Incyte Gets FDA Approval of Zynyz, Triggers Milestone Payment to MacroGenics
22 März 2023 - 7:19PM
Dow Jones News
By Colin Kellaher
Incyte Corp. said on Wednesday the U.S. Food and Drug
Administration granted accelerated approval to Zynyz for the
treatment of adults with metastatic or recurrent locally advanced
Merkel cell carcinoma, a rare and aggressive type of skin
cancer.
The Wilmington, Del., biopharmaceutical company said the
accelerated green light is based on the tumor-response rate and the
duration of response, adding that continued approval may be
contingent on verification and description of the clinical benefits
in confirmatory trials.
The approval triggers the payment of a $15 million milestone
payment from Incyte to another biopharmaceutical company,
MacroGenics Inc., which developed Zynyz and licensed it to Incyte
as part of a 2017 collaboration.
MacroGenics, based in Rockville, Md., said it remains eligible
for up to $320 million in potential additional development and
regulatory milestones, along with up to $330 million in potential
commercial milestones and royalties on sales of the drug.
Incyte noted that it is studying Zynyz in other tumor types and
in combination with other pipeline compounds.
Write to Colin Kellaher at colin.kellaher@wsj.com
(END) Dow Jones Newswires
March 22, 2023 14:04 ET (18:04 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
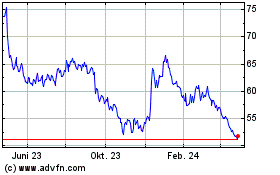
Incyte (NASDAQ:INCY)
Historical Stock Chart
Von Mär 2024 bis Apr 2024
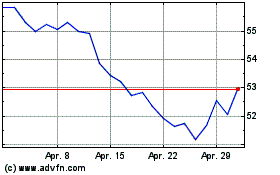
Incyte (NASDAQ:INCY)
Historical Stock Chart
Von Apr 2023 bis Apr 2024