Statement of Changes in Beneficial Ownership (4)
08 November 2019 - 11:15PM
Edgar (US Regulatory)
|
FORM 4
[ ]
Check this box if no longer subject to Section 16. Form 4 or Form 5 obligations may continue. See Instruction 1(b).
|
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
STATEMENT OF CHANGES IN BENEFICIAL OWNERSHIP OF SECURITIES
|
OMB APPROVAL
OMB Number:
3235-0287
Estimated average burden
hours per response...
0.5
|
|
Filed pursuant to Section 16(a) of the Securities Exchange Act of 1934 or Section 30(h) of the Investment Company Act of 1940
|
|
|
1. Name and Address of Reporting Person
*
Schumacher Laura J |
2. Issuer Name and Ticker or Trading Symbol
AbbVie Inc.
[
ABBV
]
|
5. Relationship of Reporting Person(s) to Issuer
(Check all applicable)
_____ Director _____ 10% Owner
__X__ Officer (give title below) _____ Other (specify below)
Vice Chairman
|
|
(Last)
(First)
(Middle)
1 N. WAUKEGAN ROAD |
3. Date of Earliest Transaction
(MM/DD/YYYY)
11/4/2019
|
|
(Street)
NORTH CHICAGO, IL 60064
(City)
(State)
(Zip)
|
4. If Amendment, Date Original Filed
(MM/DD/YYYY)
|
6. Individual or Joint/Group Filing
(Check Applicable Line)
_X
_ Form filed by One Reporting Person
___ Form filed by More than One Reporting Person
|
Table I - Non-Derivative Securities Acquired, Disposed of, or Beneficially Owned
|
1.Title of Security
(Instr. 3)
|
2. Trans. Date
|
2A. Deemed Execution Date, if any
|
3. Trans. Code
(Instr. 8)
|
4. Securities Acquired (A) or Disposed of (D)
(Instr. 3, 4 and 5)
|
5. Amount of Securities Beneficially Owned Following Reported Transaction(s)
(Instr. 3 and 4)
|
6. Ownership Form: Direct (D) or Indirect (I) (Instr. 4)
|
7. Nature of Indirect Beneficial Ownership (Instr. 4)
|
|
Code
|
V
|
Amount
|
(A) or (D)
|
Price
|
|
Common Stock, $0.01 par value
|
11/4/2019
|
|
G
|
V
|
358
|
D
|
$0
|
164480
|
D
|
|
Table II - Derivative Securities Beneficially Owned (e.g., puts, calls, warrants, options, convertible securities)
|
1. Title of Derivate Security
(Instr. 3)
|
2. Conversion or Exercise Price of Derivative Security
|
3. Trans. Date
|
3A. Deemed Execution Date, if any
|
4. Trans. Code
(Instr. 8)
|
5. Number of Derivative Securities Acquired (A) or Disposed of (D)
(Instr. 3, 4 and 5)
|
6. Date Exercisable and Expiration Date
|
7. Title and Amount of Securities Underlying Derivative Security
(Instr. 3 and 4)
|
8. Price of Derivative Security
(Instr. 5)
|
9. Number of derivative Securities Beneficially Owned Following Reported Transaction(s) (Instr. 4)
|
10. Ownership Form of Derivative Security: Direct (D) or Indirect (I) (Instr. 4)
|
11. Nature of Indirect Beneficial Ownership (Instr. 4)
|
|
Code
|
V
|
(A)
|
(D)
|
Date Exercisable
|
Expiration Date
|
Title
|
Amount or Number of Shares
|
|
Explanation of Responses:
|
Reporting Owners
|
|
Reporting Owner Name / Address
|
Relationships
|
|
Director
|
10% Owner
|
Officer
|
Other
|
Schumacher Laura J
1 N. WAUKEGAN ROAD
NORTH CHICAGO, IL 60064
|
|
|
Vice Chairman
|
|
Signatures
|
|
Steven L. Scrogham, attorney-in-fact for Laura J. Schumacher
|
|
11/8/2019
|
|
**Signature of Reporting Person
|
Date
|
|
Reminder: Report on a separate line for each class of securities beneficially owned directly or indirectly.
|
|
*
|
If the form is filed by more than one reporting person, see Instruction 4(b)(v).
|
|
**
|
Intentional misstatements or omissions of facts constitute Federal Criminal Violations. See 18 U.S.C. 1001 and 15 U.S.C. 78ff(a).
|
|
Note:
|
File three copies of this Form, one of which must be manually signed. If space is insufficient, see Instruction 6 for procedure.
|
|
Persons who respond to the collection of information contained in this form are not required to respond unless the form displays a currently valid OMB control number.
|
AbbVie (NYSE:ABBV)
Historical Stock Chart
Von Mär 2024 bis Apr 2024
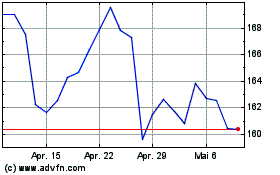
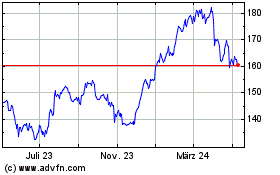
AbbVie (NYSE:ABBV)
Historical Stock Chart
Von Apr 2023 bis Apr 2024

Die zuletzt besuchten Aktien werden in diesem Feld angezeigt, so dass Sie leicht zu den Aktien zurückkehren können, die Sie zuvor angesehen haben.