CALGARY, Aug. 10, 2016 /PRNewswire/ -- Oncolytics
Biotech Inc. ("Oncolytics" or the "Company") (TSX:
ONC) (OTCQX: ONCYF) (FRA: ONY) today announced additional data from
IND 211, a randomized, Phase II clinical study of
REOLYSIN® in patients with non-small cell lung cancer
("NSCLC"). The study enrolled patients with both
non-squamous (adenocarcinoma) and squamous cell histology. Those
with adenocarcinoma (n=75) were treated with REOLYSIN®
in combination with pemetrexed in the test arm versus pemetrexed
alone in the control arm. Those with squamous cell histology (n=76)
were treated with REOLYSIN® in combination with
docetaxel in the test arm versus docetaxel alone in the control
arm. The study's primary objective was progression free survival
("PFS"). Its secondary objectives included overall survival
("OS"), safety, and measurement of biomarkers that may be
predictive of response.
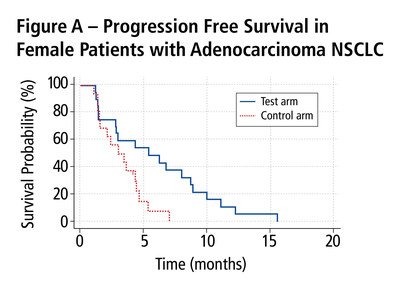
Results in Patients with Adenocarcinoma by Patient
Gender
An analysis was performed of survival outcomes for
patients with adenocarcinoma by gender. The PFS was statistically
significantly better for female patients in the test arm (n=20)
than for those in the control arm (n=16); median PFS was 5.39
months compared with 3.02 months, respectively (p=0.0201) (Figure
A). The evolving OS showed a strong trend towards survival benefit
for female patients in the test arm (n=20, six of whom remained
alive at the time of the analysis) over those in the control arm
(n=16, three of whom remained alive at the time of the analysis);
median OS was 10.68 months compared with 7.59 months, respectively
(p=0.145) (Figure B). By contrast, no PFS or OS benefit was noted
for the male patients with adenocarcinoma.
"We are excited to see a statistically significant improvement
in progression free survival for female patients with
adenocarcinoma of the lung," said Dr. Brad
Thompson, President and CEO of Oncolytics. "There is a
significant unmet need for new therapies for non-small cell lung
cancer patients."
Results in Patients with Adenocarcinoma by Patient Genetic
Status
All patients were tested for biomarkers including
those that are associated with the replication of the reovirus
(namely, EGFR, Hras, Kras, Nras, Braf and/or p53 mutations) (the
"target biomarkers"). Patients treated with
REOLYSIN® with one or more target biomarkers had a
greater PFS (p=0.039) and OS (p=0.031) than patients treated with
REOLYSIN® without any of these biomarkers. The presence
of these biomarkers may account, at least in part, for the
difference between the survival outcomes for male and female
patients; target biomarkers were present in a higher proportion of
the female patients in the study than the male patients (66.7%
versus 43.6%). As a result, pre-screening for target biomarkers in
patients with adenocarcinoma of the lung is warranted.
Results in Patients with Squamous Cell Histology
The
overall OS for patients with squamous cell histology continues to
evolve. Target biomarkers were present in a smaller proportion of
the overall patients with squamous cell histology (34.2% versus
54.7% of those with adenocarcinoma); therefore a larger patient
population would be required to make statistical conclusions about
the role of biomarkers in predicting response.
Next Steps
Based on the findings today announced from
IND 211, the Company intends to include preselection of patients
using genetic screening in future study protocols.
About IND 211
IND 211 is an open-label, randomized,
non-blinded two sided (adenocarcinoma and squamous cell carcinoma)
Phase II study of intravenously administered REOLYSIN®
in patients with advanced or metastatic non-small cell lung cancer.
Patients with squamous cell histology were treated with either
REOLYSIN® given in combination with docetaxel (test
arm) or docetaxel alone (control arm). Patients with adenocarcinoma
were treated with either REOLYSIN® given in combination
with pemetrexed (test arm) versus pemetrexed alone (control arm).
After a patient safety run-in, a total of approximately 150
response-evaluable patients were enrolled. Preliminary data from
this study were reported in May 2016.
The study was sponsored by the Canadian Cancer Trials Group
("CCTG") at Queen's University in Kingston, Ontario, formerly known as the
National Cancer Institute of Canada ("NCIC") Clinical Trials
Group.
About Non-Small Cell Lung Cancer
The American Cancer
Society ("ACS") estimates that approximately 224,390
Americans will be diagnosed with lung cancer in 2016 and that
158,080 of them will die from the disease. Approximately 80% to 85%
of lung cancer cases are non-small cell lung cancer. The ACS
identifies multiple subtypes of non-small cell lung cancer based on
the types of cells where they originate – adenocarcinoma accounts
for about 40% of lung cancers and occurs more frequently in women
than men; squamous cell carcinoma accounts for 25% to 30% of lung
cancers; and large cell carcinoma accounts for 10% to 15% of lung
cancers.
About Oncolytics Biotech Inc.
Oncolytics is a
Calgary-based biotechnology
company focused on the development of oncolytic viruses as
potential cancer therapeutics. Oncolytics' clinical program
includes a variety of later-stage, randomized human trials in
various indications using REOLYSIN®, its proprietary
formulation of the human reovirus. For further information about
Oncolytics, please visit: www.oncolyticsbiotech.com.
This press release contains forward-looking statements,
within the meaning of Section 21E of the Securities Exchange Act of
1934, as amended. Forward-looking statements, including the
Company's expectations related to the randomized Phase II study in
patients with non-small cell lung cancer, future trials in this
indication, and the Company's belief as to the potential of
REOLYSIN® as a cancer therapeutic, involve known
and unknown risks and uncertainties, which could cause the
Company's actual results to differ materially from those in the
forward-looking statements. Such risks and uncertainties include,
among others, the availability of funds and resources to pursue
research and development projects, the efficacy of
REOLYSIN® as a cancer treatment, the tolerability
of REOLYSIN® outside a controlled test, the success
and timely completion of clinical studies and trials, the Company's
ability to successfully commercialize REOLYSIN®,
uncertainties related to the research, development and
manufacturing of pharmaceuticals, changes in technology, general
changes to the economic environment and uncertainties related to
the regulatory process. Investors should consult the Company's
quarterly and annual filings with the Canadian and U.S. securities
commissions for additional information on risks and uncertainties
relating to the forward-looking statements. Investors are cautioned
against placing undue reliance on forward-looking statements. The
Company does not undertake to update these forward-looking
statements, except as required by applicable laws.
For further information: NATIONAL Equicom, Nick Hurst, 320 Front Street W, Suite 1600,
Toronto, Ontario, M5V 3B6,
Tel: 416.586.1942, nhurst@national.ca; NATIONAL Equicom,
Michael Moore, San Diego, CA, Tel: 858.886.7813,
mmoore@national.ca; Dian Griesel,
Inc., Susan Forman, 335 West 38th
Street, 3rd Floor, New York,
NY, 10018, Tel: 212.825.3210, sforman@dgicomm.com
Photo - http://photos.prnewswire.com/prnh/20160810/397063
Photo - http://photos.prnewswire.com/prnh/20160810/397064
To view the original version on PR Newswire,
visit:http://www.prnewswire.com/news-releases/oncolytics-biotech-inc-reports-additional-data-from-randomized-phase-ii-study-of-reolysin-in-non-small-cell-lung-cancer-300311680.html
SOURCE Oncolytics Biotech Inc.