China Approves Merck's Molnupiravir for Emergency Use, Regulator Says
30 Dezember 2022 - 9:39AM
Dow Jones News
China's top drug regulator said Friday that it approved Merck
& Co.'s Molnupiravir for emergency use on Wednesday, as the
country grapples with waves of infections after Beijing abruptly
reversed its stringent Covid-19 restrictions earlier this
month.
The National Medical Products Administration said it is
requiring the approval holder to continue relevant research,
complete conditional requirements and submit follow-up research
results in a timely manner, according to a statement posted on its
website Friday.
Write to Singapore Editors at singaporeeditors@dowjones.com
(END) Dow Jones Newswires
December 30, 2022 03:24 ET (08:24 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
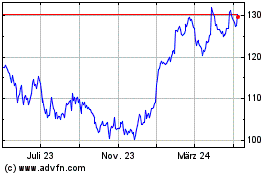
Merck (NYSE:MRK)
Historical Stock Chart
Von Mär 2024 bis Apr 2024
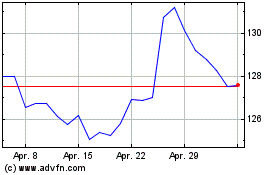
Merck (NYSE:MRK)
Historical Stock Chart
Von Apr 2023 bis Apr 2024