Theraclion: a Harvest of Good News
27 Juli 2022 - 6:00PM
Business Wire
Regulatory News:
THERACLION (ISIN: FR0010120402; Mnemo: ALTHE) (Paris:ALTHE),
an innovative company developing a robotic platform for
non-invasive ultrasound therapy, has achieved major milestones
in its development strategy in the first half of the year.
R&D at the core of the new strategy
Yann Duchesne, Executive Chairman of Theraclion since January
2022, is executing a new strategy. He is focusing Theraclion on
improving technology performance and clinical benefits to access
vein markets in the US. In Europe, the focus is on standardizing
treatment protocols among current Key Opinion Leaders (KOLs) to
pave the way for aggressive growth and U.S. clinical trials.
Theraclion will resume its commercial efforts upon finalization of
treatment protocols.
Yann Duchesne's vision is to develop Theraclion in three
therapeutic areas and three geographies: varicose veins, in the
U.S. (FDA application pending) and in Europe; thyroid, in Europe
and China; and breast cancer, primarily in the U.S. Value creation
consists of proving the potential of our disruptive technology
through robust clinical trials in each of our three indications and
geographies. Theraclion is advancing on concluding strategic
partnerships by indication/geography: e.g. Veins USA, Thyroid
China, Breast USA, Veins China, Thyroid USA... Such partnerships
will highlight our technology and show its true value. Negotiations
are underway.
First treatments in the U.S. in a feasibility trial as a
prelude to the pivotal registration study with the FDA
The first patients were treated with SONOVEIN® in the United
States at the Englewood center, New Jersey by vascular surgeons Dr.
Steven Elias, Dr. Antonios Gasparis and Prof. Nicos Labropoulos in
a feasibility trial. This is a major milestone in obtaining
marketing authorization from the Food and Drug Administration
(FDA).
These first treatments represent a further step in the strategy
of focusing on key varicose vein markets, of which the United
States is the largest with $5 billion in annual spending.
SONOVEIN® HD: first treatments with a standard system
This first half of 2022 is marked by the acceleration of
technical developments, resulting in the release of the first
SONOVEIN® HD to opinion leaders. This new version integrates the
SuperSonic Imagine (Hologic) Mach30 imager.
The SONOVEIN® HD has been very well received by surgeons and
vascular physicians and they are working on the optimization of
treatment protocols.
Inclusion of the first patients in Phase IIA breast cancer
research
The first patients were included in the Phase IIA of this
partnership with the University of Virginia Cancer Centre (UVA) in
the United States. Breast cancer research takes another step
forward.
The study aims to establish the combined effect of high
intensity focused ultrasound (HIFU) and low dose chemotherapy on
the immune response to breast cancer. After a first phase on
advanced stage subjects, this second part concerns early stage
subjects.
Results as of June 30th, 2022
A capital increase of 6.5 million euros was successfully carried
out in February and strengthened financial visibility. A move to
new premises, also in Malakoff (Paris), in June, increases the
space dedicated to R&D.
Theraclion SA had revenues of €347K in the first half of 2022, a
decrease of 61% compared to the first half of 2021. Theraclion sold
4 Echopulse® systems in 2021 compared to only one refurbished
system sold in 2022. Sales of consumables increased by 45%, driven
by sales of consumables for the treatment of varicose veins. The
expert centers have a mixed activity of developing treatment
protocols and commercial treatments.
Half-yearly sales/K€
S1 2022
S1 2021
Variation
Sales of systems
105
659
-84%
Sales of consumables
190
131
+45%
Sales of services
52
95
-45%
Theraclion SA sales
347
885
-61%
Of which breast and thyroid
212
811
-74%
Of which vein
135
74
+82%
About Theraclion
At Theraclion, we believe that surgery as we know it is
outdated. It generates excessive anxiety in patients and turns
doctors into the executors of an archaic system. On the other hand,
it subjects the health care system to tensions that are difficult
to sustain. We therefore wanted to shake up this convention by
creating an extracorporeal treatment device. Our solution replaces
surgery with a robotic treatment that directs high-intensity
focused ultrasound (HIFU) from outside the body. Our
state-of-the-art ultrasound therapy device has already obtained CE
mark for the non-invasive treatment of varicose veins with
SONOVEIN® and breast fibroadenomas and thyroid nodules with
Echopulse®.
Based in Malakoff, near Paris, our employees are constantly
seeking innovation, combining high-level clinical research with the
benefits of artificial intelligence. The varicose vein treatment
market alone generates approximately 5 million procedures per year.
It is therefore a dynamic market in which we are changing the
paradigms by making non-invasive ultrasound therapy the new
standard.
For more information, please visit www.theraclion.com and our
patient platform http://www.echotherapie.com.
Theraclion is listed on Euronext Growth Paris
Eligible for the PEA-PME scheme
Mnémonique : ALTHE - Code ISIN : FR0010120402
LEI : 9695007X7HA7A1GCYD29
View source
version on businesswire.com: https://www.businesswire.com/news/home/20220727005817/en/
Theraclion
David AUREGAN Chief Operating Officer
david.auregan@theraclion.com
Anja KLEBER VP Marketing, Market Access & Sales Francophonia
anja.kleber@theraclion.com
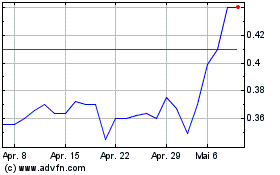
Theraclion (EU:ALTHE)
Historical Stock Chart
Von Mär 2024 bis Apr 2024
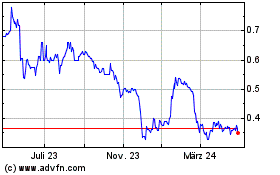
Theraclion (EU:ALTHE)
Historical Stock Chart
Von Apr 2023 bis Apr 2024