire or license new products, technologies or businesses; |
| |
● |
changes
in our expenditures to promote our product candidates; |
| |
● |
our
sale or proposed sale, or the sale by our significant stockholders, of our shares or other securities in the future; |
| |
● |
changes
in key personnel; |
| |
● |
success
or failure of our research and development projects or those of our competitors; |
| |
● |
the
trading volume of our shares; and |
| |
● |
general
economic and market conditions and other factors, including factors unrelated to our operating performance. |
These
factors and any corresponding price fluctuations may materially and adversely affect the market price of our shares and result in substantial
losses being incurred by our investors. In the past, following periods of market volatility, public company stockholders have often instituted
securities class action litigation. If we were involved in securities litigation, it could impose a substantial cost upon us and divert
the resources and attention of our management from our business.
There
is no public market for the Class A Warrants or Pre-Funded Warrants being offered in this offering.
There
is no established public trading market for the Class A Warrants or Pre-Funded Warrants being offered
in this offering, and we do not expect a market to develop. In addition, we do not intend to apply to list the Class A Warrants or Pre-Funded Warrants on any securities exchange or nationally recognized trading system, including the NYSE American.
Without an active market, the liquidity of the Class A Warrants and Pre-Funded Warrants will be limited.
This
offering may cause the trading price of our shares of common stock to decrease.
The
price per Unit, together with the number of shares of common stock and Class A Warrants and Pre-Funded Warrants we
propose to issue and ultimately will issue if this offering is completed, may result in an immediate decrease in the market price of
our shares. This decrease may continue after the completion of this offering.
Resales
of our shares of common stock in the public market by our stockholders as a result of this offering may cause the market price of our
shares of common stock to fall.
We
are registering 3,115,265 shares of common stock made part of the Units (including shares of common stock issuable upon
exercise of Pre-Funded Warrants) and an aggregate of
6,230,530 shares of common stock issuable upon the
exercise of the Class A Warrants offered under this prospectus. Sales of substantial amounts of our
shares of common stock in the public market, or the perception that such sales might occur, could adversely affect the market price
of our shares of common stock. The issuance of new shares of common stock could result in resales of our shares of common stock by
our current shareholders concerned about the potential ownership dilution of their holdings. Furthermore, in the future, we may
issue additional shares of common stock or other equity or debt securities exercisable or convertible into shares of common stock.
Any such issuance could result in substantial dilution to our existing shareholders and could cause our stock price to
decline.
Provisions
of the Class A Warrants offered pursuant to this prospectus could discourage an acquisition of us by a third-party.
Certain
provisions of the Class A Warrants offered pursuant to this prospectus could make it more difficult or expensive
for a third-party to acquire us. The Class A Warrants each prohibit us from engaging in certain transactions constituting
“fundamental transactions” unless, among other things, the surviving entity assumes our obligations under the applicable
warrants. These and other provisions of the Class A Warrants could prevent or deter a third-party from acquiring
us even where the acquisition could be beneficial to you.
The
Class A Warrants may have an adverse effect on the market price of our common stock and make it more difficult
to effect a business combination.
To
the extent we issue shares of common stock to effect a future business combination, the potential for the issuance of a substantial number
of additional shares of common stock upon exercise of the Class A Warrants could make us a less attractive acquisition
vehicle in the eyes of a target business. Such Class A Warrants, when exercised, will increase the number of issued
and outstanding shares of common stock and reduce the value of the shares issued to complete the business combination. Accordingly, the
Class A Warrants may make it more difficult to effectuate a business combination or increase the cost of acquiring
a target business. Additionally, the sale, or even the possibility of a sale, of the shares of common stock underlying the Class A Warrants could have an adverse effect on the market price for our securities or on our ability to obtain future financing.
If and to the extent the Class A Warrants are exercised, you may experience dilution to your holdings.
The
Class A Warrants are speculative in nature.
The
Class A Warrants offered hereby as part of the Units do not confer any rights of share of common stock ownership
on their holders, such as voting rights or the right to receive dividends, but rather merely represent the right to acquire shares of
common stock at a fixed price. Following this offering, the market value of the Class A Warrants will be uncertain
and there can be no assurance that the market value of the Class A Warrants, if any, will equal or exceed their
exercise prices, and consequently, whether it will ever be profitable for holders of Class A Warrants to exercise
such Class A Warrants.
If
you purchase our securities in this offering, you may experience future dilution as a result of future equity offerings or other equity
issuances.
In
order to raise additional capital, we believe that we will offer and issue additional shares of our common stock or other securities
convertible into or exchangeable for our common stock in the future. We are generally not restricted from issuing additional securities,
including shares of common stock, securities that are convertible into or exchangeable for, or that represent the right to receive, common
stock or substantially similar securities. The issuance of securities in future offerings may cause dilution to our stockholders, including
investors in this offering. We cannot assure you that we will be able to sell shares or other securities in any other offering at a price
per share that is equal to or greater than the price per share paid by investors in this offering, and investors purchasing other securities
in the future could have rights superior to existing stockholders. The price per share at which we sell additional shares of our common
stock or other securities convertible into or exchangeable for our common stock in future transactions may be higher or lower than the
price per share in this offering. Further, we may choose to raise additional capital due to market conditions or strategic considerations
even if we believe we have sufficient funds for our current or future operating plans.
We
may not receive any meaningful amount of additional funds upon the exercise of the Class A Warrants
and Pre-Funded Warrants.
Each
Class A Warrant shall be exercisable for a period of five years from the date of issuance and there can be no assurance
of when, if ever, the Class A Warrants will be exercised. Each Pre-Funded Warrant will be exercisable
until it is fully exercised and by means of payment of the nominal cash purchase price upon exercise. Accordingly, there can be no assurance
that we will receive any meaningful additional funds upon the exercise of the Class A Warrants or Pre-Funded
Warrants.
Holders
of the Class A Warrants and Pre-Funded Warrants purchased in this offering will have no rights as common
stockholders until such holders exercise such warrants and acquire shares of our common stock.
Until
holders of the Class A Warrants or Pre-Funded Warrants acquire shares of our common stock upon exercise
thereof, holders of such Class A Warrants and Pre-Funded Warrants will have no rights with respect to
the shares of our common stock underlying such warrants. Upon exercise of the Class A Warrants or Pre-Funded
Warrants, such holders will be entitled to exercise the rights of a common stockholder only as to matters for which the record date occurs
after the exercise date.
Significant
holders or beneficial holders of shares of our common stock may not be permitted to exercise the Pre-Funded Warrants that they hold.
A
holder of the Pre-Funded Warrants will not be entitled to exercise any portion of any Pre-Funded Warrant that, upon giving effect to
such exercise, would cause: (i) the aggregate number of shares of our common stock beneficially owned by such holder (together with its
affiliates) to exceed 4.99% (or, upon election of holder, 9.99%) of the number of shares of our common stock outstanding immediately
after giving effect to the exercise; or (ii) the combined voting power of our securities beneficially owned by such holder (together
with its affiliates) to exceed 4.99% (or, upon election of holder, 9.99%) of the combined voting power of all of our securities outstanding
immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the Pre-Funded
Warrants. As a result, you may not be able to exercise your Pre-Funded Warrants for shares of our common stock at a time when it would
be financially beneficial for you to do so. In such a circumstance, you could seek to sell your Pre-Funded Warrants to realize value,
but you may be unable to do so in the absence of an established trading market and due to applicable transfer restrictions.
Our
failure to meet the continued listing requirements of the NYSE American could result in a delisting of our common stock.
If
we fail to satisfy the continued listing requirements of the NYSE American, such as the corporate governance requirements or the minimum
closing bid price requirement, the NYSE American may take steps to delist our common stock. Such a delisting would likely have a negative
effect on the price of our common stock and would impair your ability to sell or purchase our common stock when you wish to do so. In
the event of a delisting, we can provide no assurance that any action taken by us to restore compliance with listing requirements would
allow our common stock to become listed again, stabilize the market price or improve the liquidity of our common stock, prevent our common
stock from dropping below the NYSE American’s minimum bid price requirement or prevent future non-compliance with the NYSE American’s
listing requirements.
Future
capital raises may dilute your ownership and/or have other adverse effects on our operations.
If
we raise additional capital by issuing equity securities, our existing stockholders’ percentage ownership will be reduced and these
stockholders may experience substantial dilution. If we raise additional funds by issuing debt securities, these debt securities would
have rights senior to those of our common stock and the terms of the debt securities issued could impose significant restrictions on
our operations, including liens on our assets. If we raise additional funds through collaborations and licensing arrangements, we may
be required to relinquish some rights to our intellectual property or candidate products, or to grant licenses on terms that are not
favorable to us.
Shares
eligible for future sale may adversely affect the market for our common stock.
As of the date of this prospectus,
we have approximately 960,146 shares of common stock issued and outstanding, all of which are eligible for sale by means of ordinary
brokerage transactions in the open market pursuant to Rule 144, promulgated under the Securities Act, subject to certain limitations
under Rule 144, except for approximately 33,860 shares of common stock held by our officers and directors which are subject to
lock-ups expiring up to two months from the close of this offering. We have granted demand and piggyback registration rights to
the former holders of our convertible preferred stock and convertible promissory notes pursuant to which they may request the registration
for resale of up to 217,082 shares of common stock, including 5,612 shares of common stock issuable upon exercise of outstanding
warrants. On July 1, 2024, we filed with the SEC a registration statement on Form S-3 to register the resale of those 217,082 shares
of common stock. Any substantial sale of our common stock pursuant to Rule 144 or pursuant to any resale prospectus (including sales
by investors of securities acquired in connection with this offering) may have a material adverse effect on the market price of our common
stock.
We have not paid dividends
on our common stock in the past and have no immediate plans to pay such dividends.
We plan to reinvest all of our
earnings, to the extent we have earnings, to cover operating costs and otherwise become and remain competitive. We do not plan to pay
any cash dividends with respect to our common stock in the foreseeable future. We cannot assure you that we would, at any time, generate
sufficient surplus cash that would be available for distribution to the holders of our common stock as a dividend. Therefore, you should
not expect to receive cash dividends on the common stock we are offering.
If
equity research analysts do not publish research or reports about our business or if they issue unfavorable commentary or downgrade our
shares, the price of our shares could decline.
The
trading market for our shares will rely in part on the research and reports that equity research analysts publish about us and our business,
if at all. We do not have control over these analysts, and we do not have commitments from them to write research reports about
us. The price of our shares could decline if no research reports are published about us or our business, or if one or more equity research
analysts downgrades our shares or if those analysts issue other unfavorable commentary or cease publishing reports about us or our business.
CAUTIONARY
NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains or
incorporates by reference forward-looking statements. The words “believe,” “may,” “will,” “potentially,”
“estimate,” “continue,” “anticipate,” “intend,” “could,” “would,”
“project,” “plan,” “expect” and similar expressions that convey uncertainty of future events or outcomes
are intended to identify forward-looking statements. These forward-looking statements include, but are not limited to, statements concerning
the following:
| |
● |
our
future financial and operating results; |
| |
● |
our
intentions, expectations and beliefs regarding anticipated growth, market penetration and trends in our business; |
| |
● |
the
timing and success of our plan of commercialization; |
| |
● |
our
ability to successfully develop and clinically test our product candidates; |
| |
● |
our
ability to obtain FDA approval for any of our product candidates; |
| |
● |
our
ability to comply with all U.S. and foreign regulations concerning the development, manufacture and sale of our product candidates; |
| |
● |
our
reliance on third parties to manufacture our product candidates; |
| |
● |
the
adequacy of the net proceeds of this offering; |
| |
● |
the
effects of market conditions on our stock price and operating results; |
| |
● |
our
ability to maintain, protect and enhance our intellectual property; |
| |
● |
the
effects of increased competition in our market and our ability to compete effectively; |
| |
● |
our
plans to use the proceeds from this offering; |
| |
● |
costs
associated with initiating and defending intellectual property infringement and other claims; |
| |
● |
the
attraction and retention of qualified employees and key personnel; |
| |
● |
future
acquisitions of or investments in complementary companies or technologies; and |
| |
● |
our
ability to comply with evolving legal standards and regulations, particularly concerning requirements for being a public company. |
These forward-looking statements
are subject to a number of risks, uncertainties and assumptions, including those described in the “Risk Factors”
section of our 2023 Form 10-K and elsewhere in this prospectus and our 2024 Form 10-Q. Moreover, we operate in a very competitive
and rapidly changing environment, and new risks emerge from time to time. It is not possible for us to predict all risks, nor can we
assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results
to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties and assumptions,
the forward-looking events and circumstances discussed in this prospectus may not occur and actual results could differ materially and
adversely from those anticipated or implied in our forward-looking statements.
You should not rely upon forward-looking
statements as predictions of future events. Although we believe that the expectations reflected in our forward-looking statements are
reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances described in the
forward-looking statements will be achieved or occur. Moreover, neither we nor any other person assumes responsibility for the accuracy
and completeness of the forward-looking statements. We undertake no obligation to update publicly any forward-looking statements for any
reason after the date of this prospectus to conform these statements to actual results or to changes in our expectations, except as required
by law.
You should read this prospectus,
our 2023 Form 10-K and 2024 Form 10-Q which are incorporated herein by reference and the documents that we reference in this prospectus
and have filed with the SEC as exhibits to the registration statement of which this prospectus is a part with the understanding that
our actual future results, levels of activity, performance and events and circumstances may be materially different from what we expect.
TRADEMARKS,
SERVICE MARKS AND TRADE NAMES
We
own or have rights to use a number of registered and common law trademarks, service marks and/or trade names in connection with our business
in the United States and/or in certain foreign jurisdictions.
Solely
for convenience, the trademarks, service marks, logos and trade names referred to in this prospectus are without the ® and ™
symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable
law, our rights or the rights of the applicable licensors to these trademarks, service marks and trade names. This prospectus contains
additional trademarks, service marks and trade names of others, which are the property of their respective owners. All trademarks, service
marks and trade names appearing in this prospectus are, to our knowledge, the property of their respective owners. We do not intend our
use or display of other companies’ trademarks, service marks, copyrights or trade names to imply a relationship with, or endorsement
or sponsorship of us by, any other companies.
USE
OF PROCEEDS
We estimate that the net proceeds
we will receive from the sale of our securities in this offering, assuming all the securities we are offering are sold, after deducting
placement agent fees and other estimated offering expenses payable by us, and assuming no sale of any Pre-Funded Warrants and no exercise
of the Class A Warrants being issued in this offering, will be approximately $8.95 million, based on an assumed
public offering price of $3.21 per Unit.
However, because this is a best-efforts
offering and there is no minimum offering amount required as a condition to the closing of this offering, the actual offering amount,
the placement agent fees and net proceeds to us are not presently determinable and may be substantially less than the maximum amounts
set forth on the cover page of this prospectus. Assuming that we receive net proceeds of approximately $5.23 million from this
offering (assuming an offering with gross proceeds of $6 million), we believe that the net proceeds from this offering, together
with our existing cash and cash equivalents, will meet our capital needs until November 2024 under our current business plan.
Assuming that we receive net proceeds of approximately $3.37 million from this offering (assuming an offering with gross proceeds
of $4 million), we believe that the net proceeds from this offering, together with our existing cash and cash equivalents, will
satisfy our capital needs until October 2024 under our current business plan. Assuming that we receive net proceeds of approximately
$2.44 million from this offering (assuming an offering with gross proceeds of $3 million), we believe that the net proceeds
from this offering, together with our existing cash and cash equivalents, will satisfy our capital needs until September 2024
under our current business plan.
We expect to use the net proceeds
from this offering for working capital and general corporate purposes. This represents our best estimate of the manner in which we will
use the net proceeds we receive from this offering based upon the current status of our business, but we have not reserved or allocated
amounts for specific purposes and we cannot specify with certainty how or when we will use any of the net proceeds. The amounts and timing
of our actual use of the net proceeds from this offering will vary depending on numerous factors, including the factors described under
“Risk Factors” located elsewhere in this prospectus or in the information incorporated by reference herein or therein. As
a result, our management will have broad discretion in the application of the net proceeds, and investors will be relying on our judgment
regarding the application of the net proceeds from this offering.
DIVIDEND
POLICY
We
have never declared or paid any cash dividends on our common stock and we do not anticipate paying any cash dividends on our common stock
in the foreseeable future. Investors should not purchase our common stock with the expectation of receiving cash dividends. The payment
of dividends on our common stock, if any, in the future is within the discretion of our Board and will depend on our earnings, capital
requirements and financial condition and other relevant facts. We currently intend to retain all future earnings, if any, to finance
the development and growth of our business.
CAPITALIZATION
The following table sets forth
our cash and capitalization as of March 31, 2024:
| |
● |
on an actual basis; and |
| |
● |
on
a as adjusted basis to reflect our sale of 3,115,265 Units in this offering at the assumed public offering price of $3.21
per Unit, the last reported sale price of our common stock on the NYSE American on July 11, 2024, and assuming
no sale of Pre-Funded Warrants or exercise of Class A Warrants, after deducting placement agent
fees and estimated offering expenses payable by us. |
You should read the information
in this table together with our financial statements and related notes and “Management’s Discussion and Analysis of Financial
Condition and Results of Operations” sections appearing in our 2023 Form 10-K and 2024 Form 10-Q, each of which are
incorporated herein by reference.
| | |
March
31, 2024 | |
| (in
thousands) | |
Actual | | |
As
Adjusted | |
| Cash
and cash equivalents | |
$ | 3,001 | | |
$ | 12,251 | |
| Common
stock, $0.0001 par value, 100,000,000 shares authorized, 960,146 shares issued and outstanding, actual; 4,075,411 shares
issued and outstanding, as adjusted | |
| 3 | | |
| 13 | |
| Additional
paid-in capital | |
$ | 55,853 | | |
$ | 64,803 | |
| Accumulated
deficit | |
$ | (51,531 | ) | |
$ | (51,531 | ) |
| Total
stockholders’ equity | |
$ | 4,324 | | |
$ | 13,274 | |
| Total
capitalization | |
$ | 4,324 | | |
$ | 13,274 | |
DESCRIPTION
OF SECURITIES
General
The
following description summarizes the most important terms of our capital stock. Because it is only a summary, it does not contain all
the information that may be important to you. For a complete description of the matters set forth in this “Description of Securities,”
you should refer to our amended and restated certificate of incorporation and amended and restated bylaws and investor rights agreement,
which are included as exhibits to the registration statement of which this prospectus forms a part, and to the applicable provisions
of Delaware law.
Our authorized capital stock consists
of 100,000,000 shares of common stock, $0.0001 par value per share, and 10,000,000 shares of undesignated preferred stock, $0.0001 par
value per share.
There are 960,146
shares of our common stock outstanding and no shares of our preferred stock outstanding as of the date of this prospectus. On
July 1, 2024, we effected a 1-for-30 reverse stock split of our issued and outstanding shares of common stock. All historical share
and per share amounts reflected in this prospectus have been adjusted to reflect the reverse stock split.
As of the date of this prospectus,
we had 17 stockholders of record.
Common
Stock
The
holders of common stock are entitled to one vote for each share of common stock. The holders of common stock are entitled to any dividends
that may be declared by the Board out of funds legally available for payment of dividends at such times and in such amounts as the Board
in its discretion. In the event of any liquidation, dissolution or winding up of the Company, holders of common stock are entitled to
receive the assets of the Company available for distribution to its stockholders ratably in proportion to the number of shares of common
stock held by the holders of common stock. The holders of shares of common stock have no preemptive, conversion, subscription rights
or cumulative voting rights.
Preferred
Stock
As
of the date of this prospectus, there are a total of 10,000,000 shares of undesignated preferred stock authorized for issuance, none
of which are outstanding.
Our
Board is authorized, without further action by our stockholders, to provide from time to time out of the unissued shares of preferred
stock for one or more series of preferred stock, and with respect to each such series, to fix the number of shares constituting such
series and the designation of such series, the powers (including voting powers), if any, of the shares of such series and the preferences
and relative, participating, optional, special or other rights, if any, and the qualifications, limitations, or restrictions, if any,
of the shares of such series. The issuance of our preferred stock could adversely affect the voting power of holders of our common stock
and the likelihood that such holders will receive dividend payments and payments upon liquidation. In addition, the issuance of preferred
stock could have the effect of delaying, deferring, or preventing a change of control or other corporate action.
Class A Warrants
The following
is a summary of certain terms and provisions of the Class A Warrants that are being offered hereby and is not complete
and is subject to, and qualified in its entirety by, the provisions of the Class A Warrants, the form of which is
filed as an exhibit to the registration statement of which this prospectus forms a part. Prospective investors should carefully review
the terms and provisions of the form of Class A Warrants for a complete description of the terms and conditions of the
Class A Warrants.
Duration and
Exercise Price
Each Class A
Warrant offered hereby is exercisable upon issuance at an initial exercise price of $3.21 (assuming
an offering price of $3.21 per Unit) per share of common stock, and will expire five (5) years from the date of issuance. However, on the date that is 30 calendar days immediately following
the date of issuance of the Class A Warrants (the “Reset Date”), if the Reset Price, as defined below, is less than the exercise
price at such time, the exercise price will be decreased to the Reset Price. “Reset Price” shall mean 100% of the trailing
five-day VWAP immediately preceding the Reset Date, provided, that in no event shall the Reset Price be less than 20% of the most recent
closing price at the time of execution of the placement agency agreement (subject to adjustment for reverse and forward stock splits,
recapitalizations and similar transactions following the date of the securities purchase agreement). Following the determination of the Reset Price, the exercise price and number of shares of common stock issuable upon exercise of
the Class A Warrants will be subject to appropriate adjustment in the event of stock dividends, stock splits, reorganizations
or similar events affecting our common stock. The Class A Warrants will be issued separately from the common stock or Pre-Funded
Warrants, respectively.
Exercisability
The Class
A Warrants will be exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed exercise
notice accompanied by payment in full for the number of shares of our common stock purchased upon such exercise (except in the case of
a cashless exercise as discussed below). A holder (together with its affiliates) may not exercise any portion of such holder’s
Class A Warrants to the extent that the holder would own more than 4.99% (or, at the election of the holder, 9.99%) of
the outstanding common stock immediately after exercise. However, upon notice from the holder to us, the holder may decrease or increase
the holder’s beneficial ownership limitation, which may not exceed 9.99% of the number of outstanding shares of common stock immediately
after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the warrants, provided
that any increase in the beneficial ownership limitation will not take effect until 61 days following notice to us.
Cashless Exercise
If, at the time a
holder exercises its Class A Warrants, a registration statement registering the issuance or resale of the shares of common
stock underlying the Class A Warrants under the Securities Act is not then effective or available for the issuance of such
shares, then in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate
exercise price, the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of shares of common
stock determined according to a formula set forth in the Class A Warrants.
Fundamental
Transactions
In the event of any
fundamental transaction, as described in the Class A Warrants and generally including any merger or consolidation with
or into another entity, sale of all or substantially all of our assets, tender offer or exchange offer, or reclassification of our common
stock, then upon any subsequent exercise of the Class A Warrants, the holder will have the right to receive as alternative
consideration, for each share of our common stock that would have been issuable upon such exercise immediately prior to the occurrence
of such fundamental transaction, the number of shares of common stock of the successor or acquiring corporation or of our Company, if
it is the surviving corporation, and any additional consideration receivable upon or as a result of such transaction by a holder of the
number of shares of our common stock for which the Class A Warrants are exercisable immediately prior to such event. Notwithstanding
the foregoing, in the event of a fundamental transaction, the holders of the Class A Warrants have the right to require
us or a successor entity to redeem the Class A Warrants for cash in the amount of the Black-Scholes Value (as defined in
the Common Warrants) of the remaining unexercised portion of the Class A Warrants on the date of the consummation of such
fundamental transaction, concurrently with or within 30 days following the consummation of a fundamental transaction.
However, in the event
of a fundamental transaction which is not in our control, including a fundamental transaction not approved by our Board, the holders
of the Class A Warrants will only be entitled to receive from us or our successor entity, as of the date of consummation
of such fundamental transaction the same type or form of consideration (and in the same proportion), at the Black Scholes Value of the
unexercised portion of the Class A Warrants that are being offered and paid to the holders of our common stock in connection
with the fundamental transaction, whether that consideration is in the form of cash, stock or any combination of cash and stock, or whether
the holders of our common stock are given the choice to receive alternative forms of consideration in connection with the fundamental
transaction.
Transferability
Subject to applicable
laws, the Class A Warrants may be transferred at the option of the holder upon surrender of the Class A Warrants
to us together with the appropriate instruments of transfer.
Fractional
Shares
No fractional shares
of common stock will be issued upon the exercise of the Class A Warrants. Rather, the number of shares of common stock
to be issued will, at our election, either be rounded up to the nearest whole number or we will pay a cash adjustment in respect of such
final fraction in an amount equal to such fraction multiplied by the exercise price.
Trading Market
There is no established
trading market for the Class A Warrants, and we do not expect an active trading market to develop. We do not intend to
apply to list the Class A Warrants on any securities exchange or other trading market. Without a trading market, the liquidity
of the Class A Warrants will be extremely limited. The shares of common stock issuable upon exercise of the Class
A Warrants are currently traded on the NYSE American under the symbol “AZTR”.
Right as a
Stockholder
Except as otherwise
provided in the Class A Warrants or by virtue of the holder’s ownership of shares of our common stock, such holder
of Class A Warrants does not have the rights or privileges of a holder of our common stock, including any voting rights,
until such holder exercises such holder’s Class A Warrants. The Class A Warrants provide that holders
have the right to participate in distributions or dividends paid on our shares of common stock.
Waivers and
Amendments
No
term of the Class A Warrants may be amended or waived without the written consent of the holder.
Governing
Law.
The
Class A Warrants are governed by New York law.
Pre-Funded
Warrants
The
following summary of certain terms and provisions of the Pre-Funded Warrants that are being offered hereby is not complete and is subject
to, and qualified in its entirety by, the provisions of the Pre-Funded Warrants, the form of which is filed as an exhibit to the registration
statement of which this prospectus forms a part. Prospective investors should carefully review the terms and provisions of the form of
Pre-Funded Warrants for a complete description of the terms and conditions of the Pre-Funded Warrants.
Duration
and Exercise Price
Each
Pre-Funded Warrant offered hereby will have an initial exercise price equal to $0.0001 per share of common stock. The Pre-Funded
Warrants will be exercisable immediately, and may be exercised at any time until the Pre-Funded Warrants are exercised in full. The exercise
price and number of shares of common stock issuable upon exercise is subject to appropriate proportional adjustment in the event of share
dividends, share splits, reorganizations or similar events affecting our shares of common stock and the exercise price.
Exercisability
The
Pre-Funded Warrants will be exercisable, at the option of each holder, in whole or in part by delivering to us a duly executed exercise
notice and within the earlier of (i) two trading days and (ii) the number of trading days comprising the standard settlement period with
respect to the shares of common stock as in effect on the date of delivery of the notice of exercise thereafter, payment in full for
the number of shares of common stock purchased upon such exercise (except in the case of a cashless exercise as discussed below). A holder
may not exercise any portion of the Pre-Funded Warrant to the extent that the holder, together with its affiliates and any other persons
acting as group together with any such persons, would own more than 4.99% (or, at the election of the purchaser, 9.99%) of the number
of shares of our common stock outstanding immediately after exercise (the “Beneficial Ownership Limitation”); provided that
a holder with Beneficial Ownership Limitation of 4.99%, upon notice to use and effective 61 days after the date such notice is delivered
to us may increase the Beneficial Ownership Limitation so long as it in no event exceeds 9.99% of the number of shares of common stock
outstanding immediately after exercise.
Cashless
Exercise
The
Pre-Funded Warrants may also be exercised, in whole or in part, at such time by means of “cashless exercise” in which the
holder shall be entitled to receive upon such exercise (either in whole or in part) the net number of shares of common stock determined
according to a formula set forth in the Pre-Funded Warrants, which generally provides for a number of shares of common stock equal to
(A)(1) the last closing trade price for such security on the NYSE American on (x) the trading day immediately preceding the date
of the applicable notice of exercise, if the notice of exercise is executed and delivered on day that is not a trading day or prior to
the opening of “regular trading hours” on a trading day or (y) the trading day of the notice of exercise, if the notice of
exercise is executed and delivered after the close of “regular trading hours” on such trading day; (2) at the option
of the holder, either (x) the volume weighted average price on the trading day immediately preceding the date of the applicable notice
of exercise or (y) the bid price of our common stock on the NYSE American as of the time of the holder’s execution of the
applicable notice of exercise if such notice of exercise is executed during “regular trading hours” on a trading day and
is delivered within two hours thereafter (including until two hours after the close of “regular trading hours” on a trading
day); or (3) the last closing trade price for such security on the NYSE American on the day of the notice of exercise, if the
notice of exercise is executed and delivered after “regular trading hours” on a trading day, less (B) the exercise price,
multiplied by (C) the number of shares of common stock the Pre-Funded Warrant was exercisable into, with such product then divided by
the number determined under clause (A) in the this sentence.
Fractional
Shares
No
fractional shares of common stock will be issued upon the exercise of the Pre-Funded Warrants. Rather, we will, at our election, either
pay a cash adjustment in respect of such final fraction in an amount equal to such fraction multiplied by the exercise price or round
up to the next whole shares of common stock.
Transferability
Subject
to applicable laws, a Pre-Funded Warrant may be transferred at the option of the holder upon surrender of the Pre-Funded Warrant to us
together with the appropriate instruments of transfer and funds sufficient to pay any transfer taxes payable upon such transfer.
Trading
Market
There
is no trading market available for the Pre-Funded Warrants on any securities exchange or nationally recognized trading system. We do
not intend to list the Pre-Funded Warrants on any securities exchange or nationally recognized trading system. The shares of common stock
issuable upon exercise of the Pre-Funded Warrants are currently listed on the NYSE American under the symbol “AZTR.”
Rights
as a Shareholder
Except
as otherwise provided in the Pre-Funded Warrants or by virtue of such holder’s ownership of the underlying shares of common stock,
the holders of the Pre-Funded Warrants do not have the rights or privileges of holders of our ordinary shares represented by shares of
common stock, including any voting rights, until they exercise their Pre-Funded Warrants.
Fundamental
Transaction
In
the event of a fundamental transaction, as described in the Pre-Funded Warrants and generally including any reorganization, recapitalization
or reclassification of our shares of common stock, the sale, transfer or other disposition of all or substantially all of our properties
or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding shares of common
stock or 50% or more of the voting power of the common equity of the Company, the holders of the Pre-Funded Warrants will be entitled
to receive upon exercise of the Pre-Funded Warrants the kind and amount of securities, cash or other property that the holders would
have received had they exercised the Pre-Funded Warrants immediately prior to such fundamental transaction.
Waivers
and Amendments
No
term of the Pre-Funded Warrants may be amended or waived without the written consent of the holder.
Governing
Law.
The
Pre-Funded Warrants are governed by New York law.
Warrants
We
have outstanding the following warrants to purchase shares of our common stock:
| ● | Warrants
issued in connection with our April 2018 placement of unsecured convertible promissory notes
to purchase up to an aggregate of 1,596 shares of our common stock, at a per share
exercise price equal to $14.40. The warrants expire in April 2028. |
| ● | Warrants
issued in connection with our February 2019 placement of Series A-1 convertible preferred
shares to purchase up to an aggregate of 7,195 shares of our common stock, at a per
share exercise price equal to $158.40. The warrants expire in February 2026. |
| ● | Warrants
issued to the underwriter of our IPO to purchase 2,000 shares of our common stock.
These warrants are exercisable at $187.50 per share. The warrants expire in June 2028. |
| | ● | Warrants
issued to the underwriter of our February 2024 public offering to purchase 22,222
shares of our common stock. These warrants are exercisable at $11.25 per share. The
warrants expire in February 2029. |
Stock
Incentive Plans
We have adopted the Azitra, Inc.
2016 Stock Incentive Plan, or 2016 Plan, providing for the grant of non-qualified stock options and incentive stock options to purchase
shares of our common stock and for the grant of restricted and unrestricted share grants and restricted stock units. We currently have
reserved 8,078 shares of our common stock under the 2016 Plan. The purpose of the 2016 Plan is to provide eligible participants
with an opportunity to acquire an ownership interest in our company. All officers, directors, employees and consultants to our company
are eligible to participate under the 2016 Plan. The 2016 Plan provides that options may not be granted at an exercise price less than
the fair market value of our shares of common stock on the date of grant. As of the date of this prospectus, we have outstanding options
granted under the 2016 Plan to purchase an aggregate of 40,275 shares of our common stock at an average exercise price of $40.92
per share.
In March 2023, our Board and
stockholders approved and adopted the Azitra, Inc. 2023 Stock Incentive Plan, or 2023 Plan, providing for the grant of non-qualified
stock options and incentive stock options to purchase shares of our common stock and for the grant of restricted and unrestricted share
grants and restricted stock units. We currently have reserved 66,666 shares of our common stock under the 2023 Plan. The purpose
of the 2023 Plan is to provide eligible participants with an opportunity to acquire an ownership interest in our company. All officers,
directors, employees and consultants to our company are eligible to participate under the 2023 Plan. The 2023 Plan provides that options
may not be granted at an exercise price less than the fair market value of our shares of common stock on the date of grant. As of the
date of this prospectus, we have outstanding options granted under the 2023 Plan to purchase an aggregate of 1,333 shares of
our common stock at an average exercise price of $60.10 per share.
Dividends
We
do not anticipate the payment of cash dividends on our common stock in the foreseeable future.
Registration
Rights
Certain
holders of our common stock, or their permitted transferees, are entitled to the registration rights described below. The registration
of shares of our common stock pursuant to the exercise of registration rights described below would enable the holders to sell these
shares without restriction under the Securities Act when the applicable registration statement is declared effective. We will pay the
registration expenses, other than the underwriting discounts and commissions, of the shares registered pursuant to the registrations
described below. The registration rights described below will expire upon the earlier of June 20, 2026 or when all investors, considered
with their affiliates, can sell all of their shares in a three-month period under Rule 144.
Convertible
Preferred Stock Registration Rights. In connection with our convertible preferred stock financings, we entered into an investor rights
agreement, as amended, pursuant to which we have granted the purchasers of our convertible preferred stock certain demand and piggyback
registration rights. Those parties beneficially hold approximately 217,082 shares of our common stock, including 5,612
shares of our common stock issuable upon exercise of warrants issued to the parties in connection with our 2018 placement of our unsecured
convertible promissory notes and our 2019 placement of Series A-1 convertible preferred shares.
Pursuant
to the investor rights agreement, we are required, upon the written request by the holders of at least 50% of the shares that are entitled
to registration rights under the investor rights agreement, to register, as soon as practicable, all or a portion of these shares for
public resale. We are required to effect two demand registrations pursuant to a registration statement on Form S-1.
Subject to our eligibility to use a registration statement on Form S-3, we are required to effect an unlimited number of demand registrations
pursuant to Form S-3, provided such requests for registration be for an aggregate offering price, net of the underwriting discounts and
commissions, equal or greater than $1 million. Pursuant to the investor rights agreement, we have also granted to the piggyback registration
rights and demand registration rights. These demand and piggyback registration rights terminate as to each investor when their shares
subject to the registration rights agreement may be sold by the investor pursuant to Rule 144 under the Securities Act without regard
to both the volume limitations for sales as provided in Rule 144.
On July 1, 2024,
we filed with the SEC a registration statement on Form S-3 to register the resale of the 217,082 shares of common stock held by the stockholders
holding demand registration rights.
Underwriter Registration Rights.
In connection with our IPO and February 2024 public offering, we issued to the representative of the underwriters or its designees
warrants, referred to as the Representative’s Warrants, to purchase up to a total of 2,000 shares and 22,222 shares
of our common stock, respectively. The Representative’s Warrants provide for registration rights (including a one-time demand registration
right and unlimited piggyback rights) consistent with FINRA Rule 5110.05. The demand for registration may be made at any time beginning
on the initial exercise date of the Representative’s Warrants and expiring on the fifth anniversary of the date of the public offering
prospectus to which the warrants relate in accordance with FINRA Rule 5110(g)(8)(C). In addition to the one-time demand registration
right, the Representative’s Warrants have unlimited piggyback rights, for a period of no more than two years from the initial exercise
date of the Representative’s Warrants in accordance with FINRA Rule 5110(g)(8)(D).
Anti-Takeover
Effects of Certain Provisions of Delaware Law and Our Charter Documents
The
following is a summary of certain provisions of Delaware law and our amended and restated certificate of incorporation and amended and
restated bylaws. This summary does not purport to be complete and is qualified in its entirety by reference to the corporate law of Delaware
and our amended and restated certificate of incorporation and amended and restated bylaws.
Delaware
Law
We
are subject to Section 203 of the Delaware General Corporation Law, an anti-takeover law. In general, Section 203 prohibits a Delaware
corporation from engaging in any business combination (as defined below) with any interested stockholder (as defined below) for a period
of three years following the date that the stockholder became an interested stockholder, unless:
| ● | prior
to that date, the board of directors of the corporation approved either the business combination
or the transaction that resulted in the stockholder becoming an interested stockholder; |
| ● | upon
consummation of the transaction that resulted in the stockholder becoming an interested stockholder,
the interested stockholder owned at least 85% of the voting stock of the corporation outstanding
at the time the transaction commenced, excluding for purposes of determining the number of
shares of voting stock outstanding (but not the voting stock owned by the interested stockholder)
those shares owned by persons who are directors and officers and by excluding employee stock
plans in which employee participants do not have the right to determine whether shares held
subject to the plan will be tendered in a tender or exchange offer; or |
| ● | on
or subsequent to that date, the business combination is approved by the board of directors
of the corporation and authorized at an annual or special meeting of stockholders, and not
by written consent, by the affirmative vote of at least 66 2/3% of the outstanding voting
stock that is not owned by the interested stockholder. |
Section
203 defines “business combination” to include the following:
| ● | any
merger or consolidation involving the corporation and the interested stockholder; |
| ● | any
sale, transfer, pledge or other disposition of 10% or more of the assets of the corporation
involving the interested stockholder; |
| ● | subject
to certain exceptions, any transaction that results in the issuance or transfer by the corporation
of any stock of the corporation to the interested stockholder; |
| ● | subject
to limited exceptions, any transaction involving the corporation that has the effect of increasing
the proportionate share of the stock of any class or series of the corporation beneficially
owned by the interested stockholder; or |
| ● | the
receipt by the interested stockholder of the benefit of any loans, advances, guarantees,
pledges or other financial benefits provided by or through the corporation. |
In
general, Section 203 defines an interested stockholder as any entity or person beneficially owning 15% or more of the outstanding voting
stock of the corporation, or who beneficially owns 15% or more of the outstanding voting stock of the corporation at any time within
a three-year period immediately prior to the date of determining whether such person is an interested stockholder, and any entity or
person affiliated with or controlling or controlled by any of these entities or persons.
Our
Charter Documents
Our
charter documents include provisions that could have the effect of discouraging others from making tender offers for our shares and may
have the effect of deterring hostile takeovers or delaying changes in our control or management. These provisions are intended to enhance
the likelihood of continued stability in the composition of our Board and its policies and to discourage certain types of transactions
that may involve an actual or threatened acquisition of us. These provisions are also designed to reduce our vulnerability to an unsolicited
acquisition proposal and to discourage certain tactics that may be used in proxy fights. However, such provisions may have the effect
of discouraging, delaying or preventing a change in control or an unsolicited acquisition proposal that a stockholder might consider
favorable, including a proposal that might result in the payment of a premium over the market price for the shares held by our stockholders.
Certain of these provisions are summarized in the following paragraphs.
Effects
of authorized but unissued common stock and preferred stock. One of the effects of the existence of authorized but unissued common
stock and preferred stock may be to enable our Board to make more difficult or to discourage an attempt to obtain control of our Company
by means of a merger, tender offer, proxy contest or otherwise, and thereby to protect the continuity of management. If, in the due exercise
of its fiduciary obligations, the Board were to determine that a takeover proposal was not in our best interest, such shares could be
issued by the Board without stockholder approval in one or more transactions that might prevent or render more difficult or costly the
completion of the takeover transaction by diluting the voting or other rights of the proposed acquirer or insurgent stockholder group,
by putting a substantial voting block in institutional or other hands that might undertake to support the position of the incumbent Board,
by effecting an acquisition that might complicate or preclude the takeover, or otherwise.
Cumulative
Voting. Our amended and restated certificate of incorporation does not provide for cumulative voting in the election of directors,
which would allow holders of less than a majority of the stock to elect some directors.
Vacancies.
Our amended and restated bylaws provide that all vacancies may be filled by the affirmative vote of a majority of directors then in office,
even if less than a quorum.
Special
Meeting of Stockholders and Stockholder Action by Written Consent. A special meeting of stockholders may only be called by our Board
or the chairperson of our Board. All stockholder actions must be effected at a duly called meeting of stockholders and not by written
consent.
Advance
Notice Provisions. Our amended and restated bylaws provide advance notice procedures for stockholders seeking to bring business before
our annual meeting of stockholders or to nominate candidates for election as directors at our annual meeting of stockholders. Our amended
and restated bylaws will also specify certain requirements regarding the form and content of a stockholder’s notice. These provisions
might preclude our stockholders from bringing matters before our annual meeting of stockholders or from making nominations for directors
at our annual meeting of stockholders if the proper procedures are not followed. We expect that these provisions may also discourage
or deter a potential acquirer from conducting a solicitation of proxies to elect the acquirer’s own slate of directors or otherwise
attempting to obtain control of our company.
Choice
of Forum. Our amended and restated certificate of incorporation and amended and restated bylaws will provide that the Court of Chancery
of the State of Delaware will be the exclusive forum for any derivative action or proceeding brought on our behalf; any action asserting
a breach of fiduciary duty; any action asserting a claim against us arising pursuant to the Delaware General Corporation Law, our amended
and restated certificate of incorporation or our bylaws; or any action asserting a claim against us that is governed by the internal
affairs doctrine.
Transfer
Agent and Registrar
The
transfer agent and registrar for our shares of common stock is VStock Transfer, LLC. The transfer agent and registrar’s address
is 18 Lafayette Place, Woodmere, New York 11598.
National
Securities Exchange Listing
Our
common stock is listed on the NYSE American under the symbol “AZTR.”
SHARES
ELIGIBLE FOR FUTURE SALE
Future
sales of substantial amounts of shares of common stock, including shares issued upon the exercise of outstanding warrants and options,
in the public market after this offering, or the possibility of these sales occurring, could adversely affect the prevailing market price
for our common stock or impair our ability to raise equity capital.
Upon the completion of this offering,
a total of 4,075,411 shares of common stock will be outstanding. All 3,115,265 shares of common stock sold in this offering
by us will be freely tradable in the public market
without restriction or further registration under the Securities Act, unless these shares are held by “affiliates,” as that
term is defined in Rule 144 under the Securities Act. In addition, of the 960,146 shares of our common stock outstanding prior
to this offering, approximately 704,547 shares will be freely tradable in the public market without restriction or further registration
under the Securities Act.
The remaining 255,5992
shares of common stock will be “restricted securities,” as that term is defined in Rule 144 under the Securities Act. These
restricted securities are eligible for public sale only if they are registered under the Securities Act or if they qualify for an exemption
from registration under Rule 144 under the Securities Act, which is summarized below.
Subject
to the lock-up agreements described below and the provisions of Rule 144 under the Securities Act, these restricted securities are available
for sale in the public market.
Rule
144
In
general, under Rule 144 as currently in effect, a person who is not deemed to have been one of our affiliates for purposes of the Securities
Act at any time during the 90 days preceding a sale and who has beneficially owned the shares proposed to be sold for at least six months,
including the holding period of any prior owner other than our affiliates, is entitled to sell such shares without complying with the
manner of sale, volume limitation, or notice provisions of Rule 144, subject to compliance with the public information requirements of
Rule 144. If such a person has beneficially owned the shares proposed to be sold for at least one year, including the holding period
of any prior owner other than our affiliates, then such person is entitled to sell such shares without complying with any of the requirements
of Rule 144.
In
general, under Rule 144, as currently in effect, our affiliates or persons selling shares on behalf of our affiliates are entitled to
sell upon expiration of the lock-up agreements described below, within any three-month period a number of shares that does not exceed
the greater of:
| ● | 1%
of the number of shares of common stock then outstanding; or |
| ● | the
average weekly trading volume of the common stock during the four calendar weeks preceding
the filing of a notice on Form 144 with respect to such sale. |
Sales
under Rule 144 by our affiliates or persons selling shares on behalf of our affiliates are also subject to certain manner of sale provisions
and notice requirements and to the availability of current public information about us.
Lock-Up
Agreements
We and each of our directors,
officers and 5% stockholders have agreed, subject to certain exceptions, not to offer, pledge, sell, contract to sell, grant,
lend, or otherwise transfer or dispose of, directly or indirectly, or enter into any swap or other arrangement that transfers to another,
in whole or in part, any of the economic consequences of ownership of any shares of our capital stock or any securities convertible into
or exercisable or exchangeable for shares of our common stock, for a period of time following this offering. Please see “Plan
of Distribution – Lock-Up Agreements.”
Equity
Plans
We
have filed with the SEC a registration statement on Form S-8 under the Securities Act to register all of the shares of common stock to
be issued or reserved for issuance under our 2016 Plan and 2023 Plan. Shares covered by this registration statement are eligible for
sale in the public market, upon the expiration or release from the terms of the lock-up agreements and subject to vesting of such shares.
PLAN
OF DISTRIBUTION
We
have engaged Maxim Group LLC to act as our exclusive placement agent pursuant to a placement agency agreement in connection with this
offering, dated as of July, 2024. The placement agent is not purchasing or selling any of
the securities, nor is it required to arrange for the purchase and sale of any specific number or dollar amount of securities, but have
agreed to use their reasonable “best-efforts” to arrange for the sale of the securities offered by this prospectus supplement.
Therefore, we may not sell the entire amount of securities being offered.
We
may enter into a securities purchase agreement directly with certain of the institutional investors, at the investor’s option,
who purchase our securities in this offering. Investors who do not enter into a securities purchase agreement shall rely solely on this
prospectus in connection with the purchase of our securities in this offering. The placement agent may engage one or more subagents or
selected dealers in connection with this offering.
The
placement agency agreement provides that the placement agent’s obligations are subject to conditions contained in the placement
agency agreement.
Delivery
of the securities offered hereby is expected to occur on or about , 2024, subject to satisfaction or waiver of customary closing conditions.
Placement
Agent Fees, Commissions and Expenses
We
have agreed to pay the placement agent a cash fee equal to 7% of the gross proceeds received from the sale of securities in the offering.
We have also agreed to reimburse the placement agent in connection with this offering for its out-of-pocket expenses incurred
in connection with this offering, including the fees and expenses of the counsel for the placement agent, in an amount up to $100,000,
of which $10,000 has been paid as an advance to be applied towards reasonable out-of-pocket expenses.
The
following table shows the public offering price, placement agent fees and proceeds, before expenses, to us, assuming the purchase of all the securities we are offering.
| |
|
Per Unit |
|
|
Total |
|
| Offering
price(1) |
|
$ |
|
|
|
$ |
|
|
| Placement
agent fees |
|
$ |
|
|
|
$ |
|
|
| Proceeds,
before expenses, to us |
|
$ |
|
|
|
$ |
|
|
| (1) |
Assumes
sale of Units including one share of common stock. The public offering price of Units including one Pre-Funded Warrant will be $3.2099. |
We
estimate the total offering expenses of this offering that will be payable by us, excluding the placement agent’s fees and expenses,
will be approximately $250,000.
Placement
Agent Warrants
We
have agreed to issue to the placement agent or its designees warrants to purchase shares of our common stock in an amount equal to 4%
of the aggregate number of shares of common stock (and/or Pre-Funded Warrants) made part of the Units sold in this offering),
or the Placement Agent’s Warrants. The Placement Agent’s Warrants will be exercisable at a per share exercise price equal
to 125% of the public offering price per share of the shares of common stock sold in this offering. The Placement Agent’s Warrants
are exercisable at any time, from time to time, in whole or in part, during the four and one half year period commencing 180 days from
the commencement of sales of the securities in this offering.
The
Placement Agent’s Warrants and the shares of common stock underlying the Placement Agent’s Warrants have been deemed compensation
by FINRA and are, therefore, subject to a 180-day lock-up pursuant to FINRA Rule 5110(e)(1) except as permitted under FINRA Rule 5110(e)(2).
The Placement Agent’s Warrants and the securities underlying the Placement Agent’s Warrants may not be sold,
transferred, assigned, pledged, or hypothecated, nor may they be the subject of any hedging, short sale, derivative, put,
or call transaction that would result in the effective economic disposition of the Placement Agent’s Warrants or the underlying
shares of common stock for a period of 180 days from the commencement of sales in this offering. The Placement Agent’s Warrants
will provide for cashless exercise and for registration rights (including a one-time demand registration right and unlimited piggyback
rights) consistent with FINRA Rule 5110(g)(8). The Placement Agent’s Warrants will also provide for customary anti-dilution
provisions (for stock dividends and splits and recapitalizations) consistent with FINRA Rule 5110.
Indemnification
We
have agreed to indemnify the placement agent and specified other persons against certain liabilities, including liabilities arising under
the Securities Act, relating to or arising out of the placement agent’s activities under the engagement letter and to contribute
to payments that the placement agent may be required to make in respect of such liabilities.
Lock-Up
Agreements
We
have agreed that for a period of up to three months from the closing of this offering, neither we nor any subsidiary may, without
the prior written consent of the placement agent (i) issue, enter into any agreement to issue or announce the issuance or proposed
issuance of any shares of common stock or common stock equivalents or (ii) file any registration statement or prospectus, or any
amendment or supplement thereto, subject to certain exceptions. The Company has also agreed not to effect or enter into an agreement
to effect any issuance of common stock or common stock equivalents involving a Variable Rate Transaction, as defined in the Securities
Purchase Agreement, for a period of up to six months following the closing of this offering (or, in the case of an “at-the-market”
offering with the placement agent, up to three months from the closing of this offering).
Each
of our officers, directors and any other holder(s) of five percent (5%) or more of the outstanding shares of common stock have agreed
to be subject to a lock-up period of up to two months from the closing of the offering pursuant to this prospectus.
This means that, during the applicable lock-up period, such persons may not offer for sale, contract to sell, sell, distribute, grant
any option, right or warrant to purchase, pledge, hypothecate or otherwise dispose of, directly or indirectly, any of our shares of common
stock or any securities convertible into, or exercisable or exchangeable for, shares of common stock, subject to customary exceptions.
The
placement agent may in its sole discretion and at any time without notice release some or all of the shares subject to lock-up agreements
prior to the expiration of the lock-up period. When determining whether or not to release shares from the lock-up agreements, the placement
agent will consider, among other factors, the security holder’s reasons for requesting the release, the number of shares for which
the release is being requested and market conditions at the time.
Regulation
M Compliance
The
placement agent may be deemed to be an underwriter within the meaning of Section 2(a)(11) of the Securities Act, and any commissions
received by it and any profit realized on the resale of the securities sold by it while acting as principal might be deemed to be underwriting
discounts or commissions under the Securities Act. As an underwriter, the placement agent would be required to comply with the requirements
of the Securities Act and the Exchange Act, including, without limitation, Rule 415(a)(4) under the Securities Act and Rule 10b-5 and
Regulation M under the Exchange Act. These rules and regulations may limit the timing of purchases and sales of common shares by the
placement agent acting as principal. Under these rules and regulations, the placement agent:
| |
● |
may
not engage in any stabilization activity in connection with our securities; and |
| |
● |
may
not bid for or purchase any of our securities or attempt to induce any person to purchase any of our securities, other than as permitted
under the Exchange Act, until it has completed its participation in the distribution. |
Determination
of Offering Price and Warrant Exercise Price
The
actual offering price of the securities we are offering, including the exercise price of the Class A Warrants, were negotiated
between us, the placement agent and the investors in the offering based on the trading of our shares of common stock prior to the offering,
among other things. Other factors considered in determining the public offering price of the securities we are offering, as well as the
exercise price of the Class A Warrants, include our history and prospects, the stage of development of our business, our
business plans for the future and the extent to which they have been implemented, an assessment of our management, the general conditions
of the securities markets at the time of the offering and such other factors as were deemed relevant.
The
Securities Purchase Agreement is included as an exhibit to a Current Report on Form 8-K that we have filed with the SEC and
that is incorporated by reference into the registration statement of which this prospectus supplement forms a part.
From
time to time, Maxim may provide in the future various advisory, investment and commercial banking and other services to us in the ordinary
course of business, for which they have received and may continue to receive customary fees and commissions. However, except as disclosed
in this prospectus supplement, we have no present arrangements with Maxim for any further services.
Electronic
Distribution
A
prospectus in electronic format may be made available on a website maintained by the placement agent. In connection with the offering,
the placement agent or selected dealers may distribute prospectuses electronically. No forms of electronic prospectus other than prospectuses
that are printable as Adobe® PDF will be used in connection with this offering.
Other
than the prospectus in electronic format, the information on each of the placement agent’s websites and any information contained
in any other website maintained by the placement agent is not part of the prospectus or the registration statement of which this prospectus
forms a part, has not been approved and/or endorsed by us or either placement agent in its capacity as placement agent and should not
be relied upon by investors.
Certain
Relationships
The
placement agent and its affiliates have and may in the future provide, from time to time, investment banking and financial advisory services
to us in the ordinary course of business, for which they may receive customary fees and commissions.
Transfer
Agent
The
transfer agent and registrar for our common stock is VStock Transfer, LLC.
Listing
Our
shares of common stock are listed on the NYSE American under the symbol “AZTR.”
Selling
Restrictions
Canada.
The securities may be sold in Canada only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited
investors, as defined in National Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities
Act (Ontario), and are permitted clients, as defined in National Instrument 31-103 Registration Requirements, Exemptions and
Ongoing Registrant Obligations. Any resale of the securities must be made in accordance with an exemption from, or in a
transaction not subject to, the prospectus requirements of applicable securities laws.
Securities
legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus
supplement (including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised
by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser
should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory for particulars
of these rights or consult with a legal advisor.
Pursuant
to section 3A.3 of National Instrument 33-105 Underwriting Conflicts (NI 33-105), the placement agents are not required
to comply with the disclosure requirements of NI 33-105 regarding placement agents conflicts of interest in connection with this offering.
European
Economic Area . In relation to each Member State of the European Economic Area which has implemented the Prospectus Directive
(each, a “Relevant Member State”) an offer to the public of any securities may not be made in that Relevant Member State,
except that an offer to the public in that Relevant Member State of any securities may be made at any time under the following exemptions
under the Prospectus Directive, if they have been implemented in that Relevant Member State:
| |
● |
to
any legal entity which is a qualified investor as defined in the Prospectus Directive; |
| |
● |
to
fewer than 100 or, if the Relevant Member State has implemented the relevant provision of the 2010 PD Amending Directive, 150, natural
or legal persons (other than qualified investors as defined in the Prospectus Directive), as permitted under the Prospectus Directive,
subject to obtaining the prior consent of the representatives for any such offer; or |
| |
● |
in
any other circumstances falling within Article 3(2) of the Prospectus Directive, provided that no such offer of securities
shall result in a requirement for the publication by us or any placement agents of a prospectus pursuant to Article 3 of the
Prospectus Directive. |
For
the purposes of this provision, the expression an “offer to the public” in relation to any securities in any Relevant Member
State means the communication in any form and by any means of sufficient information on the terms of the offer and any securities to
be offered so as to enable an investor to decide to purchase any securities, as the same may be varied in that Member State by any measure
implementing the Prospectus Directive in that Member State, the expression “Prospectus Directive” means Directive 2003/71/EC
(and amendments thereto, including the 2010 PD Amending Directive, to the extent implemented in the Relevant Member State), and includes
any relevant implementing measure in the Relevant Member State, and the expression “2010 PD Amending Directive” means Directive
2010/73/EU.
Israel.
This document does not constitute a prospectus under the Israeli Securities Law, 5728-1968, or the Securities Law, and has not been filed
with or approved by the Israel Securities Authority. In the State of Israel, this document is being distributed only to, and is directed
only at, and any offer of the shares is directed only at, investors listed in the first addendum, or the Addendum, to the Israeli Securities
Law, consisting primarily of joint investment in trust funds, provident funds, insurance companies, banks, portfolio managers, investment
advisors, members of the Tel Aviv Stock Exchange, placement agents, venture capital funds, entities with equity in excess of NIS 50 million
and “qualified individuals”, each as defined in the Addendum (as it may be amended from time to time), collectively referred
to as qualified investors (in each case purchasing for their own account or, where permitted under the Addendum, for the accounts of
their clients who are investors listed in the Addendum). Qualified investors will be required to submit written confirmation that they
fall within the scope of the Addendum, are aware of the meaning of same and agree to it.
United
Kingdom. Each placement agent has represented and agreed that:
| |
● |
it
has only communicated or caused to be communicated and will only communicate or cause to be communicated an invitation or inducement
to engage in investment activity (within the meaning of Section 21 of the Financial Services and Markets Act 2000 (the FSMA)
received by it in connection with the issue or sale of the securities in circumstances in which Section 21(1) of the FSMA
does not apply to us; and |
| |
● |
it
has complied and will comply with all applicable provisions of the FSMA with respect to anything done by it in relation to the securities
in, from or otherwise involving the United Kingdom. |
Switzerland.
The securities may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange (the SIX) or on any other
stock exchange or regulated trading facility in Switzerland. This document has been prepared without regard to the disclosure standards
for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses
under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in
Switzerland. Neither this document nor any other offering or marketing material relating to the securities or the offering may be publicly
distributed or otherwise made publicly available in Switzerland.
Neither
this document nor any other offering or marketing material relating to the offering, or the securities have been or will be filed with
or approved by any Swiss regulatory authority. In particular, this document will not be filed with, and the offer of securities will
not be supervised by, the Swiss Financial Market Supervisory Authority FINMA, and the offer of securities has not been and will not be
authorized under the Swiss Federal Act on Collective Investment Schemes (CISA). Accordingly, no public distribution, offering or advertising,
as defined in CISA, its implementing ordinances and notices, and no distribution to any non-qualified investor, as defined in CISA, its
implementing ordinances and notices, shall be undertaken in or from Switzerland, and the investor protection afforded to acquirers of
interests in collective investment schemes under CISA does not extend to acquirers of securities.
Australia.
No placement document, prospectus, product disclosure statement or other disclosure document has been lodged with the Australian Securities
and Investments Commission (ASIC), in relation to the offering.
This
prospectus does not constitute a prospectus, product disclosure statement or other disclosure document under the Corporations Act 2001
(the Corporations Act) and does not purport to include the information required for a prospectus, product disclosure statement or other
disclosure document under the Corporations Act.
Any
offer in Australia of the securities may only be made to persons (the Exempt Investors) who are “sophisticated investors”
(within the meaning of section 708(8) of the Corporations Act), “professional investors” (within the meaning of section
708(11) of the Corporations Act) or otherwise pursuant to one or more exemptions contained in section 708 of the Corporations Act so
that it is lawful to offer the securities without disclosure to investors under Chapter 6D of the Corporations Act.
The
securities applied for by Exempt Investors in Australia must not be offered for sale in Australia in the period of 12 months after the
date of allotment under the offering, except in circumstances where disclosure to investors under Chapter 6D of the Corporations Act
would not be required pursuant to an exemption under section 708 of the Corporations Act or otherwise or where the offer is pursuant
to a disclosure document which complies with Chapter 6D of the Corporations Act. Any person acquiring securities must observe such Australian
on-sale restrictions.
This
prospectus contains general information only and does not take account of the investment objectives, financial situation or particular
needs of any particular person. It does not contain any securities recommendations or financial product advice. Before making an investment
decision, investors need to consider whether the information in this prospectus is appropriate to their needs, objectives and circumstances,
and, if necessary, seek expert advice on those matters.
Notice
to Prospective Investors in the Cayman Islands. No invitation, whether directly or indirectly, may be made to the public in the
Cayman Islands to subscribe for our securities.
Taiwan.
The securities have not been and will not be registered with the Financial Supervisory Commission of Taiwan pursuant to relevant securities
laws and regulations and may not be sold, issued or offered within Taiwan through a public offering or in circumstances which constitutes
an offer within the meaning of the Securities and Exchange Act of Taiwan that requires a registration or approval of the Financial Supervisory
Commission of Taiwan. No person or entity in Taiwan has been authorized to offer, sell, give advice regarding or otherwise intermediate
the offering and sale of the securities in Taiwan.
Notice
to Prospective Investors in Hong Kong. The contents of this prospectus have not been reviewed by any regulatory authority in
Hong Kong. You are advised to exercise caution in relation to the offer. If you are in any doubt about any of the contents of this prospectus,
you should obtain independent professional advice. Please note that (i) our shares may not be offered or sold in Hong Kong, by means
of this prospectus or any document other than to “professional investors” within the meaning of Part I of Schedule 1
of the Securities and Futures Ordinance (Cap.571, Laws of Hong Kong) (SFO) and any rules made thereunder, or in other circumstances
which do not result in the document being a “prospectus” within the meaning of the Companies Ordinance (Cap.32, Laws of Hong
Kong) (CO) or which do not constitute an offer or invitation to the public for the purpose of the CO or the SFO, and (ii) no advertisement,
invitation or document relating to our shares may be issued or may be in the possession of any person for the purpose of issue (in each
case whether in Hong Kong or elsewhere) which is directed at, or the contents of which are likely to be accessed or read by, the public
in Hong Kong (except if permitted to do so under the securities laws of Hong Kong) other than with respect to the shares which are or
are intended to be disposed of only to persons outside Hong Kong or only to “professional investors” within the meaning of
the SFO and any rules made thereunder.
Notice
to Prospective Investors in the People’s Republic of China. This prospectus may not be circulated or distributed in
the PRC and the shares may not be offered or sold, and will not offer or sell to any person for re-offering or resale directly or indirectly
to any resident of the PRC except pursuant to applicable laws, rules and regulations of the PRC. For the purpose of this paragraph
only, the PRC does not include Taiwan and the special administrative regions of Hong Kong and Macau.
LEGAL
MATTERS
Greenberg
Traurig, LLP, Irvine, California, will pass upon the validity of the shares of common stock and Pre-Funded Warrants offered hereby. Ellenoff
Grossman & Schole LLP, New York, New York, has acted as counsel for the placement agent in connection with certain legal
matters related to this offering.
EXPERTS
The
financial statements as of and for the fiscal years ended December 31, 2023 and 2022 included in this prospectus have been
so included in reliance on the report of Grassi & Co., CPAs, P.C., an independent registered public accounting firm, given on the
authority of said firm as experts in auditing and accounting.
INCORPORATION
OF CERTAIN DOCUMENTS BY REFERENCE
The
SEC permits us to “incorporate by reference” the information and reports we file with it. This means that we can disclose
important information to you by referring to another document. The information that we incorporate by reference is considered to be part
of this prospectus, and later information that we file with the SEC automatically updates and supersedes this information. We incorporate
by reference the documents listed below, except to the extent information in those documents is different from the information contained
in this prospectus, and all future documents filed with the SEC under Sections 13(a), 13(c), 14, or 15(d) of the Exchange Act (other
than current reports furnished under Item 2.02 or Item 7.01 of Form 8-K and exhibits filed on such form that are related to such items
unless such Form 8-K expressly provides to the contrary) until we terminate the offering of these securities:
| |
● |
Our
Annual Report on Form 10-K for the fiscal year ended December 31, 2023 filed with the SEC on March 15, 2024; |
| |
|
|
| |
● |
Our
Amendment No. 1 to our Annual Report on Form 10-K for the fiscal year ended December 31, 2023 filed with the SEC on April 29, 2024; |
| |
|
|
| |
● |
Our
Quarterly Report on Form 10-Q for the three months ended March 31, 2024 filed with the SEC on May 9, 2024; |
| |
|
|
| |
● |
Our
Current Reports on Form
8-K filed with the SEC on February 14, 2024 and July 3, 2024; and |
| |
|
|
| |
● |
The
description of our common stock set forth in our registration statement on Form 8-A12B filed with the SEC on May 16, 2023. |
To the extent that any statement
in this prospectus is inconsistent with any statement that is incorporated by reference and that was made on or before the date of this
prospectus, the statement in this prospectus shall supersede such incorporated statement and the incorporated statement shall not be
deemed, except as modified or superseded, to constitute a part of this prospectus or the registration statement. Statements contained
in this prospectus as to the contents of any contract or other document are not necessarily complete and, in each instance, we refer
you to the copy of each contract or document filed as an exhibit to our various filings made with the SEC.
You
may request a copy of these filings, at no cost, by writing or telephoning us at the following address or telephone number:
Azitra,
Inc.
21
Business Park Drive,
Branford,
Connecticut 06405
Attention:
Corporate Secretary
Telephone:
(203) 646-6446
WHERE
YOU CAN FIND MORE INFORMATION
We
have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to the shares of common stock offered
by this prospectus. This prospectus, which constitutes a part of the registration statement, does not contain all the information set
forth in the registration statement, some of which is contained in exhibits to the registration statement as permitted by the rules and
regulations of the SEC. For further information with respect to us and our shares of common stock, we refer you to the registration statement,
including the exhibits filed as a part of the registration statement. Statements contained in this prospectus concerning the contents
of any contract or any other document is not necessarily complete. If a contract or document has been filed as an exhibit to the registration
statement, please see the copy of the contract or document that has been filed. Each statement is this prospectus relating to a contract
or document filed as an exhibit is qualified in all respects by the filed exhibit. The SEC maintains an Internet website that contains
reports, proxy statements and other information about issuers, like us, that file electronically with the SEC. The address of that website
is www.sec.gov.
In
addition, we file annual, quarterly and current reports and proxy statements and other information with the SEC. Our SEC filings are
available on the SEC’s website at www.sec.gov. These filings are also available free of charge to the public on, or accessible
through, our corporate website at www.azitrainc.com. Our website and the information contained on, or that can be accessed through, our
website is not deemed to be incorporated by reference in, and is not considered part of, this prospectus. You should not rely on any
such information in making your decision whether to purchase our common stock.
We
have not authorized anyone to give you any information or to make any representations about us or the transactions we discuss in this
prospectus other than those contained in this prospectus. If you are given any information or representations about these matters that
is not discussed in this prospectus, you must not rely on that information. This prospectus is not an offer to sell or a solicitation
of an offer to buy securities anywhere or to anyone where or to whom we are not permitted to offer or sell securities under applicable
law.
Up
to 3,115,265 Units
Each
Unit consisting of one Share of Common Stock, or one Pre-Funded Warrant to Purchase one share of Common Stock, and Two Class A Warrants
each to Purchase One Share of Common Stock
Up
to 6,230,530 Shares of Common Stock Underlying the Class A Warrants
Up
to 3,115,265 Shares of Common Stock Underlying the Pre-Funded Warrants
Up
to 124,610 Placement
Agent Warrants to Purchase Shares of Common Stock
Up
to 124,610 Shares of Common Stock Underlying the Placement Agent
Warrants

PRELIMINARY
PROSPECTUS
Maxim
Group LLC
,
2024
PART
II - INFORMATION NOT REQUIRED IN PROSPECTUS
Item
13. Other Expenses of Issuance and Distribution.
The
following table sets forth the various expenses to be incurred in connection with the sale and distribution of our common stock being
registered hereby, all of which will be borne by us (except any placement agent fees and expenses incurred for brokerage, accounting,
tax or legal services or any other expenses incurred in disposing of the shares). All amounts are estimated except the SEC registration
fee and the FINRA filing fee.
| Description |
|
Amount |
|
| SEC Registration Fee |
|
$ |
4,502 |
|
| FINRA Filing Fee |
|
|
5,075 |
|
| Expenses
of the Placement Agent |
|
|
100,000 |
|
| Printing Expenses |
|
|
10,000 |
|
| Accounting Fees and Expenses |
|
|
25,000 |
|
| Legal Fees and Expenses |
|
|
150,000 |
|
| Transfer
and Warrant Agent’s and Registrar’s Fees and Expenses |
|
|
25,000* |
|
| Miscellaneous Fees |
|
|
30,423 |
|
| Total |
|
$ |
350,000 |
|
Item
14. Indemnification of Directors and Officers.
The
following summary is qualified in its entirety by reference to the complete text of any statutes referred to below and the Second Amended
and Restated Certificate of Incorporation, or Certificate of Incorporation, of Azitra, Inc., a Delaware corporation.
Section
145 of the General Corporation Law of the State of Delaware (the “DGCL”) permits a Delaware corporation to indemnify any
person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding,
whether civil, criminal, administrative or investigative (other than an action by or in the right of the corporation) by reason of the
fact that the person is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the
corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise,
against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred
by the person in connection with such action, suit or proceeding if the person acted in good faith and in a manner the person reasonably
believed to be in or not opposed to the best interests of the corporation, and, with respect to any criminal action or proceeding, had
no reasonable cause to believe the person’s conduct was unlawful.
In
the case of an action by or in the right of the corporation, Section 145 of the DGCL permits a Delaware corporation to indemnify any
person who was or is a party or is threatened to be made a party to any threatened, pending or completed action or suit by reason of
the fact that the person is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of
the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise,
against expenses (including attorneys’ fees) actually and reasonably incurred by the person in connection with such action, suit
or proceeding if the person acted in good faith and in a manner the person reasonably believed to be in or not opposed to the best interests
of the corporation and except that no indemnification shall be made in respect of any claim, issue or matter as to which such person
shall have been adjudged to be liable to the corporation unless and only to the extent that the Court of Chancery or the court in which
such action or suit was brought shall determine upon application that, despite the adjudication of liability but in view of all the circumstances
of the case, such person is fairly and reasonably entitled to indemnity for such expenses that the Court of Chancery or such other court
shall deem proper.
Section
145 of the DGCL also permits a Delaware corporation to purchase and maintain insurance on behalf of any person who is or was a director,
officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee
or agent of another corporation, partnership, joint venture, trust or other enterprise against any liability asserted against such person
and incurred by such person in any such capacity, or arising out of such person’s status as such, whether or not the corporation
would have the power to indemnify such person against such liability under Section 145 of the DGCL.
Our
Certificate of Incorporation states that to the fullest extent permitted by the DGCL our directors shall not be personally liable to
us or to our stockholders for monetary damages for breach of fiduciary duty as a director. If the DGCL is amended after the date hereof
to authorize corporate action further eliminating or limiting the personal liability of directors, then the liability of our directors
shall be eliminated or limited to the fullest extent permitted by the DGCL, as so amended.
Our
Certificate of Incorporation requires us, to the fullest extent permitted by applicable law, to provide indemnification of (and advancement
of expenses to) our directors and officers, and authorizes us, to the fullest extent permitted by applicable law, to provide indemnification
of (and advancement of expenses to) to other employees and agents (and any other persons to which the DGCL permits us to provide indemnification)
through bylaw provisions, agreements with such directors, officers, employees, agents or other persons, vote of stockholders or disinterested
directors or otherwise, subject only to limits created by the DGCL with respect to actions for breach of duty to our corporation, our
stockholders and others.
Our
Certificate of Incorporation provides that we shall, to the maximum extent and in the manner permitted by the DGCL, indemnify each of
our directors, officers and all other persons we have the power to indemnify under Section 145 of the DGCL against expenses (including
attorneys’ fees), judgments, fines, settlements and other amounts actually and reasonably incurred in connection with any proceeding,
arising by reason of the fact that such person is or was a director of the Company. We may maintain insurance, at our expense, to protect
the Company and any of our directors, officers, employees or agents against any such expense, liability or loss, whether or not we have
the power to indemnify such person.
We
have entered into indemnification agreements with each of our directors and executive officers that may be broader than the specific
indemnification provisions contained in the DGCL. These indemnification agreements require us, among other things, to indemnify our directors
and executive officers against liabilities that may arise by reason of their status or service. These indemnification agreements also
require us to advance all expenses incurred by the directors and executive officers in investigating or defending any such action, suit,
or proceeding. We believe that these agreements are necessary to attract and retain qualified individuals to serve as directors and executive
officers.
Prior
to the closing of this offering, we plan to enter into an underwriting agreement, which will provide that the underwriters are obligated,
under some circumstances, to indemnify our directors, officers and controlling persons against specified liabilities.
Item
15. Recent Sales of Unregistered Securities.
Issuances
of capital stock
The
following list sets forth information regarding all unregistered securities sold by us over the three-year period preceding the date
of the prospectus that forms a part of this registration statement.
In
January 2022, we sold to one investor an unsecured convertible promissory note in the principal amount of $1,000,000. In January 2023,
the principal amount of the note, along with all accrued interest, was converted into 23,432 shares of our Series B convertible preferred
stock. Subsequently, these shares were converted into common stock upon the consummation of the IPO.
In
September 2022, we conducted the placement of our unsecured convertible promissory notes in the aggregate principal amount of $4.35 million
to five investors. The principal amount of the notes, along with all accrued and unpaid interest thereunder, converted into 61,534
shares of our common stock upon the consummation of the IPO.
No
underwriters were involved in the foregoing issuances of securities. We believe the offers, sales and issuances of the above securities
by us were exempt from registration under the Securities Act by virtue of Section 4(a)(2) of the Securities Act and Rule 506 thereunder
as transactions not involving a public offering. All of the investors were accredited investors as such term is defined in Rule 501 under
the Securities Act. The recipients of the securities in each of these transactions represented their intentions to acquire the securities
for investment only and not with a view to or for sale in connection with any distribution thereof, and appropriate legends were placed
upon the stock certificates, notes and warrants issued in these transactions. All recipients had adequate access, through their relationships
with us, to information about our Company. The sales of these securities were made without any general solicitation or advertising.
Item
16. Exhibits and Financial Statement Schedules.
| Exhibit
No. |
|
Description
of Document |
|
Method
of Filing |
| |
|
|
|
|
| 1.1 |
|
Form of Placement Agent Agreement. |
|
Previously filed. |
| |
|
|
|
|
| 3.1 |
|
Second
Amended and Restated Certificate of Incorporation of the Registrant. |
|
Incorporated
herein by reference to Exhibit 3.1 to the Company’s Current Report on Form 8-K filed on June 21, 2023 (File No. 001-41705). |
| |
|
|
|
|
| 3.2 |
|
Second
Amended and Restated Bylaws of the Registrant. |
|
Incorporated
herein by reference to Exhibit 3.2 to the Company’s Current Report on Form 8-K filed on June 21, 2023 (File No. 001-41705). |
| |
|
|
|
|
| 3.3 |
|
Certificate of Amendment to Second Amended and Restated Certificate of Incorporation of the Registrant. |
|
Incorporated herein by reference to Exhibit 3.3 to the Company’s Registration
Statement on Form S-3 filed on July 1, 2024 (File No. 280648). |
| |
|
|
|
|
| 4.1 |
|
Specimen
Certificate representing shares of Common Stock. |
|
Incorporated
herein by reference to Exhibit 4.1 to the Company’s Form S-1 filed on June 13, 2023 (File No. 333-269876). |
| |
|
|
|
|
| 4.2 |
|
Form
of Warrant issued to private placement investors. |
|
Incorporated
herein by reference to Exhibit 4.2 to the Company’s Form S-1 filed on June 13, 2023 (File No. 333-269876). |
| |
|
|
|
|
| 4.3 |
|
Form
of Representative’s Warrant dated June 20, 2023 issued to ThinkEquity LLC. |
|
Incorporated
herein by reference to Exhibit 4.3 to the Company’s Form S-1 filed on June 13, 2023 (File No. 333-269876). |
| |
|
|
|
|
| 4.5 |
|
Form of Representative’s Warrant dated February 13, 2024 issued to ThinkEquity LLC. |
|
Incorporated herein by reference to Exhibit 1.1 to the Company’s Current
Report on Form 8-K filed on February 14, 2024. |
| |
|
|
|
|
| 4.6 |
|
Form of Class A Warrant to be issued in this offering. |
|
Previously filed. |
| |
|
|
|
|
| 4.7 |
|
Form of Pre-Funded Common Warrant to be issued in this offering |
|
Previously filed. |
| |
|
|
|
|
| 4.8 |
|
Form of Placement agent’s warrant to be issued in this offering |
|
Previously filed. |
| |
|
|
|
|
| 4.9 |
|
Form of Warrant Agent Agreement to be entered into in connection with this offering between the Registrant and VStock Transfer, LLC |
|
Previously filed. |
| |
|
|
|
|
| 5.1 |
|
Opinion of Greenberg Traurig, LLP. |
|
Previously filed. |
| |
|
|
|
|
| 10.1+ |
|
Azitra,
Inc. 2016 Stock Incentive Plan. |
|
Incorporated
herein by reference to Exhibit 10.2 to the Company’s Form S-1 filed on June 13, 2023 (File No. 333-269876). |
| |
|
|
|
|
| 10.2+ |
|
Azitra,
Inc. 2023 Stock Incentive Plan. |
|
Incorporated
herein by reference to Exhibit 10.5 to the Company’s Form S-1 filed on June 13, 2023 (File No. 333-269876). |
| |
|
|
|
|
| 10.3+ |
|
Executive
Employment Agreement dated April 22, 2021 between the Registrant and Francisco D. Salva. |
|
Incorporated
herein by reference to Exhibit 10.4 to the Company’s Form S-1 filed on June 13, 2023 (File No. 333-269876). |
| 10.4+ |
|
Executive Employment Agreement dated July 5, 2023 between the Registrant and Travis Whitfill. |
|
Incorporated herein by reference to Exhibit 10.4 to the Company’s
Form S-1 filed on January 19, 2024 (File No. 333-276598). |
| |
|
|
|
|
| 10.5+ |
|
Form
of Indemnity Agreement between the Registrant and each of its directors and executive officers. |
|
Incorporated
herein by reference to Exhibit 10.1 to the Company’s Form S-1 filed on June 13, 2023 (File No. 333-269876). |
| |
|
|
|
|
| 10.6 |
|
Second
Amended and Restated Investors’ Rights Agreement dated September 10, 2020 between the Registrant and each of the investors
named therein. |
|
Incorporated
herein by reference to Exhibit 10.3 to the Company’s Form S-1 filed on June 13, 2023 (File No. 333-269876). |
| |
|
|
|
|
| 10.7 |
|
Form of Securities Purchase Agreement to be entered into by the Registrant in this offering |
|
Previously filed. |
| |
|
|
|
|
| 21.1 |
|
List
of Subsidiaries of the Registrant. |
|
Incorporated
herein by reference to Exhibit 21.1 to the Company’s Form S-1 filed on June 13, 2023 (File No. 333-269876). |
| |
|
|
|
|
| 23.1 |
|
Consent of Greenberg Traurig, LLP. |
|
Previously filed. |
| |
|
|
|
|
| 23.2 |
|
Consent of Grassi & Co., CPAs, P.C., Independent Registered Public Accounting Firm. |
|
Electronically filed herewith. |
| |
|
|
|
|
| 24.1 |
|
Power of Attorney. |
|
Previously filed. |
| |
|
|
|
|
| 107 |
|
Filing Fee Table. |
|
Previously filed. |
+
Indicates management compensatory plan, contract or arrangement.
(b)
Financial Statement Schedules.
All
schedules have been omitted because they are not required or are not applicable.
Item
17. Undertakings.
The
undersigned registrant hereby undertakes to provide to the underwriter at the closing specified in the underwriting agreements, certificates
in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser.
Insofar
as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons
of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities
and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred
or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is
asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless
in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the
question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final
adjudication of such issue.
The
undersigned registrant undertakes that:
(1)
For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus as filed as part
of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule
424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared
effective.
(2)
For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus
shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at
that time shall be deemed to be the initial bona fide offering thereof.
SIGNATURES
Pursuant
to the requirements of the Securities Act of 1933, the registrant has duly caused this registration statement to be signed on its behalf
by the undersigned, thereunto duly authorized, in the City of Branford, State of Connecticut, on July 19, 2024.
| |
AZITRA,
INC. |
| |
|
| |
/s/
Francisco D. Salva |
| |
Francisco
D. Salva |
| |
Chief
Executive Officer and Director |
Pursuant
to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities
and on the dates indicated.
Signature |
|
Title |
|
Date |
| |
|
|
|
|
| /s/
Francisco D. Salva |
|
President, |
|
|
|
Francisco
D. Salva |
|
Chief
Executive Officer and Director
(Principal
Executive Officer) |
|
July
19, 2024 |
| |
|
|
|
|
| /s/
Norman Staskey |
|
Chief
Financial Officer, |
|
|
|
Norman
Staskey |
|
Treasurer
and Secretary
(Principal
Financial and Accounting Officer) |
|
July
19, 2024 |
| |
|
|
|
|
| * |
|
|
|
|
|
Travis
Whitfill |
|
Chief
Operating Officer and Director |
|
July
19, 2024 |
| |
|
|
|
|
| * |
|
|
|
|
|
Andrew
McClary |
|
Director |
|
July
19, 2024 |
| |
|
|
|
|
| * |
|
|
|
|
|
Barbara
Ryan |
|
Director |
|
July
19, 2024 |
| |
|
|
|
|
| * |
|
|
|
|
|
John
Schroer |
|
Director |
|
July
19, 2024 |
| |
|
|
|
|
| */s/ Francisco D. Salva |
|
|
|
|
| Francisco D. Salva |
|
|
|
|
| Attorney-in-Fact |
|
|
|
|
Exhibit
23.2

Consent
of Independent Registered Public Accounting Firm
We
consent to the incorporation by reference in this Registration Statement on Form S-1 of our report dated February 23, 2024 with
respect to our audits of the financial statements of Azitra, Inc. as of December 31, 2023 and 2022 and for each of the two years in the
period ended December 31, 2023, which report is included in this Annual Report on Form 10-K of Azitra, Inc. for the year ended December
31, 2023. Our report contains an explanatory paragraph regarding the Company’s ability to continue as a going concern. We also
consent to the reference to our firm under the heading “Experts” appearing therein.

Grassi
& Co., CPAs, P.C.
Jericho,
New York
July
19, 2024

Azitra (AMEX:AZTR)
Historical Stock Chart
Von Dez 2024 bis Jan 2025

Azitra (AMEX:AZTR)
Historical Stock Chart
Von Jan 2024 bis Jan 2025
