Novavax Vaccine Approved for Adolescents in South Korea
12 August 2022 - 4:10PM
Dow Jones News
By Connor Hart
Novavax Inc. on Friday said the Korea Ministry of Food and Drug
Safety approved partner SK Bioscience's Covid-19 vaccine for
adolescents aged 12 through 17 in South Korea.
The Gaithersburg, Md.-based biotech company said the approval
was based on data from ongoing pediatric trials across 73 sites in
the U.S.
Preliminary safety data from the trial showed the vaccine to be
generally well-tolerated, and adverse events were low in number and
balanced between vaccine and placebo groups not considered related
to the vaccine, the company said.
In the 12 through 17 year-old population, the Novavax's vaccine
has been granted authorization in India, the European Union,
Australia, Thailand, and Japan, and is actively under review in
other markets, the company said.
Write to Connor Hart at connor.hart@wsj.com
(END) Dow Jones Newswires
August 12, 2022 09:55 ET (13:55 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
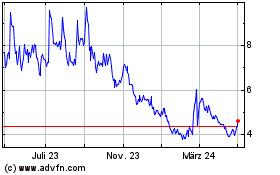
Novavax (NASDAQ:NVAX)
Historical Stock Chart
Von Mär 2024 bis Apr 2024
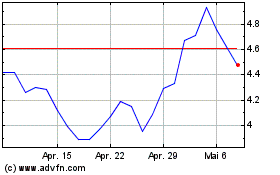
Novavax (NASDAQ:NVAX)
Historical Stock Chart
Von Apr 2023 bis Apr 2024