AstraZeneca Says Nirsevimab Recommended for Approval in EU
16 September 2022 - 8:45AM
Dow Jones News
By Kyle Morris
AstraZeneca PLC said Friday that nirsevimab, a treatment for the
prevention of respiratory syncytial virus lower respiratory tract
disease in infants, has been recommended for approval in the
EU.
The Anglo-Swedish pharma major said the Committee for Medicinal
Products for Human Use of the European Medicines Authority
recommended the treatment, know commercially as Beyfortus, based on
a clinical trial program which demonstrated protection against RSV
disease during the RSV season with a single dose.
Beyfortus is being developed jointly by AstraZeneca and Sanofi
and is an investigational long-acting antibody designed for all
infants for protection against RSV disease from birth. Beyfortus
would be the first preventative option for newborn and infant
population.
In trials, Beyfortus met its primary endpoint of reducing
infections caused by RSV during the season versus placebo.
Write to Kyle Morris at kyle.morris@dowjones.com
(END) Dow Jones Newswires
September 16, 2022 02:30 ET (06:30 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
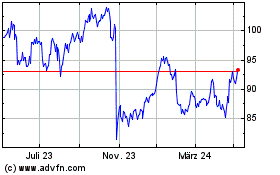
Sanofi (EU:SAN)
Historical Stock Chart
Von Mär 2024 bis Apr 2024
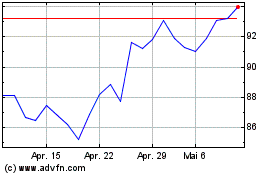
Sanofi (EU:SAN)
Historical Stock Chart
Von Apr 2023 bis Apr 2024