Sanofi: U.S. FDA Gives Authorization to Expedite Development of Hemophilia Drug
01 Juni 2022 - 7:52AM
Dow Jones News
By Cecilia Butini
Sanofi SA said Wednesday that the U.S. Food and Drug
Administration has granted breakthrough therapy designation to
hemophilia A drug efanesoctocog alfa, a move meant to expedite the
development and review of drugs that address serious illnesses.
The designation is based on positive trial results that show the
drug's ability to prevent bleeds and superiority in preventing them
compared with previous treatments. The Phase 3 trial in question,
named XTEND-1, has met its primary endpoint of showing a clinically
meaningful prevention of bleeds, the company said.
Hemophilia is a condition targeting males especially, in which
the ability for blood to coagulate is impaired, leading to abnormal
bleeding.
Write to Cecilia Butini at cecilia.butini@wsj.com
(END) Dow Jones Newswires
June 01, 2022 01:37 ET (05:37 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
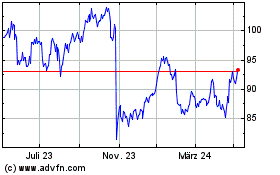
Sanofi (EU:SAN)
Historical Stock Chart
Von Mär 2024 bis Apr 2024
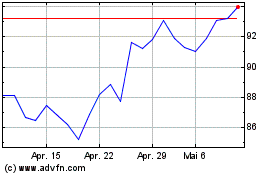
Sanofi (EU:SAN)
Historical Stock Chart
Von Apr 2023 bis Apr 2024