India Panel Recommends Covovax, Corbevax and Merck's Covid-19 Pill for Emergency Use, Economic Times Reports
28 Dezember 2021 - 7:07AM
Dow Jones News
--An expert panel under India's drug regulator recommended
emergency use authorization for Novavax Inc.'s Covovax vaccine
produced by the Serum Institute of India and Indian pharmaceutical
company Biological E's Corbevax vaccine, India's Economic Times
reports.
--The subject expert committee also gave the nod to Merck &
Co.'s Covid-19 pill molnupiravir, according to the report.
--The recommendations have been sent to the Drugs Controller
General of India for approval, the Economic Times reports, citing
unnamed sources.
Full story: https://bit.ly/3evZir6
Write to Singapore Editors at singaporeeditors@dowjones.com
(END) Dow Jones Newswires
December 28, 2021 00:52 ET (05:52 GMT)
Copyright (c) 2021 Dow Jones & Company, Inc.
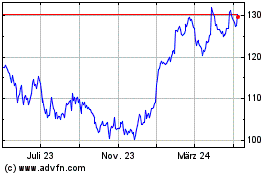
Merck (NYSE:MRK)
Historical Stock Chart
Von Mär 2024 bis Apr 2024
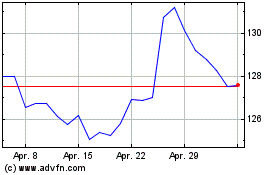
Merck (NYSE:MRK)
Historical Stock Chart
Von Apr 2023 bis Apr 2024