Bristol Myers Gets EU Approval of Opdivo in Esophageal Cancer
24 November 2020 - 2:09PM
Dow Jones News
By Colin Kellaher
Bristol Myers Squibb Co. on Tuesday said the European Commission
approved its cancer drug Opdivo as a second-line treatment for
certain forms of esophageal squamous cell carcinoma, making it the
first immunotherapy to be approved for a gastroesophageal cancer in
the European Union.
The New York biopharmaceutical company said the approval covers
Opdivo for the treatment of adults with unresectable advanced,
recurrent or metastatic esophageal squamous cell carcinoma after
prior fluoropyrimidine- and platinum-based combination
chemotherapy.
Opdivo, which harnesses the body's own immune system to fight
cancer, is currently approved in more than 65 countries across
multiple cancers, the company said. In addition to the EU, the drug
has been approved in five countries, including the U.S. and Japan,
for the second-line treatment of patients with unresectable
advanced, recurrent or metastatic esophageal squamous cell
carcinoma.
Write to Colin Kellaher at colin.kellaher@wsj.com
(END) Dow Jones Newswires
November 24, 2020 07:54 ET (12:54 GMT)
Copyright (c) 2020 Dow Jones & Company, Inc.
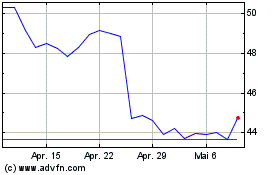
Bristol Myers Squibb (NYSE:BMY)
Historical Stock Chart
Von Mär 2024 bis Apr 2024
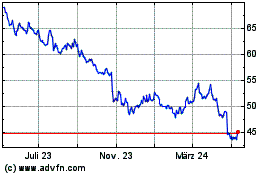
Bristol Myers Squibb (NYSE:BMY)
Historical Stock Chart
Von Apr 2023 bis Apr 2024