Immunocore Shares Rise 10% on Collaboration With Sanofi
03 Juni 2022 - 5:21PM
Dow Jones News
By Chris Wack
Immunocore Holdings Plc shares rose 10% to $33.87 after the
company said it has entered into a clinical trial collaboration and
supply agreement with Sanofi.
Under the agreement, Sanofi will evaluate SAR444245 in
combination with Kimmtrak, Immunocore's novel bispecific protein
targeting gp100, in HLA-A*02:01 positive patients with advanced
unresectable or metastatic skin cancers as part of Sanofi's ongoing
Phase 1/2 study.
Sanofi will be responsible for clinical development and will
assume all costs associated with the study, other than expenses
related to the manufacturing and supply of Kimmtrak, for which
Immunocore is responsible.
In January 2022 and April 2022, the U.S. Food and Drug
Administration and the European Commission, respectively, approved
Kimmtrak tebentafusp for the treatment of HLA-A*02:01-positive
adult patients with unresectable or metastatic uveal melanoma.
Immunocore is planning a randomized study of Kimmtrak with or
without anti-PD1 therapy in patients with metastatic melanoma and
anticipates initiating the trial in the fourth quarter of 2022.
Write to Chris Wack at chris.wack@wsj.com
(END) Dow Jones Newswires
June 03, 2022 11:06 ET (15:06 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
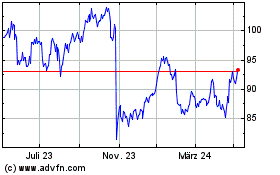
Sanofi (EU:SAN)
Historical Stock Chart
Von Mär 2024 bis Apr 2024
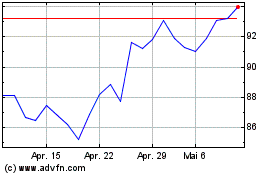
Sanofi (EU:SAN)
Historical Stock Chart
Von Apr 2023 bis Apr 2024